Colletotrichum higginsianum as a Model for Understanding Host–Pathogen Interactions: A Review
Abstract
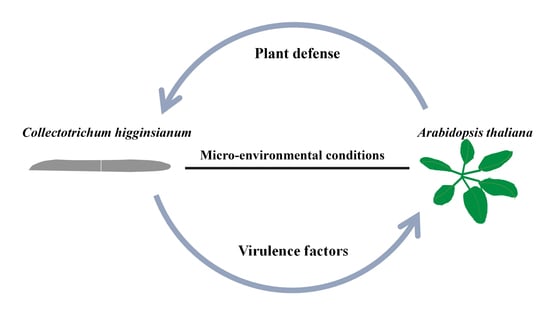
:1. Introduction
2. Infection Strategies
3. Genomics and Genetics
3.1. Genome Sequencing and Assembly
3.2. Transcriptome Analyses
3.3. Genetic Transformation
4. Virulence Factors
4.1. Mitogen-Activated Protein (MAP) Kinase and cAMP/PKA Signaling Pathway
4.2. Nutrition, Transporter and Amino Acid Biosynthesis
4.3. Effectors
5. Molecular Interactions
5.1. Primary Metabolic Pathways
5.2. Phytohormones
5.3. Resistance Genes
6. Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Jayawardena, R.S.; Hyde, K.D.; Damm, U.; Cai, L.; Liu, M.; Li, X.H.; Zhang, W.; Zhao, W.; Yan, J.Y. Notes on currently accepted species of Colletotrichum. Mycosphere 2016, 7, 1192–1260. [Google Scholar] [CrossRef]
- Damm, U.; O’Connell, R.J.; Groenewald, J.Z.; Crous, P.W. The Colletotrichum destructivum [italicize] species complex–hemibiotrophic pathogens of forage and field crops. Stud. Mycol. 2014, 79, 49–84. [Google Scholar] [CrossRef] [PubMed]
- Crouch, J.; O’Connell, R.; Gan, P.; Buiate, E.; Torres, M.F.; Beirn, L.; Shirasu, K.; Vaillancourt, L. The genomics of Colletotrichum. In Genomics of Plant-Associated Fungi: Monocot Pathogens; Dean, R.A., Lichens-Park, A., Kole, C., Eds.; Springer: Berlin, Germany, 2014; pp. 69–102. [Google Scholar]
- Narusaka, M.; Abe, H.; Kobayashi, M.; Kubo, Y.; Narusaka, Y. Comparative analysis of expression profiles of counterpart gene sets between Brassica rapa and Arabidopsis thaliana during fungal pathogen Colletotrichum higginsianum infection. Plant Biotechnol. J. 2006, 23, 503–508. [Google Scholar] [CrossRef]
- Shimada, C.; Lipka, V.; O’Connell, R.; Okuno, T.; Schulze-Lefert, P.; Takano, Y. Nonhost resistance in Arabidopsis-Colletotrichum interactions acts at the cell periphery and requires actin filament function. Mol. Plant-Microbe Interact. 2006, 19, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Zampounis, A.; Pigné, S.; Dallery, J.F.; Wittenberg, A.H.; Zhou, S.; Schwartz, D.C.; Thon, M.R.; O’Connell, R.J. Genome sequence and annotation of Colletotrichum higginsianum, a causal agent of crucifer anthracnose disease. Genome Announc. 2016, 4, e00821-16. [Google Scholar] [CrossRef] [PubMed]
- De Silva, D.D.; Crous, P.W.; Ades, P.K.; Hyde, K.D.; Taylor, P.W. Life styles of Colletotrichum species and implications for plant biosecurity. Fungal Biol. Rev. 2017, 31, 155–168. [Google Scholar] [CrossRef]
- Latunde-Dada, A.O.; O’connell, R.J.; Nash, C.; Pring, R.J.; Lucas, J.A.; Bailey, J.A. Infection process and identity of the hemibiotrophic anthracnose fungus (Colletotrichum destructivum) from cowpea (Vigna unguiculata). Mycol. Res. 1996, 100, 1133–1141. [Google Scholar] [CrossRef]
- Münch, S.; Lingner, U.; Floss, D.S.; Ludwig, N.; Sauer, N.; Deising, H.B. The hemibiotrophic lifestyle of Colletotrichum species. J. Plant Physiol. 2008, 165, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Prasanth, C.N.; Viswanathan, R.; Krishna, N.; Malathi, P.; Sundar, A.R.; Tiwari, T. Unraveling the genetic complexities in gene set of sugarcane red rot pathogen Colletotrichum falcatum through transcriptomic approach. Sugar Tech. 2017, 19, 604–615. [Google Scholar] [CrossRef]
- Crouch, J.A. Colletotrichum caudatum s.l. is a species complex. IMA Fungus 2014, 5, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Buiate, E.A.; Xavier, K.V.; Moore, N.; Torres, M.F.; Farman, M.L.; Schardl, C.L.; Vaillancourt, L.J. A comparative genomic analysis of putative pathogenicity genes in the host-specific sibling species Colletotrichum graminicola and Colletotrichum sublineola. BMC Genom. 2017, 18, 67. [Google Scholar] [CrossRef] [PubMed]
- Perfect, S.E.; Green, J.R. Infection structures of biotrophic and hemibiotrophic fungal plant pathogens. Mol. Plant Pathol. 2001, 2, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Foster, A.J.; Ryder, L.S.; Kershaw, M.J.; Talbot, N.J. The role of glycerol in the pathogenic lifestyle of the rice blast fungus Magnaporthe oryzae. Environ. Microbiol. 2017, 19, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, R.J.; Thon, M.R.; Hacquard, S.; Amyotte, S.G.; Kleemann, J.; Torres, M.F.; Damm, U.; Buiate, E.A.; Epstein, L.; Alkan, N.; et al. Lifestyle transitions in plant pathogenic Colletotrichum fungi deciphered by genome and transcriptome analyses. Nat. Genet. 2012, 44, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Gan, P.; Ikeda, K.; Irieda, H.; Narusaka, M.; O’Connell, R.J.; Narusaka, Y.; Takano, Y.; Kubo, Y.; Shirasu, K. Comparative genomic and transcriptomic analyses reveal the hemibiotrophic stage shift of Colletotrichum fungi. New Phytol. 2013, 197, 1236–1249. [Google Scholar] [CrossRef] [PubMed]
- Dallery, J.F.; Lapalu, N.; Zampounis, A.; Pigné, S.; Luyten, I.; Amselem, J.; Wittenberg, A.H.; Zhou, S.; Queiroz, M.V.; Robin, G.P. Gapless genome assembly of Colletotrichum higginsianum reveals chromosome structure and association of transposable elements with secondary metabolite gene clusters. BMC Genom. 2017, 18, 667. [Google Scholar] [CrossRef] [PubMed]
- Faino, L.; Seidl, M.F.; Datema, E.; van den Berg, G.C.; Janssen, A.; Wittenberg, A.H.; Thomma, B.P. Single-molecule real-time sequencing combined with optical mapping yields completely finished fungal genome. Mbio 2015, 6, e00936-15. [Google Scholar] [CrossRef] [PubMed]
- Van Kan, J.A.; Stassen, J.H.; Mosbach, A.; Van Der Lee, T.A.; Faino, L.; Farmer, A.D.; Papasotiriou, D.G.; Zhou, S.G.; Seidl, M.F.; Cottam, E. A gapless genome sequence of the fungus Botrytis cinerea. Mol. Plant Pathol. 2017, 18, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Derbyshire, M.; Denton-Giles, M.; Hegedus, D.; Seifbarghy, S.; Rollins, J.; Van Kan, J.; Raffaele, S. The complete genome sequence of the phytopathogenic fungus Sclerotinia sclerotiorum reveals insights into the genome architecture of broad host range pathogens. Genome Biol. Evol. 2017, 9, 593–618. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, S.B.; M’barek, S.B.; Dhillon, B.; Wittenberg, A.H.; Crane, C.F.; Hane, J.K.; Foster, A.J.; Van der Lee, T.A.; Grimwood, J.; Aerts, A. Finished genome of the fungal wheat pathogen Mycosphaerella graminicola reveals dispensome structure, chromosome plasticity, and stealth pathogenesis. PLoS Genet. 2011, 7, e1002070. [Google Scholar] [CrossRef] [PubMed]
- King, R.; Urban, M.; Hammond-Kosack, M.C.; Hassani-Pak, K.; Hammond-Kosack, K.E. The completed genome sequence of the pathogenic ascomycete fungus Fusarium graminearum. BMC Genom. 2015, 16, 544. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, K.; Fang, A.; Han, Y.; Yang, J.; Xue, M.; Bao, J.; Hu, D.; Zhou, B.; Sun, X.; et al. Specific adaptation of Ustilaginoidea virens in occupying host florets revealed by comparative and functional genomics. Nat. Commun. 2014, 5, 3849. [Google Scholar] [CrossRef] [PubMed]
- Wiemann, P.; Sieber, C.M.; Von Bargen, K.W.; Studt, L.; Niehaus, E.M.; Espino, J.J.; Huß, K.; Michielse, C.B.; Albermann, S.; Wagner, D. Deciphering the cryptic genome: Genome-wide analyses of the rice pathogen Fusarium fujikuroi reveal complex regulation of secondary metabolism and novel metabolites. PLoS Pathog. 2013, 9, e1003475. [Google Scholar] [CrossRef] [PubMed]
- Giles, S.S.; Soukup, A.A.; Lauer, C.; Shaaban, M.; Lin, A.; Oakley, B.R.; Wang, C.C.; Keller, N.P. Cryptic Aspergillus nidulans antimicrobials. Appl. Environ. Microb. 2011, 77, 3669–3675. [Google Scholar] [CrossRef] [PubMed]
- Inglis, D.O.; Binkley, J.; Skrzypek, M.S.; Arnaud, M.B.; Cerqueira, G.C.; Shah, P.; Wymore, F.; Wortman, J.R.; Sherlock, G. Comprehensive annotation of secondary metabolite biosynthetic genes and gene clusters of Aspergillus nidulans, A. fumigatus, A. niger and A. oryzae. BMC Microbiol. 2013, 13, 91. [Google Scholar] [CrossRef] [PubMed]
- Chooi, Y.H.; Muria-Gonzalez, M.J.; Solomon, P.S.A. Genome-wide survey of the secondary metabolite biosynthesis genes in the wheat pathogen Parastagonospora nodorum. Mycology 2014, 5, 192–206. [Google Scholar] [CrossRef] [PubMed]
- Yaegashi, J.; Oakley, B.R.; Wang, C.C. Recent advances in genome mining of secondary metabolite biosynthetic gene clusters and the development of heterologous expression systems in Aspergillus nidulans. J. Ind. Microbiol. Biotechnol. 2014, 41, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Plaumann, P.L.; Schmidpeter, J.; Dahl, M.; Taher, L.; Koch, C. A dispensable chromosome is required for virulence in the hemibiotrophic plant pathogen Colletotrichum higginsianum. Front. Microbiol. 2018, 9, 1005. [Google Scholar] [CrossRef] [PubMed]
- Takahara, H.; Dolf, A.; Endl, E.; O’Connell, R. Flow cytometric purification of Colletotrichum higginsianum biotrophic hyphae from Arabidopsis leaves for stage-specific transcriptome analysis. Plant J. 2009, 59, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Huser, A.; Takahara, H.; Schmalenbach, W.; O’Connell, R. Discovery of pathogenicity genes in the crucifer anthracnose fungus Colletotrichum higginsianum, using random insertional mutagenesis. Mol. Plant-Microbe Int. 2009, 22, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhao, D.; Zheng, L.; Hsiang, T.; Wei, Y.; Fu, Y.; Huang, J. Identification of virulence genes in the crucifer anthracnose fungus Colletotrichum higginsianum by insertional mutagenesis. Microb. Pathog. 2013, 64, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Korn, M.; Schmidpeter, J.; Dahl, M.; Müller, S.; Voll, L.M.; Koch, C. A genetic screen for pathogenicity genes in the hemibiotrophic fungus Colletotrichum higginsianum identifies the plasma membrane proton pump Pma2 required for host penetration. PLoS ONE 2015, 10, e0125960. [Google Scholar] [CrossRef] [PubMed]
- Guisbert, K.S.; Li, H.; Guthrie, C. Alternative 3′ pre-mRNA processing in Saccharomyces cerevisiae is modulated by Nab4/Hrp1 in vivo. PLoS Biol. 2006, 5, e6. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.J.; Cansizoglu, A.E.; Süel, K.E.; Louis, T.H.; Zhang, Z.; Chook, Y.M. Rules for nuclear localization sequence recognition by karyopherin β2. Cell 2006, 126, 543–558. [Google Scholar] [CrossRef] [PubMed]
- Araújo-Bazán, L.; Dhingra, S.; Chu, J.; Fernández-Martínez, J.; Calvo, A.M.; Espeso, E.A. Importin α is an essential nuclear import carrier adaptor required for proper sexual and asexual development and secondary metabolism in Aspergillus nidulans. Fungal Genet. Biol. 2009, 46, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ding, F.; Zhang, L.; Sheng, Y.; Zheng, X.; Wang, Y. The importin α subunit PsIMPA1 mediates the oxidative stress response and is required for the pathogenicity of Phytophthora sojae. Fungal Genet. Biol. 2015, 82, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Ghassemi, S.; Lichius, A.; Bidard, F.; Lemoine, S.; Rossignol, M.N.; Herold, S.; Seiboth, V.S.; Seiboth, B.; Espeso, E.A.; Margeot, A. The β-importin KAP8 (Pse1/Kap121) is required for nuclear import of the cellulase transcriptional regulator XYR1, asexual sporulation and stress resistance in Trichoderma reesei. Mol. Microbiol. 2015, 96, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Ushimaru, T.; Terada, H.; Tsuboi, K.; Kogou, Y.; Sakaguchi, A.; Tsuji, G.; Kubo, Y. Development of an efficient gene targeting system in Colletotrichum higginsianum using a non-homologous end-joining mutant and Agrobacterium tumefaciens-mediated gene transfer. Mol. Genet. Genom. 2010, 284, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Masuda, T.; Koyama, Y. Enhanced gene targeting frequency in ku70 and ku80 disruption mutants of Aspergillus sojae and Aspergillus oryzae. Mol. Genet. Genom. 2006, 275, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Villalba, F.; Collemare, J.; Landraud, P.; Lambou, K.; Brozek, V.; Cirer, B.; Morin, D.; Brue, C.; Beffa, B.; Lebrun, M.H. Improved gene targeting in Magnaporthe grisea by inactivation of MgKU80 required for non-homologous end joining. Fungal Genet. Biol. 2008, 45, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Takahara, H.; Huser, A.; O’Connell, R. Two arginine biosynthesis genes are essential for pathogenicity of Colletotrichum higginsianum on Arabidopsis. Mycology 2012, 3, 54–64. [Google Scholar]
- Takahara, H.; Hacquard, S.; Kombrink, A.; Hughes, H.B.; Halder, V.; Robin, G.P.; Hiruma, K.; Neumann, U.; Shinya, T.; Kombrink, E.; et al. Colletotrichum higginsianum extracellular LysM proteins play dual roles in appressorial function and suppression of chitin-triggered plant immunity. New Phytol. 2016, 211, 1323–1337. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Chen, M.; Yan, Y.; Gu, Q.; Huang, J.; Zheng, L. ChSte7 is required for vegetative growth and various plant infection processes in Colletotrichum higginsianum. BioMed Res. Int. 2016, 2016, 1–11. [Google Scholar]
- Gu, Q.; Chen, M.; Huang, J.; Wei, Y.; Hsiang, T.; Zheng, L. Multifaceted roles of the Ras guanine-nucleotide exchange factor ChRgf in development, pathogenesis, and stress responses of Colletotrichum higginsianum. Phytopathology 2017, 107, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Xiong, Y.; Zhu, W.; Wang, N.; Yang, G.; Peng, F. Colletotrichum higginsianum mitogen-activated protein kinase ChMK1: Role in growth, cell wall integrity, colony melanization, and pathogenicity. Front. Microbiol. 2016, 7, 1212. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yan, Y.; Huang, J.; Hsiang, T.; Wei, Y.; Li, Y.; Gao, J.; Zheng, L. A novel MFS transporter gene ChMfs1 is important for hyphal morphology, conidiation, and pathogenicity in Colletotrichum higginsianum. Front. Microbiol. 2017, 8, 1953. [Google Scholar] [CrossRef] [PubMed]
- Schmidpeter, J.; Dahl, M.; Hofmann, J.; Koch, C. ChMob2 binds to ChCbk1 and promotes virulence and conidiation of the fungal pathogen Colletotrichum higginsianum. BMC Microbiol. 2017, 17, 22. [Google Scholar] [CrossRef] [PubMed]
- Kubo, Y.; Harata, K.; Kodama, S.; Fukada, F. Development of the infection strategy of the hemibiotrophic plant pathogen, Colletotrichum orbiculare, and plant immunity. Physiol. Mol. Plant P 2016, 95, 32–36. [Google Scholar] [CrossRef]
- Zhao, X.; Mehrabi, R.; Xu, J.R. Mitogen-activated protein kinase pathways and fungal pathogenesis. Eukaryot. Cell 2007, 6, 1701–1714. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Zhang, X.; Liu, H.; Xu, J.R. Mitogen-activated protein kinase signaling in plant pathogenic fungi. PLoS Pathog. 2018, 14, e1006875. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhou, M.; Xiong, Z.; Peng, F.; Wei, W. The cAMP-PKA signaling pathway regulates pathogenicity, hyphal growth, appressorial formation, conidiation, and stress tolerance in Colletotrichum higginsianum. Front. Microbiol. 2017, 8, 1416. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, X.; Hu, S.; Liu, H.; Xu, J.R. PKA activity is essential for relieving the suppression of hyphal growth and appressorium formation by MoSfl1 in Magnaporthe oryzae. PLoS Genet. 2017, 13, e1006954. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhao, X.; Xue, C.; Dai, Y.; Xu, J.R. Bypassing both surface attachment and surface recognition requirements for appressorium formation by overactive ras signaling in Magnaporthe oryzae. Mol. Plant-Microbe Int. 2014, 27, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Xu, J.; Zhao, Y.; Wang, K. CtPMK1, a mitogen-activated-protein kinase gene, is required for conidiation, appressorium formation, and pathogenicity of Colletotrichum truncatum on soybean. Ann. Appl. Biol. 2015, 167, 63–74. [Google Scholar] [CrossRef]
- He, P.; Wang, Y.; Wang, X.; Zhang, X.; Tian, C. The mitogen-activated protein kinase CgMK1 governs appressorium formation, melanin synthesis, and plant infection of Colletotrichum gloeosporioides. Front. Microbiol. 2017, 8, 2216. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, R.; Herbert, C.; Sreenivasaprasad, S.; Khatib, M.; Esquerré-Tugayé, M.T.; Dumas, B. A novel Arabidopsis-Colletotrichum pathosystem for the molecular dissection of plant-fungal interactions. Mol. Plant -Microbe Int. 2004, 17, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Hergovich, A.; Stegert, M.R.; Schmitz, D.; Hemmings, B.A. NDR kinases regulate essential cell processes from yeast to humans. Nat. Rev. Mol. Cell Biol. 2006, 7, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.C.; Salek, J.; McCollum, D. Mob1p interacts with the Sid2p kinase and is required for cytokinesis in fission yeast. Curr. Biol. 2000, 10, 619–622. [Google Scholar] [CrossRef]
- Hou, M.C.; Wiley, D.J.; Verde, F.; McCollum, D. Mob2p interacts with the protein kinase Orb6p to promote coordination of cell polarity with cell cycle progression. J. Cell Sci. 2003, 116, 125–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, B.; Kurischko, C.; Horecka, J.; Mody, M.; Nair, P.; Pratt, L.; Pratt, L.; Zougman, A.; McBroom, L.D.; Hughes, T.R.; et al. RAM: A conserved signaling network that regulates Ace2p transcriptional activity and polarized morphogenesis. Mol. Biol. Cell 2003, 14, 3782–3803. [Google Scholar] [CrossRef] [PubMed]
- Hotz, M.; Barral, Y. The Mitotic Exit Network: New turns on old pathways. Trends Cell Biol. 2014, 24, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, J.; Xu, J.; Shi, J. FgIlv5 is required for branched-chain amino acid biosynthesis and full virulence in Fusarium graminearum. Microbiology 2014, 160, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Shi, W.; Xu, X.W.; Li, Z.G.; Yin, C.F.; Peng, J.B.; Pan, S.; Chen, X.; Zhao, W.; Zhang, Y.; et al. Glutamate synthase MoGlt1-mediated glutamate homeostasis is important for autophagy, virulence and conidiation in the rice blast fungus. Mol. Plant Pathol. 2018, 19, 564–578. [Google Scholar] [CrossRef] [PubMed]
- Solomon, P.S.; Thomas, S.W.; Spanu, P.; Oliver, R.P. The utilisation of di/tripeptides by Stagonospora nodorum is dispensable for wheat infection. Physiol. Mol. Plant P 2003, 63, 191–199. [Google Scholar] [CrossRef]
- Horst, R.J.; Doehlemann, G.; Wahl, R.; Hofmann, J.; Schmiedl, A.; Kahmann, R.; Kämper, J.; Voll, L.M. Ustilago maydis infection strongly alters organic nitrogen allocation in maize and stimulates productivity of systemic source leaves. Plant Physiol. 2010, 152, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Bi, F.; Ment, D.; Luria, N.; Meng, X.; Prusky, D. Mutation of AREA affects growth, sporulation, nitrogen regulation, and pathogenicity in Colletotrichum gloeosporioides. Fungal Genet. Biol. 2017, 99, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Garcia, E.; Deising, H.B. Attenuation of PAMP-triggered immunity in maize requires down-regulation of the key β-1, 6-glucan synthesis genes KRE5 and KRE6 in biotrophic hyphae of Colletotrichum graminicola. Plant J. 2016, 87, 355–375. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Moye-Rowley, W.S. Multidrug resistance in fungi: Regulation of transporter-encoding gene expression. Front. Physiol. 2014, 5, 143. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, S.C.; Teixeira, M.C.; Dias, P.J.; Sá-Correia, I. MFS transporters required for multidrug/multixenobiotic (MD/MX) resistance in the model yeast: Understanding their physiological function through post-genomic approaches. Front. Physiol. 2014, 5, 180. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.H.; Tsai, H.C.; Yu, P.L.; Chung, K.R. A major facilitator superfamily transporter-Mediated resistance to oxidative stress and fungicides requires Yap1, Skn7, and MAP kinases in the citrus fungal pathogen alternaria alternata. PLoS ONE 2017, 12, e0169103. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Gao, N.; Wang, Q.; Ren, Y.; Wang, K.; Zhu, T. BcMctA, a putative monocarboxylate transporter, is required for pathogenicity in Botrytis cinereal. Curr. Genet. 2015, 61, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Crutcher, F.K.; Liu, J.; Puckhaber, L.S.; Stipanovic, R.D.; Bell, A.A.; Nichols, R.L. FUBT, a putative MFS transporter, promotes secretion of fusaric acid in the cotton pathogen Fusarium oxysporum f. sp. vasinfectum. Microbiology 2015, 161, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Sempere, A.; Martínez-Culebras, P.V.; González-Candelas, L. The loss of the inducible Aspergillus carbonarius MFS transporter MfsA leads to ochratoxin a overproduction. Int. J. Food Mcrobiol. 2014, 181, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Temme, N.; Oeser, B.; Massaroli, M.; Heller, J.; Simon, A.; Gonzalez Collado, I.; Viaud, M.; Tudzynski, P. BcAtf1, a global regulator, controls various differentiation processes and phytotoxin production in Botrytis cinerea. Mol. Plant Pathol. 2012, 13, 704–718. [Google Scholar] [CrossRef] [PubMed]
- Choquer, M.; Lee, M.H.; Bau, H.J.; Chung, K.R. Deletion of a MFS transporter-like gene in Cercospora nicotianae reduces cercosporin toxin accumulation and fungal virulence. FEBS Lett. 2007, 581, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, P.L.; Carafoli, E. Ion motive ATPases. I. Ubiquity, properties, and significance to cell function. Trends Biochem. Sci. 1987, 12, 146–150. [Google Scholar] [CrossRef]
- Portillo, F. Regulation of plasma membrane H+-ATPase in fungi and plants. BBA-Rev. Biomembranes 2000, 1469, 31–42. [Google Scholar] [CrossRef]
- Saliba, E.; Evangelinos, M.; Gournas, C.; Corrillon, F.; Georis, I.; André, B. The yeast H+-ATPase Pma1 promotes Rag/Gtr-dependent TORC1 activation in response to H+-coupled nutrient uptake. Elife 2018, 7, e31981. [Google Scholar] [CrossRef] [PubMed]
- Charoenbhakdi, S.; Dokpikul, T.; Burphan, T.; Techo, T.; Auesukaree, C. Vacuolar H+-ATPase protects Saccharomyces cerevisiae cells against ethanol-induced oxidative and cell wall stresses. Appl. Environ. Microb. 2016, 82, 3121–3130. [Google Scholar] [CrossRef] [PubMed]
- Plaza, V.; Lagües, Y.; Carvajal, M.; Pérez-García, L.A.; Mora-Montes, H.M.; Canessa, P.; Larrondo, L.F.; Castillo, L. bcpmr1 encodes a P-type Ca2+/Mn2+-ATPase mediating cell-wall integrity and virulence in the phytopathogen Botrytis cinerea. Fungal Genet. Biol. 2015, 76, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Requena, N.; Breuninger, M.; Franken, P.; Ocón, A. Symbiotic status, phosphate, and sucrose regulate the expression of two plasma membrane H+-ATPase genes from the mycorrhizal fungus Glomus mosseae. Plant Physiol. 2003, 132, 1540–1549. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Yu, N.; Bano, S.A.; Liu, C.; Miller, A.J.; Cousins, D.; Zhang, X.; Ratet, P.; Tadege, M.; Mysore, K.S.A. H+-ATPase that energizes nutrient uptake during mycorrhizal symbioses in rice and Medicago truncatula. Plant Cell 2014, 26, 1818–1830. [Google Scholar] [CrossRef] [PubMed]
- Vleeshouwers, V.G.; Oliver, R.P. Effectors as tools in disease resistance breeding against biotrophic, hemibiotrophic, and necrotrophic plant pathogens. Mol. Plant-Microbe Int. 2014, 27, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Voegele, R.T.; Mendgen, K.W. Nutrient uptake in rust fungi: How sweet is parasitic life? Euphytica 2011, 179, 41–55. [Google Scholar] [CrossRef]
- Mims, C.W.; Richardson, E.A.; Holt, B.F., III; Dangl, J.L. Ultrastructure of the host pathogen interface in Arabidopsis thaliana leaves infected by the downy mildew Hyaloperonospora parasitica. Can. J. Bot. 2004, 82, 1001–1008. [Google Scholar] [CrossRef]
- Micali, C.O.; Neumann, U.; Grunewald, D.; Panstruga, R.; O’connell, R. Biogenesis of a specialized plant–fungal interface during host cell internalization of Golovinomyces orontii haustoria. Cell Microbiol. 2011, 13, 210–226. [Google Scholar] [CrossRef] [PubMed]
- Presti, L.L.; Kahmann, R. How filamentous plant pathogen effectors are translocated to host cells. Curr. Opin. Plant Biol. 2017, 38, 19–24. [Google Scholar] [CrossRef] [PubMed]
- De Jonge, R.; Thomma, B.P. Fungal LysM effectors: Extinguishers of host immunity? Trends Microbiol. 2009, 17, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Dodds, P.N.; Rathjen, J.P. Plant immunity: Towards an integrated view of plant–pathogen interactions. Nat. Rev. Genet. 2010, 11, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Vallet, A.; Mesters, J.R.; Thomma, B.P. The battle for chitin recognition in plant-microbe interactions. FEMS Microbiol. Rev. 2015, 39, 171–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Vallet, A.; Saleem-Batcha, R.; Kombrink, A.; Hansen, G.; Valkenburg, D.J.; Thomma, B.P.; Mesters, J.R. Fungal effector Ecp6 outcompetes host immune receptor for chitin binding through intrachain LysM dimerization. Elife 2013, 2, e00790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mentlak, T.A.; Kombrink, A.; Shinya, T.; Ryder, L.S.; Otomo, I.; Saitoh, H.; Terauchi, R.; Nishizawa, Y.; Shibuya, N.; Thomma, B.P.; et al. Effector-mediated suppression of chitin-triggered immunity by Magnaporthe oryzae is necessary for rice blast disease. Plant Cell 2012, 24, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Rudd, J.J.; Hammond-Kosack, K.E.; Kanyuka, K. Mycosphaerella graminicola LysM effector-mediated stealth pathogenesis subverts recognition through both CERK1 and CEBiP homologues in wheat. Mol. Plant-Microbe Int. 2014, 27, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Kombrink, A.; Rovenich, H.; Shi-Kunne, X.; Rojas-Padilla, E.; van den Berg, G.; Domazakis, E.; Thomma, B.P. Verticillium dahliae LysM effectors differentially contribute to virulence on plant hosts. Mol. Plant Pathol. 2017, 18, 596–608. [Google Scholar] [CrossRef] [PubMed]
- Kleemann, J.; Rincon-Rivera, L.J.; Takahara, H.; Neumann, U.; van Themaat, E.V.L.; van der Does, H.C.; Hacquard, S.; Stüber, K.; Will, I.; Schmalenbach, W. Sequential delivery of host-induced virulence effectors by appressoria and intracellular hyphae of the phytopathogen Colletotrichum higginsianum. PLoS Pathog. 2012, 8, e1002643. [Google Scholar] [CrossRef]
- Robin, G.P.; Kleemann, J.; Neumann, U.; Cabre, L.; Dallery, J.F.; Lapalu, N.; O’Connell, R.J. Subcellular localization screening of Colletotrichum higginsianum effector candidates identifies fungal proteins targeted to plant peroxisomes, Golgi bodies and microtubules. Front. Plant Sci. 2018, 9, 562. [Google Scholar] [CrossRef] [PubMed]
- Heath, M.C. Hypersensitive response-related death. In Programmed Cell Death in Higher Plants; Lam, E., Fukuda, H., Greenberg., J., Eds.; Springer: Dordrecht, Holland, 2000; pp. 77–90. ISBN 978. [Google Scholar]
- Hardham, A.R.; Jones, D.A.; Takemoto, D. Cytoskeleton and cell wall function in penetration resistance. Curr. Opin. Plant Biol. 2007, 10, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, I.; Kissen, R.; Bones, A.M. Phytoalexins in defense against pathogens. Trends Plant Sci. 2012, 17, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, L.C.; Rep, M.; Pieterse, C.M. Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef] [PubMed]
- Shigenaga, A.M.; Argueso, C.T. No hormone to rule them all: Interactions of plant hormones during the responses of plants to pathogens. Semin. Cell Dev. Biol. 2016, 56, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.; André, A.; Edwards, H.; Ehrhardt, D.; Somerville, S. Arabidopsis thaliana subcellular responses to compatible Erysiphe cichoracearum infections. Plant J. 2005, 44, 516–529. [Google Scholar] [CrossRef] [PubMed]
- Kangasjärvi, S.; Neukermans, J.; Li, S.; Aro, E.M.; Noctor, G. Photosynthesis, photorespiration, and light signalling in defence responses. J. Exp. Bot. 2012, 63, 1619–1636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojas, C.M.; Senthil-Kumar, M.; Tzin, V.; Mysore, K. Regulation of primary plant metabolism during plant-pathogen interactions and its contribution to plant defense. Front. Plant Sci. 2014, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Ji, Y.; Bhuiyan, N.H.; Pilot, G.; Selvaraj, G.; Zou, J.; Wei, Y. Amino acid homeostasis modulates salicylic acid–associated redox status and defense responses in Arabidopsis. Plant Cell 2010, 22, 3845–3863. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.H.; Singhal, R.; Kachroo, A.; Kachroo, P. Fatty acid–and lipid-mediated signaling in plant defense. Annu. Rev. Phytopathol. 2017, 55, 505–536. [Google Scholar] [CrossRef] [PubMed]
- Chanda, B.; Venugopal, S.C.; Kulshrestha, S.; Navarre, D.A.; Downie, B.; Vaillancourt, L.; Kachroo, A.; Kachroo, P. Glycerol-3-phosphate levels are associated with basal resistance to the hemibiotrophic fungus Colletotrichum higginsianum in Arabidopsis. Plant Physiol. 2008, 147, 2017–2029. [Google Scholar] [CrossRef] [PubMed]
- Drincovich, M.F.; Casati, P.; Andreo, C.S. NADP-malic enzyme from plants: A ubiquitous enzyme involved in different metabolic pathways. FEBS Lett. 2001, 490, 1–6. [Google Scholar] [CrossRef]
- Voll, L.M.; Zell, M.B.; Engelsdorf, T.; Saur, A.; Wheeler, M.G.; Drincovich, M.F.; Weber, A.P.; Maurino, V.G. Loss of cytosolic NADP-malic enzyme 2 in Arabidopsis thaliana is associated with enhanced susceptibility to Colletotrichum higginsianum. New Phytol. 2012, 195, 189–202. [Google Scholar] [CrossRef] [PubMed]
- McDowell, J.M.; Dangl, J.L. Signal transduction in the plant immune response. Trends Biochem. Sci. 2000, 25, 79–82. [Google Scholar] [CrossRef]
- Thomma, B.P.; Tierens, K.F.; Penninckx, I.A.; Mauch-Mani, B.; Broekaert, W.F.; Cammue, B.P. Different micro-organisms differentially induce Arabidopsis disease response pathways. Plant Physiol. Biochem. 2001, 39, 673–680. [Google Scholar] [CrossRef]
- Lee, Y.H.; Hong, J.K. Differential defence responses of susceptible and resistant kimchi cabbage cultivars to anthracnose, black spot and black rot diseases. Plant Pathol. 2015, 64, 406–415. [Google Scholar] [CrossRef]
- Bhagwat, R.G.; Mehta, B.P.; Patil, V.A.; Sharma, H. Screening of cultivars/varieties against mango anthracnose caused by Colletotrichum gloeosporioides. Int. J. Environ. Agric. Res. 2015, 1, 21–23. [Google Scholar]
- Jacob, I.; Hartmann, S.; Schubiger, F.X.; Struck, C. Resistance screening of red clover cultivars to Colletotrichum trifolii and improving the resistance level through recurrent selection. Euphytica 2015, 204, 303–310. [Google Scholar] [CrossRef]
- Mangandi, J.; Peres, N.A.; Whitaker, V.M. Identifying resistance to crown rot caused by Colletotrichum gloeosporioides in strawberry. Plant Dis. 2015, 99, 954–961. [Google Scholar] [CrossRef]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chisholm, S.T.; Coaker, G.; Day, B.; Staskawicz, B.J. Host-microbe interactions: Shaping the evolution of the plant immune response. Cell 2006, 124, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Narusaka, Y.; Narusaka, M.; Park, P.; Kubo, Y.; Hirayama, T.; Seki, M.; Shiraishi, T.; Shida, T.; Nakashima, M.; Enju, A.; et al. RCH1, a locus in Arabidopsis that confers resistance to the hemibiotrophic fungal pathogen Colletotrichum higginsianum. Mol. Plant-Microbe Int. 2004, 17, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Narusaka, M.; Shirasu, K.; Noutoshi, Y.; Kubo, Y.; Shiraishi, T.; Iwabuchi, M.; Narusaka, Y. RRS1 and RPS4 provide a dual Resistance-gene system against fungal and bacterial pathogens. Plant J. 2009, 60, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Birker, D.; Heidrich, K.; Takahara, H.; Narusaka, M.; Deslandes, L.; Narusaka, Y.; Reymond, M.; Parker, J.E.; O’Connell, R. A locus conferring resistance to Colletotrichum higginsianum is shared by four geographically distinct Arabidopsis accessions. Plant J. 2009, 60, 602–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarris, P.F.; Duxbury, Z.; Huh, S.U.; Ma, Y.; Segonzac, C.; Sklena, J.; Derbyshire, P.; Cevik, V.; Rallapalli, G.; Saucet, S.B. A plant immune receptor detects pathogen effectors that target WRKY transcription factors. Cell 2015, 161, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, C.; Huet, G.; Jauneau, A.; Camborde, L.; Trémousaygue, D.; Kraut, A.; Zhou, B.; Levaillant, M.; Adachi, H.; Yoshioka, H. A receptor pair with an integrated decoy converts pathogen disabling of transcription factors to immunity. Cell 2015, 161, 1074–1088. [Google Scholar] [CrossRef] [PubMed]

| Mutant | Insertion a | T-DNA Insertion b | Putative Function (NCBI Accession) c | Reference |
|---|---|---|---|---|
| path-5 | 1 | In predicted open reading frame (ORF) | Unknown | [31] |
| path-7 | 2 | In ORF | Hypothetical protein (FG06146.1) | |
| 1.5 kb upstream | Hypothetical protein (FG06145.1) | |||
| path-8 | 1 | In predicted ORF | Unknown | |
| path-9 | 1 | 1 kb downstream | Endo–1,3(4)–β–glucanase (AFUA_1G05290) | |
| path-12 | 1 | In ORF | MFS transporter (NFIA_086030) | |
| path-16 | 1 | In ORF | Ornithine decarboxylase (AY602214) | |
| path-19 | 1 | In ORF | Arg–6 protein (EAA35492.1) | |
| path-23 | 2 | 620 bp upstream | Hypothetical protein (FG02446.1) | |
| In predicted ORF | Unknown | |||
| path-29 | 1 | 730 bp upstream | ATP–binding endoribonuclease (ACLA_048430) | |
| path-35 | 1 | In ORF | Carbamoyl–phosphate synthetase (EAA36214.1) | |
| path-36 | 1 | 620 bp upstream | Importin β2 subunit (AFUA_1G15900) | |
| path-38 | 1 | In ORF | Importin β2 subunit (AFUA_1G15900) | |
| T732 | 1 | 168 bp downstream | Copper amine oxidase (XP_001826965) | [32] |
| T734 | 1 | In ORF | Hypothetical protein (ELA33048) | |
| B30 | 2 | In ORF | Exosome component EXOSC1/CSL4 (EFQ29835) | |
| 850 bp upstream | DUF221 domain protein (EFY94646) | |||
| T45 | Unknown | Hypothetical protein (EFQ29552) | ||
| vir-2 | 2 | supercontig_1.2671, 583, RB | Phosphoribosylaminoimidazole carboxylase (EFQ26499.1) | [33] |
| vir-10 | 2 | contig05930, 16777, LB | Kelch domain-containing protein (EFQ26610.1) | |
| vir-12 | 2 | supercontig_1.3174,1154, LB | Plasma-membrane proton-efflux P-type ATPase | |
| vir-14 | 2 | supercontig_1.6150,870, RB | ABC transporter (EFQ25092.1) | |
| supercontig_1.903,6335, LB | Nucleoside-diphosphate-sugar epimerase | |||
| vir-22 | 1 | supercontig_1.3174,1748, RB | Plasma-membrane proton-efflux P-type ATPase | |
| vir-24 | 1 | supercontig_1.3174,1422, RB | Plasma-membrane proton-efflux P-type ATPase | |
| vir-27 | 2 | supercontig_1.6150,873, RB | ABC transporter (EFQ25092.1) | |
| supercontig_1.826,7944, RB | STE like transcription factor | |||
| vir-51 | 1 | supercontig_1.1848,6585, LB | Unknown | |
| vir-52 | 2 | contig 00557 | Alanine dehydrogenase/PNT domain containing protein (EFQ25467.1) | |
| contig 11896 | FAD dependent oxidoreductase superfamily protein (XP_007280006) | |||
| vir-53 | 2 | supercontig_1.6692, RB | Unknown | |
| vir-56 | 3 | supercontig_1.66,3878, LB | Peroxisomal membrane protein 24 | |
| vir-76 | 2 | supercontig_1.56,17248, LB | Spindle assembly checkpoint component MAD1 | |
| vir-84 | 2 | supercontig_1.3742,1175, LB | Sporulation protein RMD1 (ELA35952.1) | |
| vir-88 | 2 | supercontig_1.5277,868–879 | Mob1/phocein family protein (EFQ26211.1) | |
| vir-97 | 2 | supercontig_1.3174,812, LB | Plasma-membrane proton-efflux P-type ATPase | |
| vir-102 | 1 | supercontig_1.3174,793, LB | Plasma-membrane proton-efflux P-type ATPase |
| Gene | ID | Description | Reference |
|---|---|---|---|
| path-19 | CH063_11554 | Putative Arg6 precursor | [42] |
| path-35 | CH063_15109 | Carbamoyl–phosphate synthetase | [42] |
| Ch-MEL1 | unknown | Hypothetical protein | [32] |
| ChPma2 | CH063_09060 | Plasma-membrane proton-efflux P-type ATPase | [33] |
| ChELP1 | CH063_13023 | LysM effectors | [43] |
| ChELP2 | CH063_04445 | LysM effectors | [43] |
| ChSte7 | CH063 02455 | Serine/threonine protein kinases | [44] |
| ChRgf | CH063_04363 | Ras guanine-nucleotide exchange factor | [45] |
| ChMK1 | CH063_08490 | Fus3/Kss1-relatedMAPKgene | [46] |
| ChMfs1 | CH063_12120 | Major facilitator superfamily (MFS) transporter | [47] |
| ChMob2 | CH063_12012 | Mob1/phocein family protein | [48] |
| ChCbk1 | CH063_12968 | NDR/LATS kinase | [48] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Y.; Yuan, Q.; Tang, J.; Huang, J.; Hsiang, T.; Wei, Y.; Zheng, L. Colletotrichum higginsianum as a Model for Understanding Host–Pathogen Interactions: A Review. Int. J. Mol. Sci. 2018, 19, 2142. https://doi.org/10.3390/ijms19072142
Yan Y, Yuan Q, Tang J, Huang J, Hsiang T, Wei Y, Zheng L. Colletotrichum higginsianum as a Model for Understanding Host–Pathogen Interactions: A Review. International Journal of Molecular Sciences. 2018; 19(7):2142. https://doi.org/10.3390/ijms19072142
Chicago/Turabian StyleYan, Yaqin, Qinfeng Yuan, Jintian Tang, Junbin Huang, Tom Hsiang, Yangdou Wei, and Lu Zheng. 2018. "Colletotrichum higginsianum as a Model for Understanding Host–Pathogen Interactions: A Review" International Journal of Molecular Sciences 19, no. 7: 2142. https://doi.org/10.3390/ijms19072142
APA StyleYan, Y., Yuan, Q., Tang, J., Huang, J., Hsiang, T., Wei, Y., & Zheng, L. (2018). Colletotrichum higginsianum as a Model for Understanding Host–Pathogen Interactions: A Review. International Journal of Molecular Sciences, 19(7), 2142. https://doi.org/10.3390/ijms19072142