Early Developmental Exposure to General Anesthetic Agents in Primary Neuron Culture Disrupts Synapse Formation via Actions on the mTOR Pathway
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effects of Isoflurane Exposure for 6 h on Apoptosis
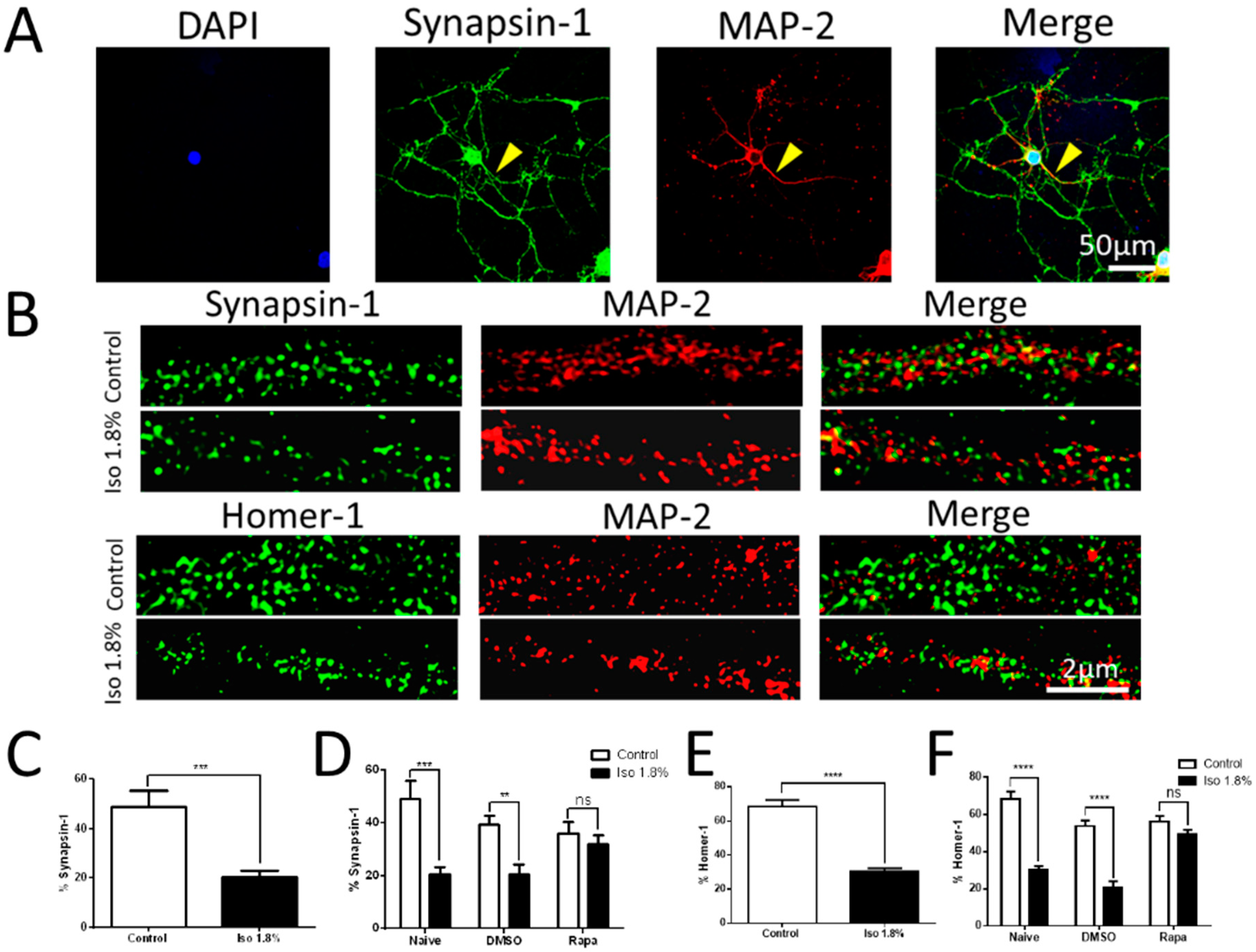
2.2. Effects of 1.8% Isoflurane Exposure for 6 h on Synaptogenesis
2.3. Parameters of Activation of mTOR by Isoflurane in Cultured Neurons
2.4. Effects of Isoflurane Exposure on the mTORC1 and mTORC2 Pathway
2.5. Effects of Sevoflurane and Propofol on the Downstream Marker of the mTOR Pathway
3. Materials and Methods
3.1. Neuronal Cultures
3.2. Anesthetic Agent Exposure
3.3. The mTOR Pathway Inhibition
3.4. TUNEL Assay
3.5. Immunocytochemistry
3.6. Imaging and Microscopic Analysis
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| GA | General anesthetics |
| mTOR | Mechanistic target of rapamycin |
| mTORC1 | mTOR 1 complex |
| mTORC2 | mTOR 2 complex |
| DIV | Days in vitro |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DMSO | Dimethyl sulfoxide |
| pS6 | Phosphorylated S6 |
| MAC | Minimum alveolar concentration |
| Ser473 | Ser473 phosphorylated Akt |
| Thr389 | Thr389 phosphorylated 70S6 |
References
- Tzong, K.Y.; Han, S.; Roh, A.; Ing, C. Epidemiology of pediatric surgical admissions in US children: Data from the HCUP kids inpatient database. J. Neurosurg. Anesthesiol. 2012, 24, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Davidson, A.J.; Disma, N.; de Graaff, J.C.; Withington, D.E.; Dorris, L.; Bell, G.; Stargatt, R.; Bellinger, D.C.; Schuster, T.; Arnup, S.J.; et al. Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS): An international multicentre, randomised controlled trial. Lancet 2016, 387, 239–250. [Google Scholar] [CrossRef]
- Sun, L.S.; Li, G.; Miller, T.L.; Salorio, C.; Byrne, M.W.; Bellinger, D.C.; Ing, C.; Park, R.; Radcliffe, J.; Hays, S.R.; et al. Association Between a Single General Anesthesia Exposure before Age 36 Months and Neurocognitive Outcomes in Later Childhood. JAMA 2016, 315, 2312–2320. [Google Scholar] [CrossRef] [PubMed]
- DiMaggio, C.; Sun, L.S.; Li, G. Early childhood exposure to anesthesia and risk of developmental and behavioral disorders in a sibling birth cohort. Anesthesia Analg. 2011, 113, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Ing, C.; Dimaggio, C.; Whitehouse, A.; Hegarty, M.K.; Brady, J.; von Ungern-Sternberg, B.S.; Davidson, A.; Wood, A.J.; Li, G.; Sun, L.S. Long-term Differences in Language and Cognitive Function After Childhood Exposure to Anesthesia. Pediatrics 2012, 130, e476–e485. [Google Scholar] [CrossRef] [PubMed]
- Wilder, R.T.; Flick, R.P.; Sprung, J.; Katusic, S.K.; Barbaresi, W.J.; Mickelson, C.; Gleich, S.J.; Schroeder, D.R.; Weaver, A.L.; Warner, D.O. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology 2009, 110, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Jevtovic-Todorovic, V.; Hartman, R.E.; Izumi, Y.; Benshoff, N.D.; Dikranian, K.; Zorumski, C.F.; Olney, J.W.; Wozniak, D.F. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J. Neurosci. Off. J. Soc. Neurosci. 2003, 23, 876–882. [Google Scholar] [CrossRef]
- Kang, E.; Jiang, D.; Ryu, Y.K.; Lim, S.; Kwak, M.; Gray, C.D.; Xu, M.; Choi, J.H.; Junn, S.; Kim, J.; et al. Early postnatal exposure to isoflurane causes cognitive deficits and disrupts development of newborn hippocampal neurons via activation of the mTOR pathway. PLoS Biol. 2017, 15, e2001246. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Chan, J.T.; Hazarika, O.; Vutskits, L.; Sall, J.W. Early exposure to volatile anesthetics impairs long-term associative learning and recognition memory. PLoS ONE 2014, 9, e105340. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.D.; Uemura, E.; Bowman, R.E. Neurobehavioral toxicology of halothane in rats. Neurotoxicol. Teratol. 1991, 13, 461–470. [Google Scholar] [CrossRef]
- Sanders, R.D.; Xu, J.; Shu, Y.; Januszewski, A.; Halder, S.; Fidalgo, A.; Sun, P.; Hossain, M.; Ma, D.; Maze, M. Dexmedetomidine attenuates isoflurane-induced neurocognitive impairment in neonatal rats. Anesthesiology 2009, 110, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Satomoto, M.; Satoh, Y.; Terui, K.; Miyao, H.; Takishima, K.; Ito, M.; Imaki, J. Neonatal exposure to sevoflurane induces abnormal social behaviors and deficits in fear conditioning in mice. Anesthesiology 2009, 110, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Stratmann, G.; Sall, J.W.; May, L.D.; Bell, J.S.; Magnusson, K.R.; Rau, V.; Visrodia, K.H.; Alvi, R.S.; Ku, B.; Lee, M.T.; et al. Isoflurane differentially affects neurogenesis and long-term neurocognitive function in 60-day-old and 7-day-old rats. Anesthesiology 2009, 110, 834–848. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Xue, Z.; Cang, J. Sevoflurane exposure in 7-day-old rats affects neurogenesis, neurodegeneration and neurocognitive function. Neurosci. Bull. 2012, 28, 499–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzales, E.L.; Yang, S.M.; Choi, C.S.; Mabunga, D.F.; Kim, H.J.; Cheong, J.H.; Ryu, J.H.; Koo, B.N.; Shin, C.Y. Repeated neonatal propofol administration induces sex-dependent long-term impairments on spatial and recognition memory in rats. Biomol. Ther. 2015, 23, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, M.C.; Murphy, K.L.; Baxter, M.G. Visual recognition memory is impaired in rhesus monkeys repeatedly exposed to sevoflurane in infancy. Br. J. Anaesth. 2017, 119, 517–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coleman, K.; Robertson, N.D.; Dissen, G.A.; Neuringer, M.D.; Martin, L.D.; Cuzon Carlson, V.C.; Kroenke, C.; Fair, D.; Brambrink, A.M. Isoflurane Anesthesia Has Long-term Consequences on Motor and Behavioral Development in Infant Rhesus Macaques. Anesthesiology 2017, 126, 74–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raper, J.; Alvarado, M.C.; Murphy, K.L.; Baxter, M.G. Multiple Anesthetic Exposure in Infant Monkeys Alters Emotional Reactivity to an Acute Stressor. Anesthesiology 2015, 123, 1084–1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raper, J.; De Biasio, J.C.; Murphy, K.L.; Alvarado, M.C.; Baxter, M.G. Persistent alteration in behavioural reactivity to a mild social stressor in rhesus monkeys repeatedly exposed to sevoflurane in infancy. Br. J. Anaesth. 2018, 120, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Vutskits, L.; Xie, Z. Lasting impact of general anaesthesia on the brain: Mechanisms and relevance. Nat. Rev. Neurosci. 2016, 17, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Li, L.; Yuan, W. Neonatal anesthetic neurotoxicity: Insight into the molecular mechanisms of long-term neurocognitive deficits. Biomed. Pharmacother. Biomedecine Pharmacother. 2017, 87, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef] [PubMed]
- Takei, N.; Nawa, H. mTOR signaling and its roles in normal and abnormal brain development. Front. Mol. Neurosci. 2014, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Costa-Mattioli, M.; Monteggia, L.M. mTOR complexes in neurodevelopmental and neuropsychiatric disorders. Nat. Neurosci. 2013, 16, 1537–1543. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Kang, E.; Mintz, C.D. Anesthetics disrupt brain development via actions on the mTOR pathway. Commun. Integr. Boil. 2018, e1451719. [Google Scholar] [CrossRef]
- Goslin, K.; Banker, G. Experimental observations on the development of polarity by hippocampal neurons in culture. J. Cell Boil. 1989, 108, 1507–1516. [Google Scholar] [CrossRef] [Green Version]
- Amrock, L.G.; Starner, M.L.; Murphy, K.L.; Baxter, M.G. Long-term effects of single or multiple neonatal sevoflurane exposures on rat hippocampal ultrastructure. Anesthesiology 2015, 122, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Lunardi, N.; Ori, C.; Erisir, A.; Jevtovic-Todorovic, V. General anesthesia causes long-lasting disturbances in the ultrastructural properties of developing synapses in young rats. Neurotox. Res. 2010, 17, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Briner, A.; Nikonenko, I.; De Roo, M.; Dayer, A.; Muller, D.; Vutskits, L. Developmental Stage-dependent persistent impact of propofol anesthesia on dendritic spines in the rat medial prefrontal cortex. Anesthesiology 2011, 115, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Jevtovic-Todorovic, V. General Anesthetics and Neurotoxicity: How Much Do We Know? Anesthesiol. Clin. 2016, 34, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Guo, Q.; Zhang, J. Mechanistic insights into neurotoxicity induced by anesthetics in the developing brain. Int. J. Mol. Sci. 2012, 13, 6772–6799. [Google Scholar] [CrossRef] [PubMed]
- Banker, G.; Goslin, K. Culturing Nerve Cells, 2nd ed.; MIT Press: Cambridge, UK, 1998; 666p. [Google Scholar]
- Zhang, W.; Benson, D.L. Stages of synapse development defined by dependence on F-actin. J. Neurosci. Off. J. Soc. Neurosci. 2001, 21, 5169–5181. [Google Scholar] [CrossRef]
- Lemkuil, B.P.; Head, B.P.; Pearn, M.L.; Patel, H.H.; Drummond, J.C.; Patel, P.M. Isoflurane neurotoxicity is mediated by p75NTR-RhoA activation and actin depolymerization. Anesthesiology 2011, 114, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Lunardi, N.; Hucklenbruch, C.; Latham, J.R.; Scarpa, J.; Jevtovic-Todorovic, V. Isoflurane impairs immature astroglia development in vitro: The role of actin cytoskeleton. J. Neuropathol. Exp. Neurol. 2011, 70, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.E., Jr. Safety considerations of anesthetic drugs in children. Expert Opin. Drug Saf. 2017, 16, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Murray, D.J.; Mehta, M.P.; Forbes, R.B. The additive contribution of nitrous oxide to isoflurane MAC in infants and children. Anesthesiology 1991, 75, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Sun, L. Early childhood general anaesthesia exposure and neurocognitive development. Br. J. Anaesth. 2010, 105 (Suppl. 1), i61–i68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanders, R.D.; Hassell, J.; Davidson, A.J.; Robertson, N.J.; Ma, D. Impact of anaesthetics and surgery on neurodevelopment: An update. Br. J. Anaesth. 2013, 110 (Suppl. 1), i53–i72. [Google Scholar] [CrossRef] [PubMed]
- Rappaport, B.A.; Suresh, S.; Hertz, S.; Evers, A.S.; Orser, B.A. Anesthetic neurotoxicity—Clinical implications of animal models. N. Engl. J. Med. 2015, 372, 796–797. [Google Scholar] [CrossRef] [PubMed]
- Dowling, R.J.; Topisirovic, I.; Fonseca, B.D.; Sonenberg, N. Dissecting the role of mTOR: Lessons from mTOR inhibitors. Biochim. Biophys. Acta 2010, 1804, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Warren, K.J.; Fang, X.; Gowda, N.M.; Thompson, J.J.; Heller, N.M. The TORC1-activated Proteins, p70S6K and GRB10, Regulate IL-4 Signaling and M2 Macrophage Polarization by Modulating Phosphorylation of Insulin Receptor Substrate-2. J. Boil. Chem. 2016, 291, 24922–24930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mintz, C.D.; Barrett, K.M.; Smith, S.C.; Benson, D.L.; Harrison, N.L. Anesthetics interfere with axon guidance in developing mouse neocortical neurons in vitro via a gamma-aminobutyric acid type A receptor mechanism. Anesthesiology 2013, 118, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Mintz, C.D.; Smith, S.C.; Barrett, K.M.; Benson, D.L. Anesthetics interfere with the polarization of developing cortical neurons. J. Neurosurg. Anesthesiol. 2012, 24, 368–375. [Google Scholar] [CrossRef] [PubMed]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Mathena, R.P.; Xu, M.; Wang, Y.; Chang, C.; Fang, Y.; Zhang, P.; Mintz, C.D. Early Developmental Exposure to General Anesthetic Agents in Primary Neuron Culture Disrupts Synapse Formation via Actions on the mTOR Pathway. Int. J. Mol. Sci. 2018, 19, 2183. https://doi.org/10.3390/ijms19082183
Xu J, Mathena RP, Xu M, Wang Y, Chang C, Fang Y, Zhang P, Mintz CD. Early Developmental Exposure to General Anesthetic Agents in Primary Neuron Culture Disrupts Synapse Formation via Actions on the mTOR Pathway. International Journal of Molecular Sciences. 2018; 19(8):2183. https://doi.org/10.3390/ijms19082183
Chicago/Turabian StyleXu, Jing, R. Paige Mathena, Michael Xu, YuChia Wang, CheJui Chang, Yiwen Fang, Pengbo Zhang, and C. David Mintz. 2018. "Early Developmental Exposure to General Anesthetic Agents in Primary Neuron Culture Disrupts Synapse Formation via Actions on the mTOR Pathway" International Journal of Molecular Sciences 19, no. 8: 2183. https://doi.org/10.3390/ijms19082183
APA StyleXu, J., Mathena, R. P., Xu, M., Wang, Y., Chang, C., Fang, Y., Zhang, P., & Mintz, C. D. (2018). Early Developmental Exposure to General Anesthetic Agents in Primary Neuron Culture Disrupts Synapse Formation via Actions on the mTOR Pathway. International Journal of Molecular Sciences, 19(8), 2183. https://doi.org/10.3390/ijms19082183




