Direct Cell–Cell Interactions in the Endometrium and in Endometrial Pathophysiology
Abstract
:1. Introduction
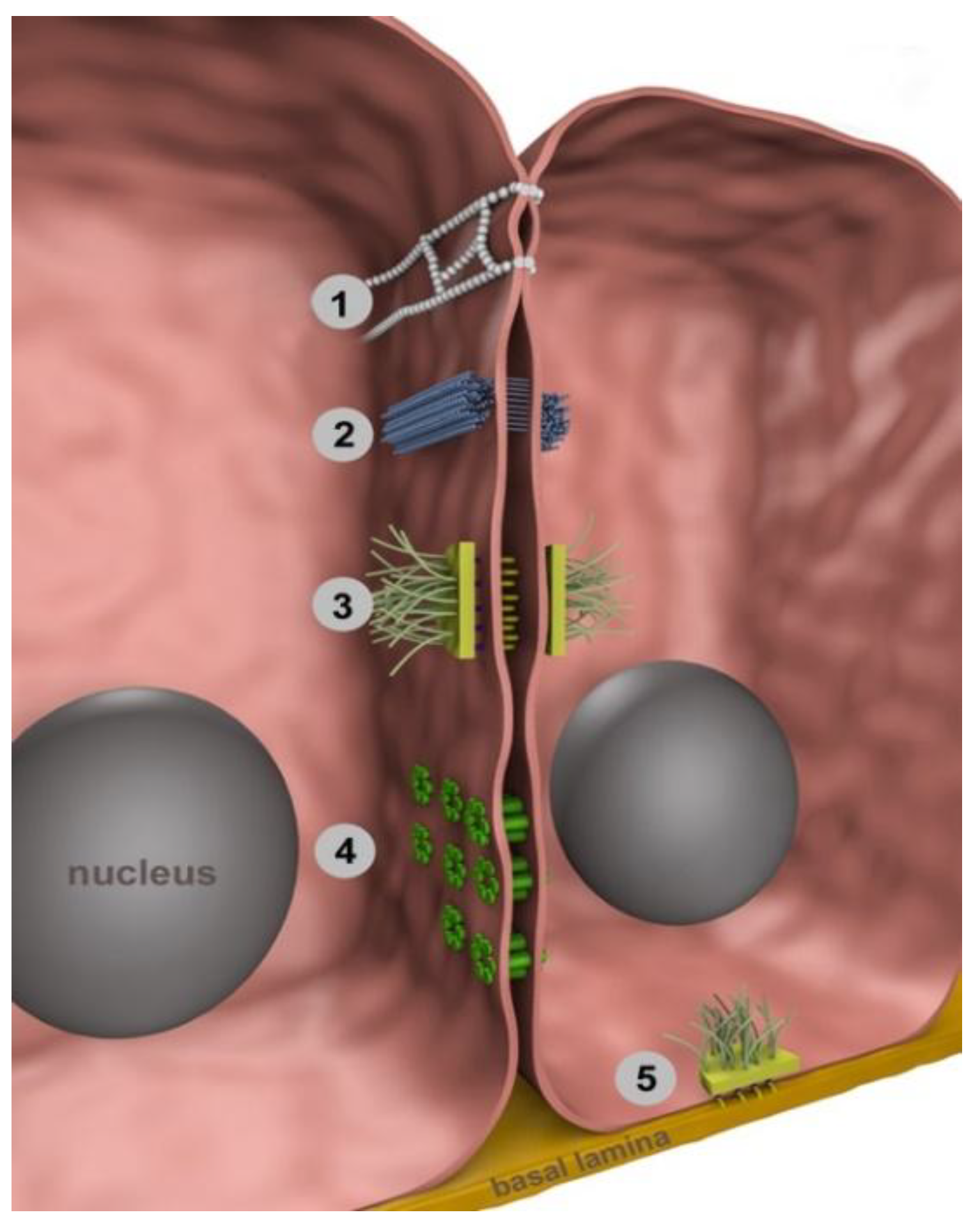
2. Intercellular Junctions
3. Cell–Cell Junctions in the Cyclic Human Endometrium
4. Hormonal Regulation of Endometrial Junctional Proteins
5. Cell–Cell Junctions during Implantation and Decidualization
5.1. Changes in Epithelial Junctions during Embryo Implantation
5.2. Changes in Stromal Junctions during Decidualization
6. Direct Cell–Cell Interactions in Endometrial Pathophysiology
6.1. Endometriosis
6.2. Endometrial Carcinoma
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CPE | Clostridium perfringens enterotoxin |
| Cx | Connexin |
| dpc | Days post coitum |
| E | Estrogen |
| GE | Glandular epithelium |
| hEEC | Human endometrial epithelial cells |
| IVF | In vitro fertilization |
| JAM | Junction adhesion molecule |
| LE | Luminal epithelium |
| LIF | Leukemia inhibitory factor |
| MUPP | Multi-PDZ domain protein |
| P | Progesterone |
| pc | Post coitum |
| PCR | Polymerase chain reaction |
| SP | Secretory phase |
| USPC | Uterine serous papillary carcinoma |
| VEGF | Vascular endothelial growth factor |
| ZO | Zonula occludens |
References
- Garcia, M.A.; Nelson, W.J.; Chavez, N. Cell-Cell Junctions Organize Structural and Signaling Networks. Cold Spring Harb. Perspect. Biol. 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.R. Junctional barrier complexes undergo major alterations during the plasma membrane transformation of uterine epithelial cells. Hum. Reprod. 2000, 15 (Suppl. 3), 182–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denker, H.-W. Cell Biology of Endometrial Receptivity and of Trophoblast-Endometrial Interactions. In Endocrinology of Embryo-Endometrium Interactions; Glasser, S.R., Mulholland, J., Psychoyos, A., Eds.; Springer: Boston, MA, USA, 1994; pp. 17–32. [Google Scholar]
- Gellersen, B.; Brosens, J.J. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr. Rev. 2014, 35, 851–905. [Google Scholar] [CrossRef] [PubMed]
- Hay, E.D. An overview of epithelio-mesenchymal transformation. Acta Anat. 1995, 154, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.H.; Liang, X.; Liang, X.H.; Wang, T.S.; Qi, Q.R.; Deng, W.B.; Sha, A.G.; Yang, Z.M. The mesenchymal-epithelial transition during in vitro decidualization. Reprod. Sci. 2013, 20, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, M.G.; Palade, G.E. Junctional complexes in various epithelia. J. Cell Biol. 1963, 17, 375–412. [Google Scholar] [CrossRef] [PubMed]
- Zihni, C.; Mills, C.; Matter, K.; Balda, M.S. Tight junctions: From simple barriers to multifunctional molecular gates. Nat. Rev. Mol. Cell Biol. 2016, 17, 564–580. [Google Scholar] [CrossRef] [PubMed]
- Hartsock, A.; Nelson, W.J. Adherens and tight junctions: Structure, function and connections to the actin cytoskeleton. Biochim. Biophys. Acta 2008, 1778, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Furuse, M.; Sasaki, H.; Fujimoto, K.; Tsukita, S. A single gene product, claudin-1 or -2, reconstitutes tight junction strands and recruits occludin in fibroblasts. J. Cell Biol. 1998, 143, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Fanning, A.S.; Jameson, B.J.; Jesaitis, L.A.; Anderson, J.M. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J. Biol. Chem. 1998, 273, 29745–29753. [Google Scholar] [CrossRef] [PubMed]
- Itoh, M.; Morita, K.; Tsukita, S. Characterization of ZO-2 as a MAGUK family member associated with tight as well as adherens junctions with a binding affinity to occludin and alpha catenin. J. Biol. Chem. 1999, 274, 5981–5986. [Google Scholar] [CrossRef] [PubMed]
- Laird, D.W. The gap junction proteome and its relationship to disease. Trends Cell Biol. 2010, 20, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Matter, K.; Balda, M.S. Signalling to and from tight junctions. Nat. Rev. Mol. Cell Biol. 2003, 4, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Campbell, H.K.; Maiers, J.L.; DeMali, K.A. Interplay between tight junctions & adherens junctions. Exp. Cell Res. 2017, 358, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Garrod, D.; Chidgey, M. Desmosome structure, composition and function. Biochim. Biophys. Acta 2008, 1778, 572–587. [Google Scholar] [CrossRef] [PubMed]
- Trosko, J.E.; Ruch, R.J. Cell-cell communication in carcinogenesis. Front. Biosci. 1998, 3, d208–d236. [Google Scholar] [CrossRef] [PubMed]
- Wincewicz, A.; Baltaziak, M.; Kanczuga-Koda, L.; Lesniewicz, T.; Rutkowski, R.; Sobaniec-Lotowska, M.; Sulkowski, S.; Koda, M.; Sulkowska, M. Aberrant distributions and relationships among E-cadherin, beta-catenin, and connexin 26 and 43 in endometrioid adenocarcinomas. Int. J. Gynecol. Pathol. 2010, 29, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Nelson, W.J.; Nusse, R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science 2004, 303, 1483–1487. [Google Scholar] [CrossRef] [PubMed]
- Perez-Moreno, M.; Fuchs, E. Catenins: Keeping cells from getting their signals crossed. Dev. Cell 2006, 11, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Mese, G.; Richard, G.; White, T.W. Gap junctions: Basic structure and function. J. Investig. Dermatol. 2007, 127, 2516–2524. [Google Scholar] [CrossRef] [PubMed]
- Yeager, M.; Harris, A.L. Gap junction channel structure in the early 21st century: Facts and fantasies. Curr. Opin. Cell Biol. 2007, 19, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.L.; Contreras, J.E. Motifs in the permeation pathway of connexin channels mediate voltage and Ca (2+) sensing. Front. Physiol. 2014, 5, 113. [Google Scholar] [CrossRef] [PubMed]
- Herve, J.C.; Derangeon, M.; Sarrouilhe, D.; Giepmans, B.N.; Bourmeyster, N. Gap junctional channels are parts of multiprotein complexes. Biochim. Biophys. Acta 2012, 1818, 1844–1865. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, T.; Dai, P.; Harada, Y.; Hino, H.; Tsukahara, F.; Maru, Y.; Otsuji, E.; Takamatsu, T. Connexin43 functions as a novel interacting partner of heat shock cognate protein 70. Sci. Rep. 2013, 3, 2719. [Google Scholar] [CrossRef] [PubMed]
- Penes, M.C.; Li, X.; Nagy, J.I. Expression of zonula occludens-1 (ZO-1) and the transcription factor ZO-1-associated nucleic acid-binding protein (ZONAB)-MsY3 in glial cells and colocalization at oligodendrocyte and astrocyte gap junctions in mouse brain. Eur. J. Neurosci. 2005, 22, 404–418. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.H.; De Vuyst, E.; Leybaert, L. The gap junction cellular internet: Connexin hemichannels enter the signalling limelight. Biochem. J. 2006, 397, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Schlafke, S.; Welsh, A.O.; Enders, A.C. Penetration of the basal lamina of the uterine luminal epithelium during implantation in the rat. Anat. Rec. 1985, 212, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Salamonsen, L.A.; Evans, J.; Nguyen, H.P.; Edgell, T.A. The Microenvironment of Human Implantation: Determinant of Reproductive Success. Am. J. Reprod. Immunol. 2016, 75, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.R.; Swift, J.G.; Need, J.A.; Mukherjee, T.M.; Rogers, A.W. A freeze-fracture electron microscopic study of tight junctions of epithelial cells in the human uterus. Anat. Embryol. 1982, 163, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.R.; Rogers, P.A.; Hosie, M.J.; Leeton, J.; Beaton, L. Tight junctions of human uterine epithelial cells change during the menstrual cycle: A morphometric study. Acta Anat. 1992, 144, 36–38. [Google Scholar] [CrossRef] [PubMed]
- Sarani, S.A.; Ghaffari-Novin, M.; Warren, M.A.; Dockery, P.; Cooke, I.D. Morphological evidence for the ‘implantation window’ in human luminal endometrium. Hum. Reprod. 1999, 14, 3101–3106. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.Y.; Wang, B.; Che, Y.C.; Weng, Z.P.; Dai, H.Y.; Peng, W. Expression of claudin-3 and claudin-4 in normal, hyperplastic, and malignant endometrial tissue. Int. J. Gynecol. Cancer 2007, 17, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Karakotchian, M.; Fraser, I.S. An ultrastructural study of microvascular inter-endothelial tight junctions in normal endometrium. Micron 2007, 38, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Sobel, G.; Nemeth, J.; Kiss, A.; Lotz, G.; Szabo, I.; Udvarhelyi, N.; Schaff, Z.; Paska, C. Claudin 1 differentiates endometrioid and serous papillary endometrial adenocarcinoma. Gynecol. Oncol. 2006, 103, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Gaetje, R.; Holtrich, U.; Engels, K.; Kissler, S.; Rody, A.; Karn, T.; Kaufmann, M. Differential expression of claudins in human endometrium and endometriosis. Gynecol. Endocrinol. 2008, 24, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Buck, V.U.; Windoffer, R.; Leube, R.E.; Classen-Linke, I. Redistribution of adhering junctions in human endometrial epithelial cells during the implantation window of the menstrual cycle. Histochem. Cell Biol. 2012, 137, 777–790. [Google Scholar] [CrossRef] [PubMed]
- Someya, M.; Kojima, T.; Ogawa, M.; Ninomiya, T.; Nomura, K.; Takasawa, A.; Murata, M.; Tanaka, S.; Saito, T.; Sawada, N. Regulation of tight junctions by sex hormones in normal human endometrial epithelial cells and uterus cancer cell line Sawano. Cell Tissue Res. 2013, 354, 481–494. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.Y.; Li, X.; Weng, Z.P.; Wang, B. Altered expression of claudin-3 and claudin-4 in ectopic endometrium of women with endometriosis. Fertil. Steril. 2009, 91, 1692–1699. [Google Scholar] [CrossRef] [PubMed]
- Serafini, P.C.; Silva, I.D.; Smith, G.D.; Motta, E.L.; Rocha, A.M.; Baracat, E.C. Endometrial claudin-4 and leukemia inhibitory factor are associated with assisted reproduction outcome. Reprod. Biol. Endocrinol. 2009, 7, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundqvist, J.; Andersson, K.L.; Scarselli, G.; Gemzell-Danielsson, K.; Lalitkumar, P.G. Expression of adhesion, attachment and invasion markers in eutopic and ectopic endometrium: A link to the aetiology of endometriosis. Hum. Reprod. 2012, 27, 2737–2746. [Google Scholar] [CrossRef] [PubMed]
- Koshiba, H.; Hosokawa, K.; Kubo, A.; Tokumitsu, N.; Watanabe, A.; Honjo, H. Junctional adhesion molecule A [corrected] expression in human endometrial carcinoma. Int. J. Gynecol. Cancer 2009, 19, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Darcha, C.; Maleysson, E.; Canis, M.; Mage, G. Impaired down-regulation of E-cadherin and beta-catenin protein expression in endometrial epithelial cells in the mid-secretory endometrium of infertile patients with endometriosis. J. Clin. Endocrinol. Metab. 2010, 95, 3437–3445. [Google Scholar] [CrossRef] [PubMed]
- Jahn, E.; Classen-Linke, I.; Kusche, M.; Beier, H.M.; Traub, O.; Grummer, R.; Winterhager, E. Expression of gap junction connexins in the human endometrium throughout the menstrual cycle. Hum. Reprod. 1995, 10, 2666–2670. [Google Scholar] [CrossRef] [PubMed]
- Winterhager, E.; Grummer, R.; Jahn, E.; Willecke, K.; Traub, O. Spatial and temporal expression of connexin26 and connexin43 in rat endometrium during trophoblast invasion. Dev. Biol. 1993, 157, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Oyamada, M.; Yamasaki, H.; Mori, M.; Kudo, R. Co-ordinated expression of connexins 26 and 32 in human endometrial glandular epithelium during the reproductive cycle and the influence of hormone replacement therapy. Int. J. Cancer 1997, 73, 479–485. [Google Scholar] [CrossRef] [Green Version]
- Granot, I.; Dekel, N.; Bechor, E.; Segal, I.; Fieldust, S.; Barash, A. Temporal analysis of connexin43 protein and gene expression throughout the menstrual cycle in human endometrium. Fertil. Steril. 2000, 73, 381–386. [Google Scholar] [CrossRef]
- Tsukita, S.; Furuse, M.; Itoh, M. Multifunctional strands in tight junctions. Nat. Rev. Mol. Cell Biol. 2001, 2, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.R.; Rogers, A.W. Effects of ovarian hormones on cell membranes in the rat uterus. III. The surface carbohydrates at the apex of the luminal epithelium. Cell Biophys. 1981, 3, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Rodriguez, C.A.; Gonzalez-Mariscal, L.; Cerbon, M. Changes in the distribution of ZO-1, occludin, and claudins in the rat uterine epithelium during the estrous cycle. Cell Tissue Res. 2005, 319, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Satterfield, M.C.; Dunlap, K.A.; Hayashi, K.; Burghardt, R.C.; Spencer, T.E.; Bazer, F.W. Tight and adherens junctions in the ovine uterus: Differential regulation by pregnancy and progesterone. Endocrinology 2007, 148, 3922–3931. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zhang, X.H.; Han, B.C.; Lei, W.; Qi, Q.R.; Wang, T.S.; Gu, X.W.; Yang, Z.M. Progesterone and heparin-binding epidermal growth factor-like growth factor regulate the expression of tight junction protein Claudin-3 during early pregnancy. Fertil. Steril. 2013, 100, 1410–1418. [Google Scholar] [CrossRef] [PubMed]
- Hata, M.; Yamanegi, K.; Yamada, N.; Ohyama, H.; Yukitatsu, Y.; Nakasho, K.; Okamura, H.; Terada, N. Estrogen decreases the expression of claudin-5 in vascular endothelial cells in the murine uterus. Endocr. J. 2014, 61, 705–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aberdeen, G.W.; Wiegand, S.J.; Bonagura, T.W., Jr.; Pepe, G.J.; Albrecht, E.D. Vascular endothelial growth factor mediates the estrogen-induced breakdown of tight junctions between and increase in proliferation of microvessel endothelial cells in the baboon endometrium. Endocrinology 2008, 149, 6076–6083. [Google Scholar] [CrossRef] [PubMed]
- Risek, B.; Klier, F.G.; Phillips, A.; Hahn, D.W.; Gilula, N.B. Gap junction regulation in the uterus and ovaries of immature rats by estrogen and progesterone. J. Cell Sci. 1995, 108 Pt 3, 1017–1032. [Google Scholar] [PubMed]
- Grummer, R.; Chwalisz, K.; Mulholland, J.; Traub, O.; Winterhager, E. Regulation of connexin26 and connexin43 expression in rat endometrium by ovarian steroid hormones. Biol. Reprod. 1994, 51, 1109–1116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grummer, R.; Traub, O.; Winterhager, E. Gap junction connexin genes cx26 and cx43 are differentially regulated by ovarian steroid hormones in rat endometrium. Endocrinology 1999, 140, 2509–2516. [Google Scholar] [CrossRef] [PubMed]
- Grummer, R.; Hewitt, S.W.; Traub, O.; Korach, K.S.; Winterhager, E. Different regulatory pathways of endometrial connexin expression: Preimplantation hormonal-mediated pathway versus embryo implantation-initiated pathway. Biol. Reprod. 2004, 71, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Heikaus, S.; Winterhager, E.; Traub, O.; Grummer, R. Responsiveness of endometrial genes Connexin26, Connexin43, C3 and clusterin to primary estrogen, selective estrogen receptor modulators, phyto- and xenoestrogens. J. Mol. Endocrinol. 2002, 29, 239–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egashira, M.; Hirota, Y. Uterine receptivity and embryo-uterine interactions in embryo implantation: Lessons from mice. Reprod. Med. Biol. 2013, 12, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Aplin, J.D.; Ruane, P.T. Embryo-epithelium interactions during implantation at a glance. J. Cell Sci. 2017, 130, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Ashary, N.; Tiwari, A.; Modi, D. Embryo Implantation: War in Times of Love. Endocrinology 2018, 159, 1188–1198. [Google Scholar] [CrossRef] [PubMed]
- Schlafke, S.; Enders, A.C. Cellular basis of interaction between trophoblast and uterus at implantation. Biol. Reprod. 1975, 12, 41–65. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.R.; Swift, J.G.; Mukherjee, T.M.; Rogers, A.W. The structure of tight junctions between uterine luminal epithelial cells at different stages of pregnancy in the rat. Cell Tissue Res. 1982, 223, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Winterhager, E.; Kuhnel, W. Alterations in intercellular junctions of the uterine epithelium during the preimplantation phase in the rabbit. Cell Tissue Res. 1982, 224, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Orchard, M.D.; Murphy, C.R. Alterations in tight junction molecules of uterine epithelial cells during early pregnancy in the rat. Acta Histochem. 2002, 104, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, M.D.; Lindsay, L.A.; Murphy, C.R. Ovarian hormones control the changing expression of claudins and occludin in rat uterine epithelial cells during early pregnancy. Acta Histochem. 2010, 112, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Schumann, S.; Buck, V.U.; Classen-Linke, I.; Wennemuth, G.; Grummer, R. Claudin-3, claudin-7, and claudin-10 show different distribution patterns during decidualization and trophoblast invasion in mouse and human. Histochem. Cell Biol. 2015, 144, 571–585. [Google Scholar] [CrossRef] [PubMed]
- Illingworth, I.M.; Kiszka, I.; Bagley, S.; Ireland, G.W.; Garrod, D.R.; Kimber, S.J. Desmosomes are reduced in the mouse uterine luminal epithelium during the preimplantation period of pregnancy: A mechanism for facilitation of implantation. Biol. Reprod. 2000, 63, 1764–1773. [Google Scholar] [CrossRef] [PubMed]
- Orchard, M.D.; Shaw, T.J.; Murphy, C.R. Junctional plaque proteins shift to the apical surface of uterine epithelial cells during early pregnancy in the rat. Acta Histochem. 1999, 101, 147–156. [Google Scholar] [CrossRef]
- Potter, S.W.; Gaza, G.; Morris, J.E. Estradiol induces E-cadherin degradation in mouse uterine epithelium during the estrous cycle and early pregnancy. J. Cell. Physiol. 1996, 169, 1–14. [Google Scholar] [CrossRef]
- Paria, B.C.; Zhao, X.; Das, S.K.; Dey, S.K.; Yoshinaga, K. Zonula occludens-1 and E-cadherin are coordinately expressed in the mouse uterus with the initiation of implantation and decidualization. Dev. Biol. 1999, 208, 488–501. [Google Scholar] [CrossRef] [PubMed]
- Reardon, S.N.; King, M.L.; MacLean, J.A., 2nd; Mann, J.L.; DeMayo, F.J.; Lydon, J.P.; Hayashi, K. CDH1 is essential for endometrial differentiation, gland development, and adult function in the mouse uterus. Biol. Reprod. 2012, 86, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Grummer, R.; Winterhager, E. Regulation of gap junction connexins in the endometrium during early pregnancy. Cell Tissue Res. 1998, 293, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Antoskiewicz, B.; Muller, G.; Grummer, R.; Winterhager, E. Induction of connexin 32 expression by potential embryonic signals in rabbit uterine epithelium. Early Pregnancy 1996, 2, 253–263. [Google Scholar] [PubMed]
- Gabriel, S.; Winterhager, E.; Pfarrer, C.; Traub, O.; Leiser, R. Modulation of connexin expression in sheep endometrium in response to pregnancy. Placenta 2004, 25, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Joswig, A.; Gabriel, H.D.; Kibschull, M.; Winterhager, E. Apoptosis in uterine epithelium and decidua in response to implantation: Evidence for two different pathways. Reprod. Biol. Endocrinol. 2003, 1, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diao, H.; Xiao, S.; Howerth, E.W.; Zhao, F.; Li, R.; Ard, M.B.; Ye, X. Broad gap junction blocker carbenoxolone disrupts uterine preparation for embryo implantation in mice. Biol. Reprod. 2013, 89, 31. [Google Scholar] [CrossRef] [PubMed]
- Denker, H.W.; Hafez, E.S. Proteases and implantation in the rabbit: Role of trophoblast vs. uterine secretion. Cytobiologie 1975, 11, 101–109. [Google Scholar] [PubMed]
- Pinsker, M.C.; Sacco, A.G.; Mintz, B. Implantation-associated proteinase in mouse uterine fluid. Dev. Biol. 1974, 38, 285–290. [Google Scholar] [CrossRef]
- Ramathal, C.Y.; Bagchi, I.C.; Taylor, R.N.; Bagchi, M.K. Endometrial decidualization: Of mice and men. Semin. Reprod. Med. 2010, 28, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.; Sun, X.; Dey, S.K. Mechanisms of implantation: Strategies for successful pregnancy. Nat. Med. 2012, 18, 1754–1767. [Google Scholar] [CrossRef] [PubMed]
- Malek, A.M.; Izumo, S. Mechanism of endothelial cell shape change and cytoskeletal remodeling in response to fluid shear stress. J. Cell Sci. 1996, 109 Pt 4, 713–726. [Google Scholar] [PubMed]
- Schutte, S.C.; Taylor, R.N. A tissue-engineered human endometrial stroma that responds to cues for secretory differentiation, decidualization, and menstruation. Fertil. Steril. 2012, 97, 997–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikoo, S.; Ebtekar, M.; Jeddi-Tehrani, M.; Shervin, A.; Bozorgmehr, M.; Vafaei, S.; Kazemnejad, S.; Zarnani, A.H. Menstrual blood-derived stromal stem cells from women with and without endometriosis reveal different phenotypic and functional characteristics. Mol. Hum. Reprod. 2014, 20, 905–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Berga, S.L.; Johnston-MacAnanny, E.B.; Sidell, N.; Bagchi, I.C.; Bagchi, M.K.; Taylor, R.N. Endometrial Stromal Decidualization Responds Reversibly to Hormone Stimulation and Withdrawal. Endocrinology 2016, 157, 2432–2446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Matsumoto, H.; Zhao, X.; Das, S.K.; Paria, B.C. Embryonic signals direct the formation of tight junctional permeability barrier in the decidualizing stroma during embryo implantation. J. Cell Sci. 2004, 117, 53–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tung, H.N.; Parr, M.B.; Parr, E.L. The permeability of the primary decidual zone in the rat uterus: An ultrastructural tracer and freeze-fracture study. Biol. Reprod. 1986, 35, 1045–1058. [Google Scholar] [CrossRef] [PubMed]
- Wolburg, H.; Wolburg-Buchholz, K.; Kraus, J.; Rascher-Eggstein, G.; Liebner, S.; Hamm, S.; Duffner, F.; Grote, E.H.; Risau, W.; Engelhardt, B. Localization of claudin-3 in tight junctions of the blood-brain barrier is selectively lost during experimental autoimmune encephalomyelitis and human glioblastoma multiforme. Acta Neuropathol. 2003, 105, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Winterhager, E.; Kidder, G.M. Gap junction connexins in female reproductive organs: Implications for women’s reproductive health. Hum. Reprod. Update 2015, 21, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Berga, S.L.; Zou, W.; Sun, H.Y.; Johnston-MacAnanny, E.; Yalcinkaya, T.; Sidell, N.; Bagchi, I.C.; Bagchi, M.K.; Taylor, R.N. Gap junction blockade induces apoptosis in human endometrial stromal cells. Mol. Reprod. Dev. 2014, 81, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Laws, M.J.; Taylor, R.N.; Sidell, N.; DeMayo, F.J.; Lydon, J.P.; Gutstein, D.E.; Bagchi, M.K.; Bagchi, I.C. Gap junction communication between uterine stromal cells plays a critical role in pregnancy-associated neovascularization and embryo survival. Development 2008, 135, 2659–2668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boeldt, D.S.; Yi, F.X.; Bird, I.M. eNOS activation and NO function: Pregnancy adaptive programming of capacitative entry responses alters nitric oxide (NO) output in vascular endothelium—New insights into eNOS regulation through adaptive cell signaling. J. Endocrinol. 2011, 210, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wu, J.; Bagchi, I.C.; Bagchi, M.K.; Sidell, N.; Taylor, R.N. Disruption of gap junctions reduces biomarkers of decidualization and angiogenesis and increases inflammatory mediators in human endometrial stromal cell cultures. Mol. Cell. Endocrinol. 2011, 344, 25–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, R.R.; Jain, M.; Singh, K. Reduced expression of gap junction gene connexin 43 in recurrent early pregnancy loss patients. Placenta 2011, 32, 619–621. [Google Scholar] [CrossRef] [PubMed]
- Nevin, R.L. Mefloquine blockade of connexin 43 (Cx43) and risk of pregnancy loss. Placenta 2011, 32, 712. [Google Scholar] [CrossRef] [PubMed]
- Piltonen, T.T.; Chen, J.C.; Khatun, M.; Kangasniemi, M.; Liakka, A.; Spitzer, T.; Tran, N.; Huddleston, H.; Irwin, J.C.; Giudice, L.C. Endometrial stromal fibroblasts from women with polycystic ovary syndrome have impaired progesterone-mediated decidualization, aberrant cytokine profiles and promote enhanced immune cell migration in vitro. Hum. Reprod. 2015, 30, 1203–1215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maybin, J.A.; Critchley, H.O.; Jabbour, H.N. Inflammatory pathways in endometrial disorders. Mol. Cell. Endocrinol. 2011, 335, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Teklenburg, G.; Salker, M.; Heijnen, C.; Macklon, N.S.; Brosens, J.J. The molecular basis of recurrent pregnancy loss: Impaired natural embryo selection. Mol. Hum. Reprod. 2010, 16, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Dey, S.K.; Fisher, S.J. Preterm labor: One syndrome, many causes. Science 2014, 345, 760–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vannuccini, S.; Clifton, V.L.; Fraser, I.S.; Taylor, H.S.; Critchley, H.; Giudice, L.C.; Petraglia, F. Infertility and reproductive disorders: Impact of hormonal and inflammatory mechanisms on pregnancy outcome. Hum. Reprod. Update 2016, 22, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Burney, R.O.; Giudice, L.C. Pathogenesis and pathophysiology of endometriosis. Fertil. Steril. 2012, 98, 511–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proestling, K.; Birner, P.; Gamperl, S.; Nirtl, N.; Marton, E.; Yerlikaya, G.; Wenzl, R.; Streubel, B.; Husslein, H. Enhanced epithelial to mesenchymal transition (EMT) and upregulated MYC in ectopic lesions contribute independently to endometriosis. Reprod. Biol. Endocrinol. 2015, 13, 75. [Google Scholar] [CrossRef] [PubMed]
- Sohler, F.; Sommer, A.; Wachter, D.L.; Agaimy, A.; Fischer, O.M.; Renner, S.P.; Burghaus, S.; Fasching, P.A.; Beckmann, M.W.; Fuhrmann, U.; et al. Tissue remodeling and nonendometrium-like menstrual cycling are hallmarks of peritoneal endometriosis lesions. Reprod. Sci. 2013, 20, 85–102. [Google Scholar] [CrossRef] [PubMed]
- Scotti, S.; Regidor, P.A.; Schindler, A.E.; Winterhager, E. Reduced proliferation and cell adhesion in endometriosis. Mol. Hum. Reprod. 2000, 6, 610–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poncelet, C.; Leblanc, M.; Walker-Combrouze, F.; Soriano, D.; Feldmann, G.; Madelenat, P.; Scoazec, J.Y.; Darai, E. Expression of cadherins and CD44 isoforms in human endometrium and peritoneal endometriosis. Acta Obstet. Gynecol. Scand. 2002, 81, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Shaco-Levy, R.; Sharabi, S.; Benharroch, D.; Piura, B.; Sion-Vardy, N. Matrix metalloproteinases 2 and 9, E-cadherin, and beta-catenin expression in endometriosis, low-grade endometrial carcinoma and non-neoplastic eutopic endometrium. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 139, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, J.; Ichigo, S.; Hori, M.; Tamaya, T. Expression of E-cadherin, alpha- and beta-catenin mRNAs in ovarian endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 1996, 67, 179–183. [Google Scholar] [CrossRef]
- Gaetje, R.; Kotzian, S.; Herrmann, G.; Baumann, R.; Starzinski-Powitz, A. Nonmalignant epithelial cells, potentially invasive in human endometriosis, lack the tumor suppressor molecule E-cadherin. Am. J. Pathol. 1997, 150, 461–467. [Google Scholar] [PubMed]
- Yu, J.; Boicea, A.; Barrett, K.L.; James, C.O.; Bagchi, I.C.; Bagchi, M.K.; Nezhat, C.; Sidell, N.; Taylor, R.N. Reduced connexin 43 in eutopic endometrium and cultured endometrial stromal cells from subjects with endometriosis. Mol. Hum. Reprod. 2014, 20, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Regidor, P.A.; Regidor, M.; Schindler, A.E.; Winterhager, E. Aberrant expression pattern of gap junction connexins in endometriotic tissues. Mol. Hum. Reprod. 1997, 3, 375–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winterhager, E.; Grummer, R.; Mavrogianis, P.A.; Jones, C.J.; Hastings, J.M.; Fazleabas, A.T. Connexin expression pattern in the endometrium of baboons is influenced by hormonal changes and the presence of endometriotic lesions. Mol. Hum. Reprod. 2009, 15, 645–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kralickova, M.; Losan, P.; Vetvicka, V. Endometriosis and cancer. Womens Health 2014, 10, 591–597. [Google Scholar] [CrossRef]
- Cho, Y.J.; Lee, S.H.; Park, J.W.; Han, M.; Park, M.J.; Han, S.J. Dysfunctional signaling underlying endometriosis: Current state of knowledge. J. Mol. Endocrinol. 2018, 60, R97–R113. [Google Scholar] [CrossRef] [PubMed]
- Murali, R.; Delair, D.F.; Bean, S.M.; Abu-Rustum, N.R.; Soslow, R.A. Evolving Roles of Histologic Evaluation and Molecular/Genomic Profiling in the Management of Endometrial Cancer. J. Natl. Compr. Cancer Netw. 2018, 16, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Vallone, C.; Rigon, G.; Gulia, C.; Baffa, A.; Votino, R.; Morosetti, G.; Zaami, S.; Briganti, V.; Catania, F.; Gaffi, M.; et al. Non-Coding RNAs and Endometrial Cancer. Genes 2018, 9, 187. [Google Scholar] [CrossRef] [PubMed]
- Kalampokas, E.; Payne, F.; Gurumurthy, M. An update on the use of immunohistochemistry and molecular pathology in the diagnosis of pre-invasive and malignant lesions in gynecological oncology. Gynecol. Oncol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.V.; Jiang, J.X.; Mesnil, M. Connexins and Pannexins: Important Players in Tumorigenesis, Metastasis and Potential Therapeutics. Int. J. Mol. Sci. 2018, 19, 1645. [Google Scholar] [CrossRef] [PubMed]
- Pal, M.; Bhattacharya, S.; Kalyan, G.; Hazra, S. Cadherin profiling for therapeutic interventions in Epithelial Mesenchymal Transition (EMT) and tumorigenesis. Exp. Cell Res. 2018, 368, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Elble, R.C. Homeostatic Signaling by Cell-Cell Junctions and Its Dysregulation during Cancer Progression. J. Clin. Med. 2016, 5, 26. [Google Scholar] [CrossRef] [PubMed]
- Balda, M.S.; Matter, K. Tight junctions and the regulation of gene expression. Biochim. Biophys. Acta 2009, 1788, 761–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knights, A.J.; Funnell, A.P.; Crossley, M.; Pearson, R.C. Holding Tight: Cell Junctions and Cancer Spread. Trends Cancer Res. 2012, 8, 61–69. [Google Scholar] [PubMed]
- Santin, A.D.; Zhan, F.; Cane, S.; Bellone, S.; Palmieri, M.; Thomas, M.; Burnett, A.; Roman, J.J.; Cannon, M.J.; Shaughnessy, J., Jr.; et al. Gene expression fingerprint of uterine serous papillary carcinoma: Identification of novel molecular markers for uterine serous cancer diagnosis and therapy. Br. J. Cancer 2005, 92, 1561–1573. [Google Scholar] [CrossRef] [PubMed]
- Katahira, J.; Sugiyama, H.; Inoue, N.; Horiguchi, Y.; Matsuda, M.; Sugimoto, N. Clostridium perfringens enterotoxin utilizes two structurally related membrane proteins as functional receptors in vivo. J. Biol. Chem. 1997, 272, 26652–26658. [Google Scholar] [CrossRef] [PubMed]
- Swisshelm, K.; Macek, R.; Kubbies, M. Role of claudins in tumorigenesis. Adv. Drug Deliv. Rev. 2005, 57, 919–928. [Google Scholar] [CrossRef] [PubMed]
- McClane, B.A. An overview of Clostridium perfringens enterotoxin. Toxicon 1996, 34, 1335–1343. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Kojima, T.; Ito, T.; Kyuno, D.; Kimura, Y.; Imamura, M.; Hirata, K.; Sawada, N. Effects of Clostridium perfringens enterotoxin via claudin-4 on normal human pancreatic duct epithelial cells and cancer cells. Cell. Mol. Biol. Lett. 2011, 16, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Winkler, L.; Gehring, C.; Wenzel, A.; Muller, S.L.; Piehl, C.; Krause, G.; Blasig, I.E.; Piontek, J. Molecular determinants of the interaction between Clostridium perfringens enterotoxin fragments and claudin-3. J. Biol. Chem. 2009, 284, 18863–18872. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.J. Emerging roles of claudins in human cancer. Int. J. Mol. Sci. 2013, 14, 18148–18180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abouhashem, N.S.; Ibrahim, D.A.; Mohamed, A.M. Prognostic implications of epithelial to mesenchymal transition related proteins (E-cadherin, Snail) and hypoxia inducible factor 1alpha in endometrioid endometrial carcinoma. Ann. Diagn. Pathol. 2016, 22, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Holcomb, K.; Delatorre, R.; Pedemonte, B.; McLeod, C.; Anderson, L.; Chambers, J. E-cadherin expression in endometrioid, papillary serous, and clear cell carcinoma of the endometrium. Obstet. Gynecol. 2002, 100, 1290–1295. [Google Scholar] [PubMed]
- Blechschmidt, K.; Kremmer, E.; Hollweck, R.; Mylonas, I.; Hofler, H.; Kremer, M.; Becker, K.F. The E-cadherin repressor snail plays a role in tumor progression of endometrioid adenocarcinomas. Diagn. Mol. Pathol. 2007, 16, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Carico, E.; Atlante, M.; Giarnieri, E.; Raffa, S.; Bucci, B.; Giovagnoli, M.R.; Vecchione, A. E-cadherin and alpha-catenin expression in normal, hyperplastic and neoplastic endometrium. Anticancer Res. 2010, 30, 4993–4997. [Google Scholar] [PubMed]
- Feng, Y.; Wang, X.; Wang, Q. Expression of SATB1 and E-cad in tissues of patients with endometrial carcinoma and the relationship with clinicopathological features. Exp. Ther. Med. 2018, 15, 4339–4343. [Google Scholar] [CrossRef] [PubMed]
- Florescu, M.M.; Pirici, D.; Simionescu, C.E.; Stepan, A.E.; Margaritescu, C.; Tudorache, S.; Ciurea, R.N. E-cadherin and beta-catenin immunoexpression in endometrioid endometrial carcinoma. Rom. J. Morphol. Embryol. 2016, 57, 1235–1240. [Google Scholar] [PubMed]
- Huszar, M.; Pfeifer, M.; Schirmer, U.; Kiefel, H.; Konecny, G.E.; Ben-Arie, A.; Edler, L.; Munch, M.; Muller-Holzner, E.; Jerabek-Klestil, S.; et al. Up-regulation of L1CAM is linked to loss of hormone receptors and E-cadherin in aggressive subtypes of endometrial carcinomas. J. Pathol. 2010, 220, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Koyuncuoglu, M.; Okyay, E.; Saatli, B.; Olgan, S.; Akin, M.; Saygili, U. Tumor budding and E-Cadherin expression in endometrial carcinoma: Are they prognostic factors in endometrial cancer? Gynecol. Oncol. 2012, 125, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Sakuragi, N.; Nishiya, M.; Ikeda, K.; Ohkouch, T.; Furth, E.E.; Hareyama, H.; Satoh, C.; Fujimoto, S. Decreased E-cadherin expression in endometrial carcinoma is associated with tumor dedifferentiation and deep myometrial invasion. Gynecol. Oncol. 1994, 53, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Scholten, A.N.; Aliredjo, R.; Creutzberg, C.L.; Smit, V.T. Combined E-cadherin, alpha-catenin, and beta-catenin expression is a favorable prognostic factor in endometrial carcinoma. Int. J. Gynecol. Cancer 2006, 16, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Yalta, T.; Atay, L.; Atalay, F.; Caydere, M.; Gonultas, M.; Ustun, H. E-cadherin expression in endometrial malignancies: Comparison between endometrioid and non-endometrioid carcinomas. J. Int. Med. Res. 2009, 37, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Mell, L.K.; Meyer, J.J.; Tretiakova, M.; Khramtsov, A.; Gong, C.; Yamada, S.D.; Montag, A.G.; Mundt, A.J. Prognostic significance of E-cadherin protein expression in pathological stage I-III endometrial cancer. Clin. Cancer Res. 2004, 10, 5546–5553. [Google Scholar] [CrossRef] [PubMed]
- Varras, M.; Skafida, E.; Vasilakaki, T.; Anastasiadis, A.; Akrivis, C.; Vrachnis, N.; Nikolopoulos, G. Expression of E-cadherin in primary endometrial carcinomas: Clinicopathological and immunohistochemical analysis of 30 cases. Eur. J. Gynaecol. Oncol. 2013, 34, 31–35. [Google Scholar] [CrossRef]
- Zyla, M.M.; Wilczynski, J.R.; Kostrzewa, M.; Ksiezakowska-Lakoma, K.; Nowak, M.; Stachowiak, G.; Szyllo, K.; Stetkiewicz, T. The significance of markers in the diagnosis of endometrial cancer. Prz Menopauzalny 2016, 15, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Fukuchi, T.; Sakamoto, M.; Tsuda, H.; Maruyama, K.; Nozawa, S.; Hirohashi, S. Beta-catenin mutation in carcinoma of the uterine endometrium. Cancer Res. 1998, 58, 3526–3528. [Google Scholar] [PubMed]
- Kobayashi, K.; Sagae, S.; Nishioka, Y.; Tokino, T.; Kudo, R. Mutations of the beta-catenin gene in endometrial carcinomas. Jpn. J. Cancer Res. 1999, 90, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Nishimura, M.; Kudo, R.; Yamasaki, H. Suppressed gap junctional intercellular communication in carcinogenesis of endometrium. Int. J Cancer 2001, 93, 317–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lesniewicz, T.; Kanczuga-Koda, L.; Baltaziak, M.; Sulkowska, M.; Rutkowski, R.; Koda, M.; Sulkowski, S. Expression of connexin 26 in endometrial adenocarcinoma--analysis of correlations with some anatomoclinical features. Folia Histochem. Cytobiol. 2008, 46, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Tanaka, R.; Wataba, K.; Kudo, R.; Yamasaki, H. Overexpression of estrogen receptor-alpha gene suppresses gap junctional intercellular communication in endometrial carcinoma cells. Oncogene 2004, 23, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Olbina, G.; Eckhart, W. Mutations in the second extracellular region of connexin 43 prevent localization to the plasma membrane, but do not affect its ability to suppress cell growth. Mol. Cancer Res. 2003, 1, 690–700. [Google Scholar] [PubMed]
- Qin, H.; Shao, Q.; Curtis, H.; Galipeau, J.; Belliveau, D.J.; Wang, T.; Alaoui-Jamali, M.A.; Laird, D.W. Retroviral delivery of connexin genes to human breast tumor cells inhibits in vivo tumor growth by a mechanism that is independent of significant gap junctional intercellular communication. J. Biol. Chem. 2002, 277, 29132–29138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.W.; Kaneda, M.; Morita, I. The gap junction-independent tumor-suppressing effect of connexin 43. J. Biol. Chem. 2003, 278, 44852–44856. [Google Scholar] [CrossRef] [PubMed]
| Junctional Component | Analyzed Parameter | Localization | Regulation | Reference |
|---|---|---|---|---|
| Claudin-1 | mRNA | Not regulated | [36] | |
| Protein | GE | Upregulated in SP | [35] | |
| Claudin-3 | mRNA | Upregulated in mid SP | [36] | |
| Protein | GE | Not regulated | [33,39] | |
| Claudin-4 | mRNA | Upregulated in mid SP | [35,36] | |
| Protein | GE | Not regulated | [33,35,37,39] | |
| Claudin-5 | mRNA | Not regulated | [36] | |
| Protein | GE | Upregulated in SP | [35] | |
| Claudin-7 | mRNA | Upregulated in mid SP | [36] | |
| ZO-1 | mRNA | Not regulated | [37] | |
| Protein | GE | Not regulated | [37] | |
| JAM-1 | mRNA | Not regulated | [41] | |
| Protein | GE | Not regulated | [41,42] | |
| Desmoplakin 1/2 | Protein | GE (functionalis) | Change of localization | [37] |
| Desmoglein 2 | Protein | GE (functionalis) | Change of localization | [37] |
| E-cadherin | mRNA | Not regulated | [37] | |
| Protein | GE | Downregulated in SP | [37] | |
| β-Catenin | Protein | GE | Downregulated in SP | [37] |
| Cx26 | Protein | LE/GE | Downregulated in SP | [44] |
| Cx32 | Protein | GE | Downregulated in mid SP | [44] |
| Upregulated in early SP/Downregulated in late SP | [46] | |||
| Cx43 | Protein | Stromal cells | Downregulated in SP | [44] |
| Upregulated in SP | [47] |
| Junctional Component | Species | Localization | Regulation | Reference |
|---|---|---|---|---|
| Claudin-1 | Human | Primary hEEC | Upregulated by P/inhibited by E | [38] |
| Rat | Epithelial cells | Change in localization | [50] | |
| Claudin-3 | Human | Primary hEEC | Upregulated by P/inhibited by E | [38] |
| Rat | Change in localization | [50] | ||
| Claudin-4 | Human | Primary hEEC | Upregulated by P/inhibited by E | [38] |
| Claudin-5 | Mouse | Endothelial cells | Downregulated by E | [53] |
| Rat | Epithelial cells | Change in localization | [50] | |
| Claudin-7 | Human | Primary hEEC | Upregulated by P/inhibited by E | [38] |
| Rat | Epithelial cells | Change in localization | [50] | |
| Zo-1 | Rat | Epithelial cells | Not regulated | [50] |
| Occludin | Rat | Epithelial cells | Upregulated by E | [50] |
| Cx26 | Rat | Epithelial cells | Upregulated by E/inhibited by P | [55,56,57] |
| Cx43 | Rat | Stromal cells | Upregulated by E/inhibited by P | [55,56,57] |
| Junctional Component | Species | Localization | Regulation | Reference |
|---|---|---|---|---|
| Claudin-1 | Rat | Epithelial cells | Increased on 6 dpc | [66] |
| Claudin-3 | Mouse | Decidual cells | Induced on 6.5 dpc | [52,68] |
| Change of localization on 4.5 dpc | [68] | |||
| Claudin-4 | Rat | Epithelial cells | Increase from 1–6 dpc | [67] |
| Claudin-10 | Mouse | Decidual cells | Induced on 4.5 dpc | [68] |
| Occludin | Rat | Epithelial cells | Induced on 6 dpc | [66] |
| Cx26 | Rat | Epithelial cells | Induced on 5 dpc | [45] |
| Stromal cells | Induced on 6 dpc | [45] | ||
| Mouse | Epithelial cells | Induced on 4.5 dpc | [58] | |
| Cx43 | Rat | Decidual cells | Increased during decidualization | [45] |
| Mouse | Decidual cells | Increased during decidualization | [58] |
| Junctional Component | Analyzed Parameter | Regulation | Reference |
|---|---|---|---|
| Claudin-1 | mRNA | Upregulated in peritoneal lesions | [104] |
| Protein | Downregulated in peritoneal lesions | [38] | |
| Claudin-3 | mRNA | Downregulated in peritoneal lesions | [104] |
| Downregulated in ovarian endometriomata | [39] | ||
| Protein | Downregulated in ovarian endometriomata | [39] | |
| Claudin-4 | mRNA | Downregulated in peritoneal lesions | [104] |
| Downregulated in ovarian endometriomata | [39] | ||
| Protein | Downregulated in ovarian endometriomata | [39] | |
| Claudin-5 | mRNA | Upregulated in peritoneal lesions | [104] |
| Protein | Downregulated in peritoneal lesions | [38] | |
| Claudin-7 | mRNA | Downregulated in peritoneal lesions | [104] |
| Claudin-11 | mRNA | Upregulated in peritoneal lesions | [104] |
| Jam-B | mRNA | Upregulated in peritoneal lesions | [104] |
| Jam-C | mRNA | Upregulated in peritoneal lesions | [104] |
| Zo-3 | mRNA | Downregulated in peritoneal lesions | [104] |
| E-Cadherin | Protein | Downregulated in peritoneal lesions | [105,106] |
| Not regulated in endometriosis | [107] | ||
| mRNA | Downregulated in ovarian endometriomata | [108] | |
| α-Catenin | Protein | Downregulated in peritoneal lesions | [105] |
| mRNA | Downregulated in ovarian endometriomata | [108] | |
| β-Catenin | Protein | Downregulated in peritoneal lesions | [105,107] |
| mRNA | Downregulated in ovarian endometriomata | [108] | |
| Cx26 | Protein | No regulation in eutopic endometrium * | [110] |
| Downregulated in peritoneal lesions | [111] | ||
| Cx43 | Protein | Downregulated in eutopic endometrium * | [110] |
| Downregulated in peritoneal lesions | [111] |
| Junctional Component | Analyzed Parameter | Tumor Staging | Regulation | Reference |
|---|---|---|---|---|
| Claudin-1 | Protein | Type II (USPC) | Upregulated | [35] |
| Claudin-2 | Protein | Type II (USPC) | Downregulated | [35] |
| Claudin-3 | mRNA | Type I | Upregulated | [33] |
| Type II (USPC) | Upregulated | [123] | ||
| Protein | Type I | Upregulated | [33] | |
| Claudin-4 | mRNA | Type I | Upregulated | [33] |
| Type II (USPC) | Upregulated | [123] | ||
| Protein | Type I | Upregulated | [33] | |
| Claudin-5 | mRNA | Type II (USPC) | Downregulated | [123] |
| E-Cadherin | Protein | Type I/Type II | Downregulated during dedifferentiation | [130,131,132,133,134,135,136,137,138,139] |
| β-Catenin | Protein | Type I/Type II | Downregulated during dedifferentiation | [143] |
| Cx26 | mRNA | Type I | Downregulated | [146] |
| Protein | Type I | Downregulated | [146,147] | |
| Cx32 | mRNA | Type I | Downregulated | [146] |
| Protein | Type I | Downregulated | [146,147] | |
| Cx43 | mRNA | Type I | Downregulated | [146] |
| Protein | Type I | Downregulated | [146,147] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grund, S.; Grümmer, R. Direct Cell–Cell Interactions in the Endometrium and in Endometrial Pathophysiology. Int. J. Mol. Sci. 2018, 19, 2227. https://doi.org/10.3390/ijms19082227
Grund S, Grümmer R. Direct Cell–Cell Interactions in the Endometrium and in Endometrial Pathophysiology. International Journal of Molecular Sciences. 2018; 19(8):2227. https://doi.org/10.3390/ijms19082227
Chicago/Turabian StyleGrund, Susanne, and Ruth Grümmer. 2018. "Direct Cell–Cell Interactions in the Endometrium and in Endometrial Pathophysiology" International Journal of Molecular Sciences 19, no. 8: 2227. https://doi.org/10.3390/ijms19082227
APA StyleGrund, S., & Grümmer, R. (2018). Direct Cell–Cell Interactions in the Endometrium and in Endometrial Pathophysiology. International Journal of Molecular Sciences, 19(8), 2227. https://doi.org/10.3390/ijms19082227





