DNA Repair Deficient Chinese Hamster Ovary Cells Exhibiting Differential Sensitivity to Charged Particle Radiation under Aerobic and Hypoxic Conditions
Abstract
:1. Introduction
2. Results
2.1. Cell Survival
2.2. RBE in Aerobic Condition
2.3. RBE for Hypoxic Condition
2.4. OER
2.5. Micronuclei Formation
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Irradiation
4.3. Cell Survival Colony Formation Assay and RBE, OER, and SF2 Calculation
4.4. Micronuclei Formation Assay
4.5. Statistics
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CHO | Chinese Hamster Ovary |
| D10 | Dose to achieve 10% cell survival |
| SF2 | Survival Fraction at 2 Gy |
| LET | Linear Energy Transfer |
| RBE | Relative Biological Effectiveness |
| OER | Oxygen Enhancement Ratio |
| NIRS | National Institute of Radiological Sciences |
| HIMAC | Heavy Ion Medical Accelerator in Chiba |
| SOBP | Spread Out Bragg peak |
| ANOVA | Analysis of valiance |
| PARP | Poly (ADP-ribose) polymerase |
| FANC | Fanconi Anemia |
| NHEJ | Non Homologous End Joining |
| HR | Homologous Recombination |
References
- Ruhm, W.; Woloschak, G.E.; Shore, R.E.; Azizova, T.V.; Grosche, B.; Niwa, O.; Akiba, S.; Ono, T.; Suzuki, K.; Iwasaki, T.; et al. Dose and dose-rate effects of ionizing radiation: A discussion in the light of radiological protection. Radiat. Environ. Biophs. 2015, 54, 379–401. [Google Scholar] [CrossRef] [PubMed]
- Morgan, W.F.; Day, J.P.; Kaplan, M.I.; McGhee, E.M.; Limoli, C.L. Genomic instability induced by ionizing radiation. Radiat. Res. 1996, 146, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, H.; Latt, S.A.; Lalande, M.E.; Little, J.B. Effects of X-irradiation on cell-cycle progression, induction of chromosomal aberrations and cell killing in ataxia telangiectasia (AT) fibroblasts. Mutat. Res. 1985, 148, 71–82. [Google Scholar] [CrossRef]
- Cornforth, M.N.; Bedford, J.S. X-Ray-Induced Breakage and Rejoining of Human Interphase Chromosomes. Science 1983, 222, 1141–1143. [Google Scholar] [CrossRef] [PubMed]
- Norppa, H.; Falck, G.C.M. What do human micronuclei contain? Mutagenesis 2003, 18, 221–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hopkins, S.R.; McGregor, G.A.; Murray, J.M.; Downs, J.A.; Savic, V. Novel synthetic lethality screening method identifies TIP60-dependent radiation sensitivity in the absence of BAF180. DNA Repair 2016, 46, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Vandevoorde, C.; Depuydt, J.; Veldeman, L.; De Neve, W.; Sebastia, N.; Wieme, G.; Baert, A.; De Langhe, S.; Philippe, J.; Thierens, H.; et al. In vitro cellular radiosensitivity in relationship to late normal tissue reactions in breast cancer patients: A multi-endpoint case-control study. Int. J. Radiat. Biol. 2016, 92, 823–836. [Google Scholar] [CrossRef] [PubMed]
- Vandevoorde, C.; Vral, A.; Vandekerckhove, B.; Philippe, J.; Thierens, H. Radiation Sensitivity of Human CD34(+) Cells Versus Peripheral Blood T Lymphocytes of Newborns and Adults: DNA Repair and Mutagenic Effects. Radiat. Res. 2016, 185, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.G.; Pezuk, J.A.; Brassesco, M.S.; de Oliveira, J.C.; Queiroz, R.G.D.; Machado, H.R.; Carlotti, C.G.; Neder, L.; de Oliveira, H.F.; Scrideli, C.A.; et al. BUB1 and BUBR1 inhibition decreases proliferation and colony formation, and enhances radiation sensitivity in pediatric glioblastoma cells. Child’s Nerv. Syst. 2013, 29, 2241–2248. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.F. DNA Damage Produced by Ionizing-Radiation in Mammalian-Cells: Identities, Mechanisms of Formation, and Reparability. Prog. Nucleic Acid Res. Mol. Biol. 1988, 35, 95–125. [Google Scholar] [PubMed]
- Riley, P.A. Free-Radicals in Biology—Oxidative Stress and the Effects of Ionizing-Radiation. Int. J. Radiat. Biol. 1994, 65, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Hamada, N.; Imaoka, T.; Masunaga, S.; Ogata, T.; Okayasu, R.; Takahashi, A.; Kato, T.A.; Kobayashi, Y.; Ohnishi, T.; Ono, K.; et al. Recent Advances in the Biology of Heavy-Ion Cancer Therapy. J. Radiat. Res. 2010, 51, 365–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okayasu, R.; Okada, M.; Okabe, A.; Noguchi, M.; Takakura, K.; Takahashi, S. Repair of DNA damage induced by accelerated heavy ions in mammalian cells proficient and deficient in the non-homologous end-joining pathway. Radiat. Res. 2006, 165, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Radford, I.R. The Level of Induced DNA Double-Strand Breakage Correlates with Cell Killing after X-Irradiation. Int. J. Radiat. Biol. 1985, 48, 45–54. [Google Scholar] [CrossRef]
- Hauth, F.; Toulany, M.; Zips, D.; Menegakis, A. Cell-line dependent effects of hypoxia prior to irradiation in squamous cell carcinoma lines. Clin. Transl. Radiat. Oncol. 2017, 5, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.F. The Complexity of DNA-Damage—Relevance to Biological Consequences. Int. J. Radiat. Biol. 1994, 66, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Scifoni, E.; Tinganelli, W.; Weyrather, W.K.; Durante, M.; Maier, A.; Kramer, M. Including oxygen enhancement ratio in ion beam treatment planning: Model implementation and experimental verification. Phys. Med. Biol. 2013, 58, 3871–3895. [Google Scholar] [CrossRef] [PubMed]
- Wenzl, T.; Wilkens, J.J. Modelling of the oxygen enhancement ratio for ion beam radiation therapy. Phys. Med. Biol. 2011, 56, 3251–3268. [Google Scholar] [CrossRef] [PubMed]
- Barendsen, G.W. Possibilities for the application of fast neutrons in radiotherapy: Recovery and oxygen enhancement of radiation induced damage in relation to linear energy transfer. Eur. J. Cancer 1966, 2, 333–345. [Google Scholar] [CrossRef]
- Weyrather, W.K.; Ritter, S.; Scholz, M.; Kraft, G. RBE for carbon track-segment irradiation in cell lines of differing repair capacity. Int. J. Radiat. Biol. 1999, 75, 1357–1364. [Google Scholar] [PubMed]
- Cartwright, I.M.; Bell, J.J.; Maeda, J.; Genet, M.D.; Romero, A.; Fujii, Y.; Fujimori, A.; Kitamuta, H.; Kamada, T.; Chen, D.J.; et al. Effects of targeted phosphorylation site mutations in the DNA-PKcs phosphorylation domain on low and high LET radiation sensitivity. Oncol. Lett. 2015, 9, 1621–1627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagasawa, H.; Chen, D.J.; Strniste, G.F. Response of X-ray-sensitive CHO mutant cells to gamma radiation. I. Effects of low dose rates and the process of repair of potentially lethal damage in G1 phase. Radiat. Res. 1989, 118, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Barker, H.E.; Paget, J.T.E.; Khan, A.A.; Harrington, K.J. The tumour microenvironment after radiotherapy: Mechanisms of resistance and recurrence (vol 15, pg 409, 2015). Nat. Rev. Cancer 2015, 15, 409–425. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.C.; Lebedev, A.; Aten, E.; Madsen, K.; Marciano, L.; Kolb, H.C. The Clinical Importance of Assessing Tumor Hypoxia: Relationship of Tumor Hypoxia to Prognosis and Therapeutic Opportunities. Antioxid. Redox Sign. 2014, 21, 1516–1554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visvader, J.E.; Lindeman, G.J. Cancer stem cells in solid tumours: Accumulating evidence and unresolved questions. Nat. Rev. Cancer 2008, 8, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Pajonk, F.; Vlashi, E.; McBride, W.H. Radiation resistance of cancer stem cells: The 4 R’s of radiobiology revisited. Stem Cells 2010, 28, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Soeda, A.; Park, M.; Lee, D.; Mintz, A.; Androutsellis-Theotokis, A.; McKay, R.D.; Engh, J.; Iwama, T.; Kunisada, T.; Kassam, A.B.; et al. Hypoxia promotes expansion of the CD133-positive glioma stem cells through activation of HIF-1alpha. Oncogene 2009, 28, 3949–3959. [Google Scholar] [CrossRef] [PubMed]
- Moulder, J.E.; Rockwell, S. Hypoxic fractions of solid tumors: Experimental techniques, methods of analysis, and a survey of existing data. Int. J. Radiat. Oncol. Biol. Phys. 1984, 10, 695–712. [Google Scholar] [CrossRef]
- Brown, J.M.; Lemmon, M.J. Tumor Hypoxia Can Be Exploited to Preferentially Sensitize Tumors to Fractionated-Irradiation. Int. J. Radiat. Oncol. 1991, 20, 457–461. [Google Scholar] [CrossRef]
- Matsuo, M.; Krishna, M.; Tanaka, H.; Yamaguchi, T.; Mitchell, J.B. Reoxygenation-Based Radiation Therapy Improve the Tumor Control. Int. J. Radiat. Oncol. 2017, 99, E608. [Google Scholar] [CrossRef]
- Withers, H.R.; Taylor, J.M.G.; Maciejewski, B. The Hazard of Accelerated Tumor Clonogen Repopulation during Radiotherapy. Acta Oncol. 1988, 27, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Tannock, I.F. Repopulation of cancer cells during therapy: An important cause of treatment failure. Nat. Rev. Cancer 2005, 5, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Shuryak, I.; Hall, E.J.; Brenner, D.J. Dose dependence of accelerated repopulation in head and neck cancer: Supporting evidence and clinical implications. Radiother. Oncol. 2018, 127, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Overgaard, J.; Horsman, M.R. Modification of hypoxia-induced radioresistance in tumors by the use of oxygen and sensitizers. Semin. Radiat. Oncol. 1996, 6, 10–21. [Google Scholar] [CrossRef]
- Tepper, J.E.; Wang, A.Z. Improving local control in rectal cancer: Radiation sensitizers or radiation dose? J. Clin. Oncol. 2010, 28, 1623–1624. [Google Scholar] [CrossRef] [PubMed]
- Carreau, A.; El Hafny-Rahbi, B.; Matejuk, A.; Grillon, C.; Kieda, C. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J. Cell. Mol. Med. 2011, 15, 1239–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhani, N.; Fyles, A.; Hedley, D.; Milosevic, M. The Clinical Significance of Hypoxia in Human Cancers. Semin. Nucl. Med. 2015, 45, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Baumann, R.; Depping, R.; Delaperriere, M.; Dunst, J. Targeting hypoxia to overcome radiation resistance in head & neck cancers: Real challenge or clinical fairytale? Expert Rev. Anticancer Ther. 2016, 16, 751–758. [Google Scholar] [PubMed]
- Prise, K.M.; Folkard, M.; Newman, H.C.; Michael, B.D. Effect of radiation quality on lesion complexity in cellular DNA. Int. J. Radiat. Biol. 1994, 66, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Niedzwiedz, W.; Mosedale, G.; Johnson, M.; Ong, C.Y.; Pace, P.; Patel, K.J. The Fanconi anaemia gene FANCC promotes homologous recombination and error-prone DNA repair. Mol. Cell 2004, 15, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Ishiai, M.; Matsushita, N.; Arakawa, H.; Lamerdin, J.E.; Buerstedde, J.M.; Tanimoto, M.; Harada, M.; Thompson, L.H.; Takata, M. Fanconi anemia FANCG protein in mitigating radiation- and enzyme-induced DNA double-strand breaks by homologous recombination in vertebrate cells. Mol. Cell Biol. 2003, 23, 5421–5430. [Google Scholar] [CrossRef] [PubMed]
- Helleday, T.; Bryant, H.E.; Schultz, N. Poly(ADP-ribose) polymerase (PARP-1) in homologous recombination and as a target for cancer therapy. Cell Cycle 2005, 4, 1176–1178. [Google Scholar] [CrossRef] [PubMed]
- Schultz, N.; Lopez, E.; Saleh-Gohari, N.; Helleday, T. Poly(ADP-ribose) polymerase (PARP-1) has a controlling role in homologous recombination. Nucleic Acids Res. 2003, 31, 4959–4964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hada, M.; Georgakilas, A.G. Formation of clustered DNA damage after high-LET irradiation: A review. J. Radiat. Res. 2008, 49, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, R.; Uzawa, A.; Obara, M.; Takase, N.; Koda, K.; Ozaki, M.; Noguchi, M.; Matsumoto, Y.; Li, H.Z.; Yamashita, K.; et al. Determination of the relative biological effectiveness and oxygen enhancement ratio for micronuclei formation using high-LET radiation in solid tumor cells: An in vitro and in vivo study. Mutat. Res. Genet Toxicol. Environ. Mutagen. 2015, 793, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Ebner, D.K.; Kamada, T. The Emerging Role of Carbon-Ion Radiotherapy. Front. Oncol. 2016, 6, 140. [Google Scholar] [CrossRef] [PubMed]
- Drean, A.; Lord, C.J.; Ashworth, A. PARP inhibitor combination therapy. Crit. Rev. Oncol. Hematol. 2016, 108, 73–85. [Google Scholar] [CrossRef] [PubMed]
- To, C.; Kim, E.H.; Royce, D.B.; Williams, C.R.; Collins, R.M.; Risingsong, R.; Sporn, M.B.; Liby, K.T. The PARP inhibitors, veliparib and olaparib, are effective chemopreventive agents for delaying mammary tumor development in BRCA1-deficient mice. Cancer Prev. Res. 2014, 7, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Jeggo, P.A.; Kemp, L.M. X-ray-sensitive mutants of Chinese hamster ovary cell line. Isolation and cross-sensitivity to other DNA-damaging agents. Mutat. Res. 1983, 112, 313–327. [Google Scholar] [CrossRef]
- Stamato, T.D.; Weinstein, R.; Giaccia, A.; Mackenzie, L. Isolation of cell cycle-dependent gamma ray-sensitive Chinese hamster ovary cell. Somat. Cell Genet. 1983, 9, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Witmer, M.V.; Aboul-Ela, N.; Jacobson, M.K.; Stamato, T.D. Increased sensitivity to DNA-alkylating agents in CHO mutants with decreased poly(ADP-ribose) polymerase activity. Mutat. Res. 1994, 314, 249–260. [Google Scholar] [CrossRef]
- Whitmore, G.F.; Varghese, A.J.; Gulyas, S. Cell cycle responses of two X-ray sensitive mutants defective in DNA repair. Int. J. Radiat. Biol. 1989, 56, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Hinz, J.M.; Tebbs, R.S.; Wilson, P.F.; Nham, P.B.; Salazar, E.P.; Nagasawa, H.; Urbin, S.S.; Bedford, J.S.; Thompson, L.H. Repression of mutagenesis by Rad51D-mediated homologous recombination. Nucleic Acids Res. 2006, 34, 1358–1368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuller, L.F.; Painter, R.B. A Chinese hamster ovary cell line hypersensitive to ionizing radiation and deficient in repair replication. Mutat. Res. 1988, 193, 109–121. [Google Scholar] [CrossRef]
- Tebbs, R.S.; Hinz, J.M.; Yamada, N.A.; Wilson, J.B.; Salazar, E.P.; Thomas, C.B.; Jones, I.M.; Jones, N.J.; Thompson, L.H. New insights into the Fanconi anemia pathway from an isogenic FancG hamster CHO mutant. DNA Repair 2005, 4, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Obata, A.; Kasamatsu, S.; Lewis, J.S.; Furukawa, T.; Takamatsu, S.; Toyohara, J.; Asai, T.; Welch, M.J.; Adams, S.G.; Saji, H.; et al. Basic characterization of 64Cu-ATSM as a radiotherapy agent. Nucl. Med. Biol. 2005, 32, 21–28. [Google Scholar] [CrossRef] [PubMed]
- McMillan, D.D.; Maeda, J.; Bell, J.J.; Genet, M.D.; Phoonswadi, G.; Mann, K.A.; Kraft, S.L.; Kitamura, H.; Fujimori, A.; Yoshii, Y.; et al. Validation of 64Cu-ATSM damaging DNA via high-LET Auger electron emission. J. Radiat. Res. 2015, 56, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Seko, Y.; Tobe, K.; Ueki, K.; Kadowaki, T.; Yazaki, Y. Hypoxia and hypoxia/reoxygenation activate Raf-1, mitogen-activated protein kinase kinase, mitogen-activated protein kinases, and S6 kinase in cultured rat cardiac myocytes. Circ. Res. 1996, 78, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Haskins, J.S.; Su, C.; Allum, A.; Haskins, A.H.; Salinas, V.A.; Sunada, S.; Inoue, T.; Aizawa, Y.; Uesaka, M.; et al. In vitro screening of radioprotective properties in the novel glucosylated flavonoids. Int. J. Mol. Med. 2016, 38, 1525–1530. [Google Scholar] [CrossRef] [PubMed]
- Maeda, J.; Cartwright, I.M.; Haskins, J.S.; Fujii, Y.; Fujisawa, H.; Hirakawa, H.; Uesaka, M.; Kitamura, H.; Fujimori, A.; Thamm, D.H.; et al. Relative biological effectiveness in canine osteosarcoma cells irradiated with accelerated charged particles. Oncol. Lett. 2016, 12, 1597–1601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, M.; Kase, Y.; Yamaguchi, H.; Kanai, T.; Ando, K. Relative biological effectiveness for cell-killing effect on various human cell lines irradiated with heavy-ion medical accelerator in Chiba (HIMAC) carbon-ion beams. Int. J. Radiat. Oncol. Biol. Phys. 1999, 48, 241–250. [Google Scholar] [CrossRef]
- Kamada, T.; Tsujii, H.; Tsuji, H.; Yanagi, T.; Mizoe, J.E.; Miyamoto, T.; Kato, H.; Yamada, S.; Morita, S.; Yoshikawa, K.; et al. Efficacy and safety of carbon ion radiotherapy in bone and soft tissue sarcomas. J. Clin. Oncol. 2002, 20, 4466–4471. [Google Scholar] [CrossRef] [PubMed]
- Maeda, J.; Roybal, E.J.; Brents, C.A.; Uesaka, M.; Aizawa, Y.; Kato, T.A. Natural and glucosyl flavonoids inhibit poly(ADP-ribose) polymerase activity and induce synthetic lethality in BRCA mutant cells. Oncol. Rep. 2014, 31, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Allum, A.J.; Aizawa, Y.; Kato, T.A. Novel glyceryl glucoside is a low toxic alternative for cryopreservation agent. Biochem. Biophys. Res. Commun. 2016, 476, 359–364. [Google Scholar] [CrossRef] [PubMed]


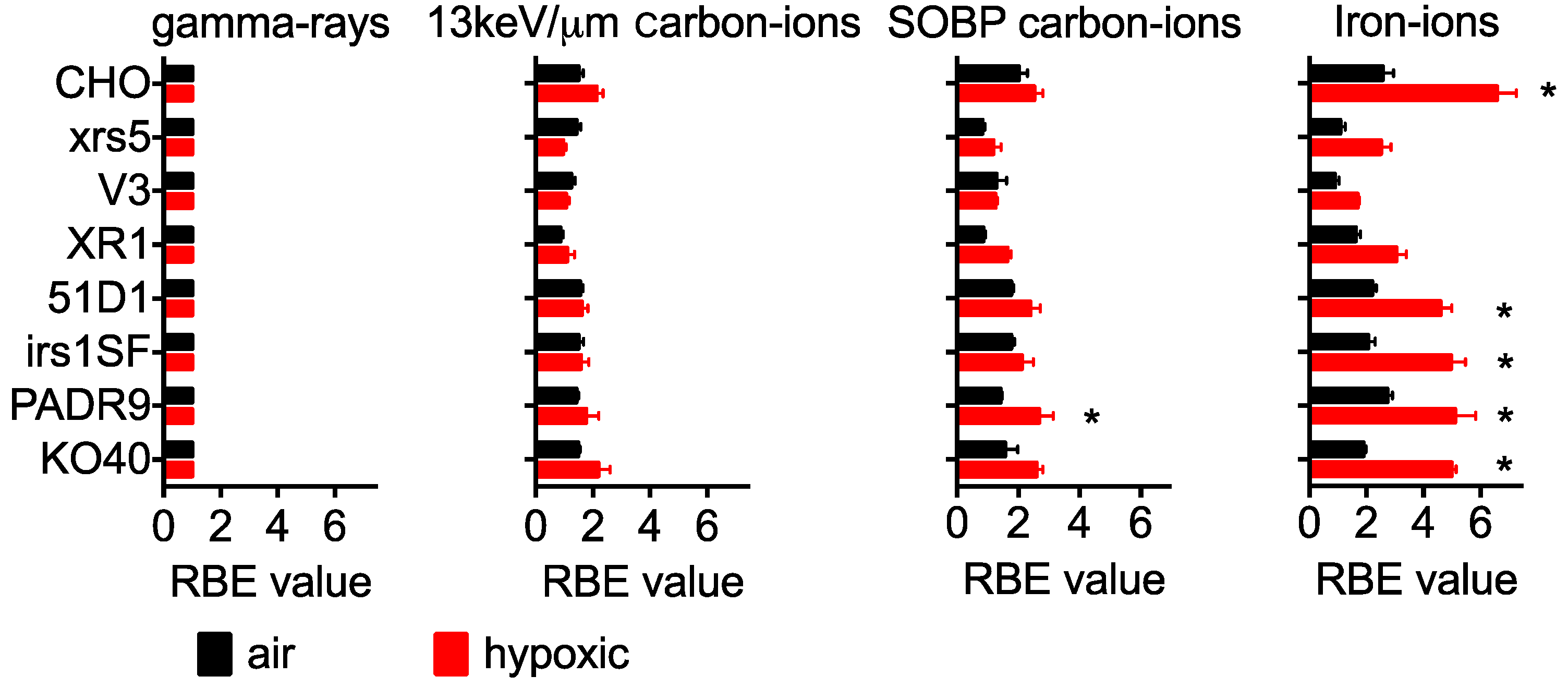



| CHO | xrs5 | V3 | XR1 | 51D1 | irs1SF | PADR9 | KO40 | ||
|---|---|---|---|---|---|---|---|---|---|
| gamma-rays | aerobic | 0.631 | 0.020 | 0.092 | 0.238 | 0.426 | 0.250 | 0.657 | 0.618 |
| hypoxic | 0.840 | 0.291 | 0.362 | 0.543 | 0.676 | 0.595 | 0.737 | 0.735 | |
| carbon-ions 13 keV/μm | aerobic | 0.538 | 0.006 | 0.041 | 0.250 | 0.154 | 0.147 | 0.423 | 0.302 |
| hypoxic | 0.898 | 0.322 | 0.340 | 0.527 | 0.452 | 0.375 | 0.638 | 0.603 | |
| carbon-ions SOBP | aerobic | 0.371 | 0.060 | 0.054 | 0.241 | 0.131 | 0.081 | 0.551 | 0.372 |
| hypoxic | 0.710 | 0.201 | 0.369 | 0.379 | 0.339 | 0.428 | 0.583 | 0.520 | |
| iron-ions 200 keV/μm | aerobic | 0.201 | 0.021 | 0.110 | 0.070 | 0.074 | 0.042 | 0.174 | 0.204 |
| hypoxic | 0.222 | 0.025 | 0.138 | 0.131 | 0.081 | 0.037 | 0.223 | 0.248 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cartwright, I.M.; Su, C.; Haskins, J.S.; Salinas, V.A.; Sunada, S.; Yu, H.; Uesaka, M.; Hirakawa, H.; Chen, D.J.; Fujimori, A.; et al. DNA Repair Deficient Chinese Hamster Ovary Cells Exhibiting Differential Sensitivity to Charged Particle Radiation under Aerobic and Hypoxic Conditions. Int. J. Mol. Sci. 2018, 19, 2228. https://doi.org/10.3390/ijms19082228
Cartwright IM, Su C, Haskins JS, Salinas VA, Sunada S, Yu H, Uesaka M, Hirakawa H, Chen DJ, Fujimori A, et al. DNA Repair Deficient Chinese Hamster Ovary Cells Exhibiting Differential Sensitivity to Charged Particle Radiation under Aerobic and Hypoxic Conditions. International Journal of Molecular Sciences. 2018; 19(8):2228. https://doi.org/10.3390/ijms19082228
Chicago/Turabian StyleCartwright, Ian M., Cathy Su, Jeremy S. Haskins, Victoria A. Salinas, Shigeaki Sunada, Hao Yu, Mitsuru Uesaka, Hirokazu Hirakawa, David J. Chen, Akira Fujimori, and et al. 2018. "DNA Repair Deficient Chinese Hamster Ovary Cells Exhibiting Differential Sensitivity to Charged Particle Radiation under Aerobic and Hypoxic Conditions" International Journal of Molecular Sciences 19, no. 8: 2228. https://doi.org/10.3390/ijms19082228
APA StyleCartwright, I. M., Su, C., Haskins, J. S., Salinas, V. A., Sunada, S., Yu, H., Uesaka, M., Hirakawa, H., Chen, D. J., Fujimori, A., & Kato, T. A. (2018). DNA Repair Deficient Chinese Hamster Ovary Cells Exhibiting Differential Sensitivity to Charged Particle Radiation under Aerobic and Hypoxic Conditions. International Journal of Molecular Sciences, 19(8), 2228. https://doi.org/10.3390/ijms19082228




