Autophagy in Human Skin Fibroblasts: Impact of Age
Abstract
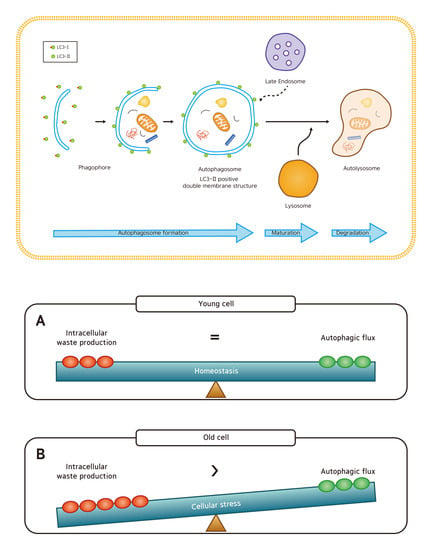
1. Introduction
2. Results
2.1. Autophagosomes and Autolysosomes under Electron Microscopy
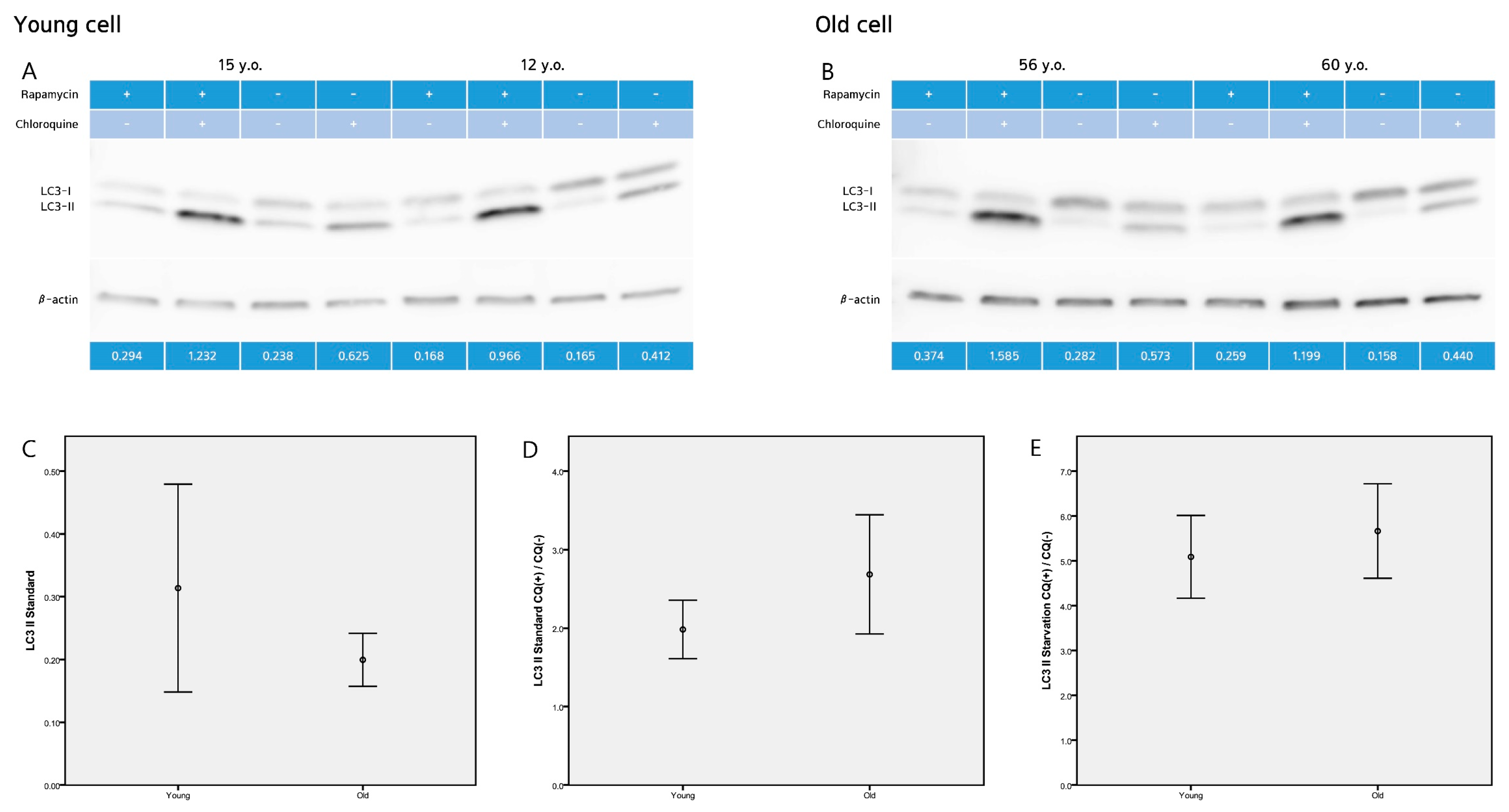
2.2. LC3-II Level and LC3-II Turnover Assay
2.3. p62 Assay
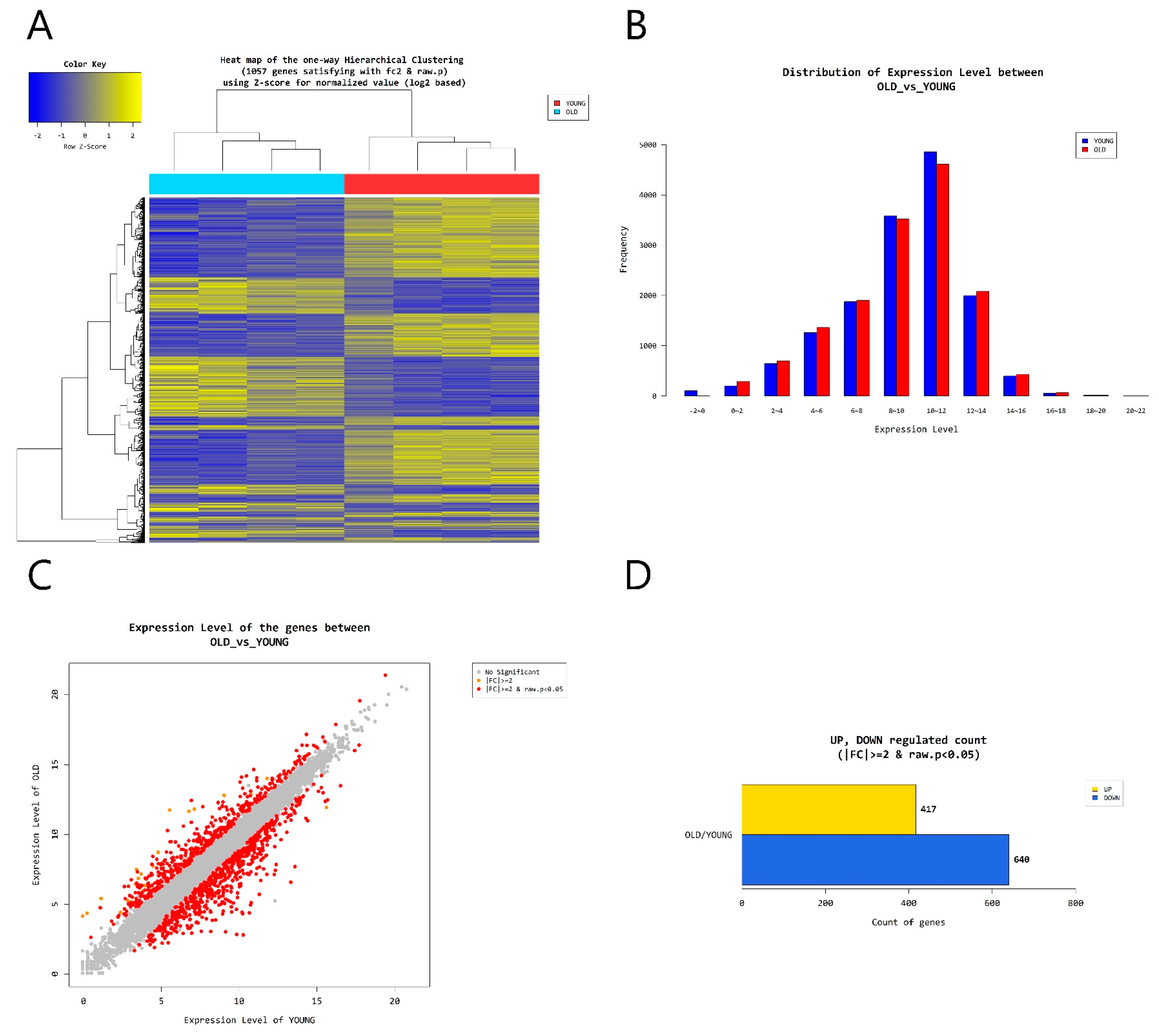
2.4. Gene Transcription Profile
3. Discussion
4. Materials and Methods
4.1. Skin Samples
4.2. Ethics
4.3. Cell Culture
4.4. Cell Lysis and Western Blotting
4.5. Enzyme-Linked Immunosorbent Assay (ELISA)
4.6. Transmission Electron Microscopy (TEM)
4.7. mRNA-Seq
4.8. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| TEM | Transmission electron microscopy |
| LC3 | Microtubule-associated protein 1 light chain 3 |
| BECN1 | Beclin-1 |
| MAP1L3B | Microtubule-associated protein 1A/1B light chain 3B |
| Atg5 | Autophagy related 5 |
| Atg7 | Autophagy related 7 |
| ULK1 | Serine/threonine-protein kinase |
| PIK3C3 | Phosphatidylinositol 3-kinase catalytic subunit type 3 |
| mTOR | Mammalian target of rapamycin |
| FBXL2 | F-box and leucine rich repeat protein 2 |
| HTR2B | 5-hydroxytryptamine receptor 2B |
| MAPT | Microtubule-associated protein Tau |
| RAB33A | Member RAS oncogene family |
| SOGA3 | Suppressor of glucose, autophagy associated 3 |
| BNIP3 | Bcl2/adenovirus E1B 19-kDa interacting protein 3 |
References
- Schulze, C.; Wetzel, F.; Kueper, T.; Malsen, A.; Muhr, G.; Jaspers, S.; Blatt, T.; Wittern, K.P.; Wenck, H.; Kas, J.A. Stiffening of human skin fibroblasts with age. Biophys. J. 2010, 99, 2434–2442. [Google Scholar] [CrossRef] [PubMed]
- Fatima, F.; Nawaz, M. Long distance metabolic regulation through adipose-derived circulating exosomal miRNAs: A trail for RNA-based therapies? Front. Physiol. 2017, 8, 545. [Google Scholar] [CrossRef] [PubMed]
- Demirovic, D.; Nizard, C.; Rattan, S.I. Basal level of autophagy is increased in aging human skin fibroblasts in vitro, but not in old skin. PLoS ONE 2015, 10, e0126546. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, B.; Sarkar, S.; Davies, J.E.; Flutter, M.; Garcia-Arencibia, M.; Green-Thompson, Z.W.; Jimenez-Sanchez, M.; Korolchuk, V.I.; Lichtenberg, M.; Luo, S.; et al. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol. Rev. 2010, 90, 1383–1435. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J. The molecular machinery of autophagy: Unanswered questions. J. Cell Sci. 2005, 118, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.J.; Chen, S.; Huang, K.X.; Le, W.D. Why should autophagic flux be assessed? Acta Pharmacol. Sin. 2013, 34, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Pernodet, N.; Dong, K.; Pelle, E. Autophagy in human skin fibroblasts: Comparison between young and aged cells and evaluation of its cellular rhythm and response to ultraviolet A radiation. J. Cosmet. Sci. 2016, 67, 13–20. [Google Scholar] [PubMed]
- Tashiro, K.; Shishido, M.; Fujimoto, K.; Hirota, Y.; Yo, K.; Gomi, T.; Tanaka, Y. Age-related disruption of autophagy in dermal fibroblasts modulates extracellular matrix components. Biochem. Biophys. Res. Commun. 2014, 443, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Cuervo, A.M. Autophagy and aging: Keeping that old broom working. Trends Genet. 2008, 24, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Rajawat, Y.S.; Hilioti, Z.; Bossis, I. Aging: Central role for autophagy and the lysosomal degradative system. Ageing Res. Rev. 2009, 8, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Takabatake, Y.; Kimura, T.; Takahashi, A.; Namba, T.; Matsuda, J.; Minami, S.; Kaimori, J.Y.; Matsui, I.; Kitamura, H.; et al. Time-dependent dysregulation of autophagy: Implications in aging and mitochondrial homeostasis in the kidney proximal tubule. Autophagy 2016, 12, 801–813. [Google Scholar] [CrossRef] [PubMed]
- Masoro, E. Challenges of Biological Aging; Springer Publishing Co.: New York, NY, USA, 1999; pp. 1–210. [Google Scholar]
- Stadtman, E. Protein oxidation in aging and age-related diseases. Ann. N. Y. Acad. Sci. 2001, 928, 22–38. [Google Scholar] [CrossRef] [PubMed]
- Ryazanov, A.G.; Nefsky, B.S. Protein turnover plays a key role in aging. Mech. Ageing Dev. 2002, 123, 207–213. [Google Scholar] [CrossRef]
- Cuervo, A.M.; Bergamini, E.; Brunk, U.T.; Droge, W.; Ffrench, M.; Terman, A. Autophagy and aging: The importance of maintaining “clean” cells. Autophagy 2015, 1, 131–140. [Google Scholar] [CrossRef]
- Gershon, H.; Gershon, D. Detection of inactive enzyme molecules in aging organisms. Nature 1970, 227, 1214–1217. [Google Scholar] [CrossRef] [PubMed]
- Miquel, J.; Tapperl, A.L.; Dillard, C.J.; Herman, M.M.; Bensch, K.G. Fluorescent products and lysosomal components in aging Drosophila melanogaster. J. Gerontol. 1974, 29, 622–637. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.; Stotland, D.; Cordeiro, R.A. Decreased proteolysis and increased amino acid efflux in aging human fibroblasts. Mech. Ageing Dev. 1976, 5, 221–233. [Google Scholar] [CrossRef]
- Bergamini, E.; Kovacs, J. Exploring the age-related changes in hormone-regulated protein breakdown by the use of a physiologic model of stimulation of liver autophagy. In Protein Metabolism in Aging Modern Aging Research; Segal, H., Rothstein, M., Bergamini, E., Eds.; Wiley-Liss: New York, NY, USA, 1990; pp. 361–370. [Google Scholar]
- Terman, A. The effect of age on formation and elimination of autophagic vacuoles in mouse hepatocytes. Gerontology 1995, 41, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Stupina, A.S.; Terman, A.D.; Kvitmitskaia-Ryzhova, T.L.U.; Mezhiborskaia, N.A.; Zherebitskii, V.A. The age-related characteristics of autophagocytosis in different tissues of laboratory animals. Tsitol. Genet. 1994, 28, 15–20. [Google Scholar] [PubMed]
- Lorenzi, P.L.; Claerhout, S.; Mills, G.B.; Weinstein, J.N. A curated census of autophagy-modulating proteins and small-molecules: Candidate targets for cancer therapy. Autophagy 2014, 10, 1316–1326. [Google Scholar] [CrossRef] [PubMed]
- Ingram, D.K.; Anson, R.M.; de Cabo, R.; Mamczarz, J.; Zhu, M.; Mattison, J.; Lane, M.A.; Roth, G.S. Development of calorie restriction mimetics as a prolongevity strategy. Ann. N. Y. Acad. Sci. 2004, 1019, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Yoshimori, T.; Levine, B. Methods in mammalian autophagy research. Cell 2010, 140, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, S.R.; Mizushima, N. Monitoring and measuring autophagy. Int. J. Mol. Sci. 2017, 18, 1865. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef] [PubMed]



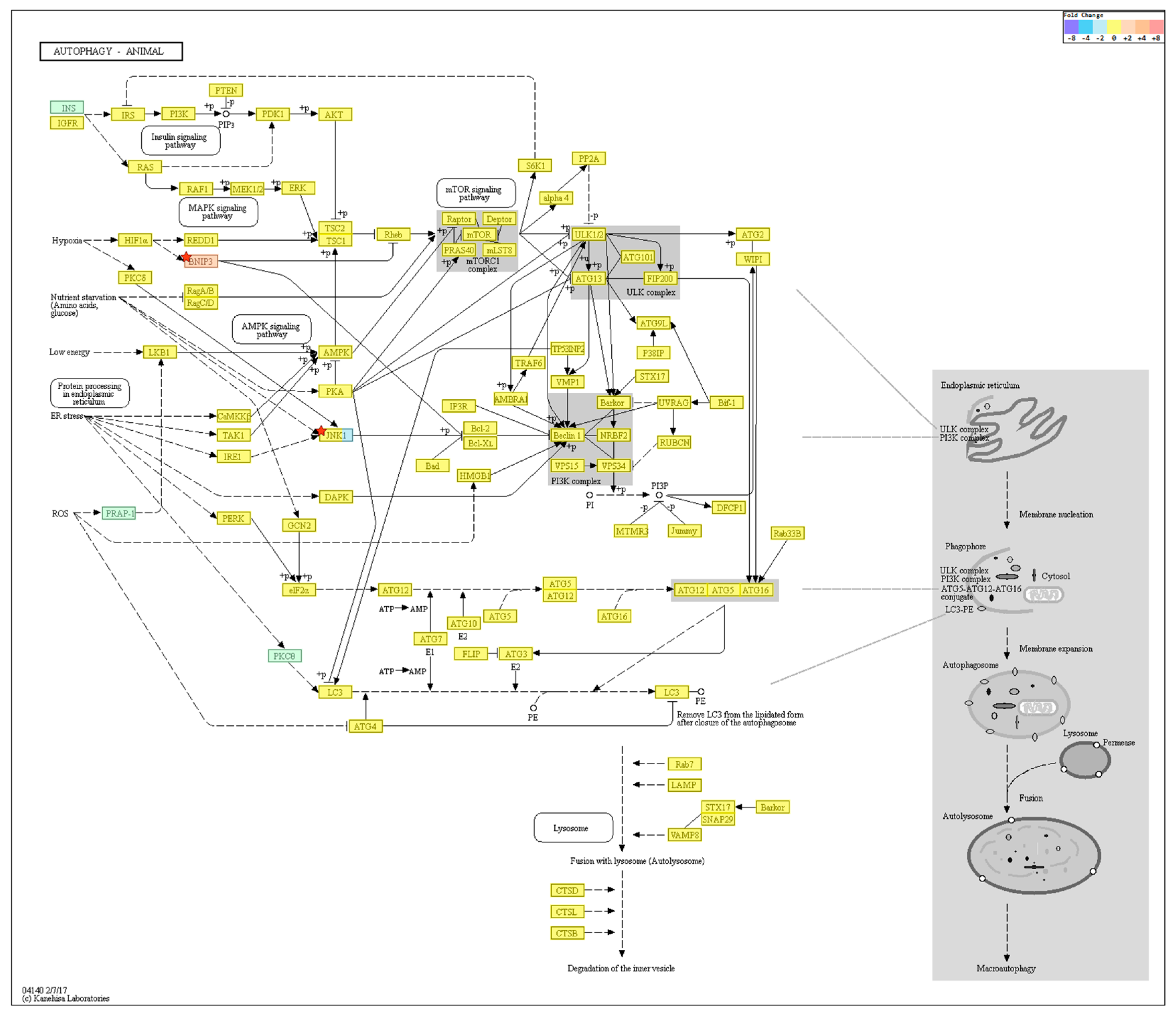

| Autophagy-Regulating Genes Downregulated in the Old Cells | ||
| OLD/YOUNG. fc | p-value | |
| FBXL2 | −4.021224 | >0.05 |
| HTR2B | −2.349009 | >0.05 |
| MAPT | −4.279171 | >0.05 |
| RAB33A | −5.610289 | >0.05 |
| Autophagy-Regulating Genes Upregulated in the Old Cells | ||
| OLD/YOUNG. fc | p-value | |
| HK2 | 2.100391 | >0.05 |
| SOGA3 | 2.114569 | >0.05 |
| BNIP3 | 2.776557 | >0.05 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.S.; Park, S.-Y.; Moon, S.H.; Lee, J.D.; Kim, S. Autophagy in Human Skin Fibroblasts: Impact of Age. Int. J. Mol. Sci. 2018, 19, 2254. https://doi.org/10.3390/ijms19082254
Kim HS, Park S-Y, Moon SH, Lee JD, Kim S. Autophagy in Human Skin Fibroblasts: Impact of Age. International Journal of Molecular Sciences. 2018; 19(8):2254. https://doi.org/10.3390/ijms19082254
Chicago/Turabian StyleKim, Hei Sung, Seo-Yeon Park, Seok Hoon Moon, Jeong Deuk Lee, and Sungjoo Kim. 2018. "Autophagy in Human Skin Fibroblasts: Impact of Age" International Journal of Molecular Sciences 19, no. 8: 2254. https://doi.org/10.3390/ijms19082254
APA StyleKim, H. S., Park, S.-Y., Moon, S. H., Lee, J. D., & Kim, S. (2018). Autophagy in Human Skin Fibroblasts: Impact of Age. International Journal of Molecular Sciences, 19(8), 2254. https://doi.org/10.3390/ijms19082254