Combination ART-Induced Oxidative/Nitrosative Stress, Neurogenic Inflammation and Cardiac Dysfunction in HIV-1 Transgenic (Tg) Rats: Protection by Mg
Abstract
:1. Introduction
2. Results
2.1. Effects of HIV-1 Expression ± cART on Body Weight Gain, Food and Drug Consumption
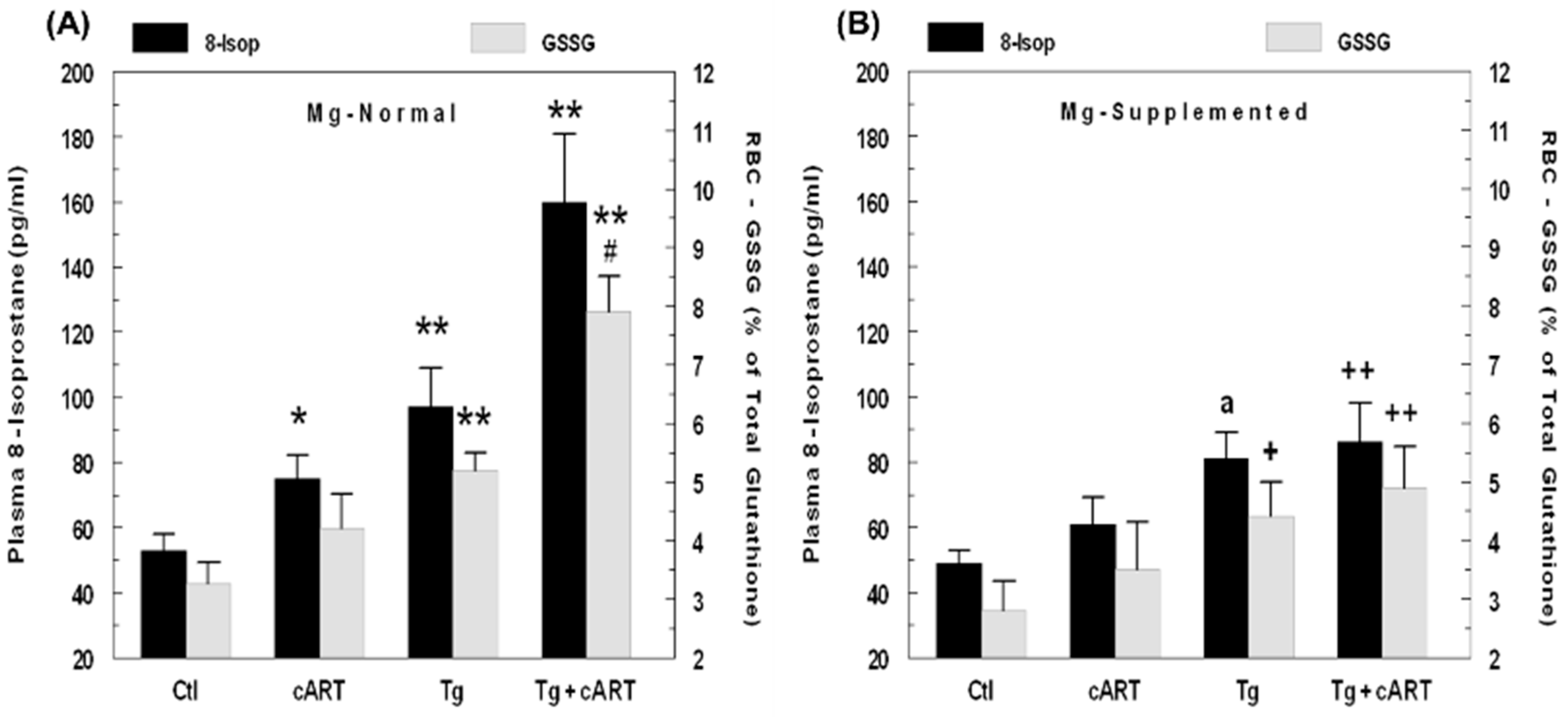
2.2. Effects of HIV-1 Expression ± cART on Indices of Oxidative Stress
2.3. Effect of cART on Neutrophil Activation
2.4. Impact of cART on Plasma and Cardiac Nitrotyrosine (NT) Elevations in Tg and Control Rats on Normal or Mg Supplemented Diets
2.5. Effects of HIV-1 Expression ± cART on Cardiac Function/Hemodynamics
2.6. Mg-Supplementation on Cardiac Function/Hemodynamics of cART−Treated HIV-Tg and Control Rats
2.7. Cardiac Anatomical Parameters and Effects of Mg
2.8. Time-Course Changes in Plasma Mg during cART Treatment of Tg and Control Rats
2.9. Kidney Nitrosative Stress and Decreases in Function
2.10. Effects of HIV-1 ± cART on Neurogenic Inflammation and Impact of Mg Supplementation
3. Discussion
4. Materials and Methods
4.1. HIV-1 Transgenic Rat Model, cART Treatment and Mg-Diets
4.2. Blood Sample Collection
4.3. Plasma Magnesium
4.4. Determination of Systemic Oxidative/Nitrosative Indices, Neutrophil ROS Activity, Plasma Creatinine, Urea and Substance P
4.5. Immunohistochemical Analysis of 3-Nitrotyrosine
4.6. Non-Invasive Ultra-High Frequency Echocardiography
4.7. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Calza, L.; Manfredi, R.; Verucchi, G. Myocardial infarction risk in HIV-infected patients: Epidemiology, pathogenesis, and clinical management. AIDS 2010, 24, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Dube, M.P.; Lipshultz, S.E.; Fichtenbaum, C.J.; Greenberg, R.; Schecter, A.D.; Fisher, S.D.; Working Group 3. Effects of HIV infection and antiretroviral therapy on the heart and vasculature. Circulation 2008, 118, e36–e40. [Google Scholar] [CrossRef] [PubMed]
- Freiberg, M.S.; So-Armah, K. HIV and cardiovascular disease: We need a mechanism, and we need a plan. J. Am. Heart Assoc. 2016, 5, e003411. [Google Scholar] [CrossRef] [PubMed]
- Rathbun, R.C. Antiretroviral Therapy for HIV Infection: Overview, FDA-Approved Antivirals and Regimens. Medscape, 2018. Available online: https://emedicine.Medscape.com/article/1533218 (accessed on 14 March 2018).
- Valdez, H. Immune restoration after treatment of HIV-1 infection with highly active antiretroviral therapy HAART. AIDS Rev. 2002, 4, 157–164. [Google Scholar] [PubMed]
- Kramer, J.H.; Spurney, C.F.; Chmielinska, J.J.; Weglicki, W.B.; Mak, I.T. Mg-supplementation protects against oxidative stress and cardiac dysfunction in chronic HAART-treated rats. In HIV/AIDS: Oxidative Stress and Dietary Antioxidants; Preedy, V.R.A., Watson, R.R., Eds.; Academic Press: London, UK, 2017; Part 2, Chapter 16; pp. 183–196. [Google Scholar]
- Mak, I.T.; Chmielinska, J.J.; Kramer, J.H.; Weglicki, W.B. AZT-induced cardiovascular toxicity-Attenuation by Mg-supplementation. Cardiovasc. Toxicol. 2009, 9, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Mak, I.T.; Kramer, J.H.; Chen, X.; Chmielinska, J.J.; Spurney, C.F.; Weglicki, W.B. Mg-supplementation attenuates Ritonavir-induced hyperlipidemia, oxidative stress and cardiac dysfunction in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R1102–R1111. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mak, I.T. Mg supplementation protects against ritonavir-mediated endothelial oxidative stress and hepatic eNOS downregulation. Free Radioc. Biol. Med. 2014, 69, 77–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kline, E.R.; Sutliff, R.L. The roles of HIV-1 proteins and antiretroviral drug therapy in HIV-1-associated endothelial dysfunction. J. Investig. Med. 2008, 56, 752–769. [Google Scholar] [CrossRef] [PubMed]
- Otis, J.S.; Ashikhmin, Y.I.; Brown, L.A.; Guidot, D.M. Effect of HIV-1-related protein expression on cardiac and skeletal muscles from transgenic rats. Aids Res. Ther. 2008, 5, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scarpino, M.; Santoro, M.; Pellicanò, G. HIV infection and kidney disease: Literature review. Infect. Dis. Trop. Med. 2015, 1, E195. [Google Scholar]
- Cortez, K.J.; Maldarelli, F. Clinical management of HIV drug resistance. Viruses 2011, 3, 347–378. [Google Scholar] [CrossRef] [PubMed]
- Kline, E.R.; Kleihenz, D.J.; Liang, B.; Sutliff, R.L. Vascular oxidative stress and nitric oxide depletion in HIV-1 transgenic rats are reversed by glutathione restoration. Am. J. Physiol. 2008, 294, H2792–H2804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reid, W.; Sadowska, M.; Denaro, F.; Rao, S.; Foulke, J., Jr.; Hayes, N.; Jones, O.; Doodnauth, D.; Davis, H.; Sill, A.; et al. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc. Natl. Acad. Sci. USA 2001, 98, 9271–9276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, J.; Vigorito, M.; Liu, X.; Zhou, D.; Wu, X.; Chang, S.L. The HIV-1 transgenic rat as a model for HIV-1 infected individuals on HAART. J. Neuroimmunol. 2010, 218, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Bogden, J.D.; Baker, H.; Frank, O.; Perez, G.; Kemp, F.; Bruening, K.; Louria, D. Micronutrient status and human immunodeficiency virus (HIV) infection. Ann. N. Y. Acad. Sci. 1990, 587, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Brod-Miller, C. Hypomagnesemia (Hmg) in acquired immune deficiency syndrome (AIDS). J. Am. Soc. Nephrol. 1990, 1, 329–331. [Google Scholar]
- Isnard Bagnis, C.; Du Montcel, S.T.; Fonfrede, M.; Jaudon, M.C.; Thibault, V.; Carcelain, G.; Valantin, M.A.; Izzedine, H.; Servais, A.; Katlama, C.; et al. Changing electrolyte and acido-basic profile in HIV-infected patients in the HAART era. Nephron Physiol. 2006, 103, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Biagioni Santos, M.S.; Seguro, A.C.; Andrade, L. Hypomagnesemia is a risk factor for non-recovery of renal function and mortality in AIDS patients. Braz. J. Med. Biol. Res. 2010, 43, 316–323. [Google Scholar] [CrossRef]
- Moreno Díaz, M.T.; Ruiz López, M.D.; Navarro Alarcón, M.; Artacho Martín-Lagos, R.; Martínez Atienza, M.; Pérez de la Cruz, A. Magnesium deficiency in patients withHIV-AIDS. Nutr. Hosp. 1997, 12, 304–308. [Google Scholar] [PubMed]
- Mudzinge, D.; Nyazika, T.K.; Chisango, T.J.; Zhou, D.T. Differences in serum levels of magnesium, phosphate, and albumin for HAART-experienced and HAART-naïve female patients attending Parirenyatwa opportunistic infections in clinic in Harare, Zimbabwe. ISRN AIDS 2013, 2013, 383214. [Google Scholar] [CrossRef] [PubMed]
- Redhage, L.A.; Shintani, A.; Haas, D.W.; Emeagwali, N.; Markovic, M.; Oboho, I.; Mwenya, C.; Erdem, H.; Acosta, E.P.; Morrow, J.D.; et al. Clinical factors associated with plasma F2-isoprostane levels in HIV-infected adults. HIV Clin. Trials 2009, 10, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Masiá, M.; Padilla, S.; Fernández, M.; Rodríguez, C.; Moreno, A.; Oteo, J.A.; Antela, A.; Moreno, S.; Del Amo, J.; Gutiérrez, F.; et al. Oxidative stress predicts all-cause mortality inHIV-infected patients. PLoS ONE 2016, 11, e0153456. [Google Scholar] [CrossRef] [PubMed]
- Kramer, J.H.; Phillips, T.M.; Weglicki, W.B. Magnesium-deficiency enhanced postischemic myocardial injury is reduced by substance P receptor blockade. J. Mol. Cell. Cardiol. 1997, 29, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration, U.S Department of Health and Human Services, Center for Drug Evaluation and Research. Guidance for Industry Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers; Center for Drug Evaluation and Research (CDER): Rockville, MD, USA, 2005; pp. 1–27.
- Ataka, K.; Chen, D.; McCully, J.; Levitsky, S.; Feinberg, H. Magnesium cardioplegia prevents accumulation of cytosolic calcium in ischemic myocardium. J. Mol. Cell. Cardiol. 1993, 25, 1387–1390. [Google Scholar] [CrossRef] [PubMed]
- Houston, M. The role of magnesium in hypertension and cardiovascular disease. J. Clin. Hypertens. 2011, 13, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Brechard, S.; Tschirhart, E.J. Regulation of superoxide production in neutrophils: Role of calcium influx. J. Leukoc. Biol. 2008, 84, 1223–1237. [Google Scholar] [CrossRef] [PubMed]
- Choudlary, S.; Kumar, A.; Kale, R.K.; Raisz, L.G.; Pilbeam, C.C. Extracellular calcium induces COX-2 in osteoblasts via a PKA pathway. Biochem. Biophys. Res. Commun. 2004, 322, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Mak, I.T.; Komarov, A.M.; Kramer, J.H.; Weglicki, W.B. Protective mechanisms of Mg-gluconate against oxidative endothelial cytotoxicity. Cell. Mol. Biol. 2000, 46, 1337–1344. [Google Scholar] [PubMed]
- Mak, I.T.; Nedelec, L.F.; Weglicki, W.B. Pro-oxidant properties and cytotoxicity of AZT-monophosphate and AZT. Cardiovasc. Toxicol. 2004, 4, 109–115. [Google Scholar] [CrossRef]
- Murthi, S.B.; Wise, R.M.; Weglicki, W.B.; Komarov, A.M.; Kramer, J.H. Mg-Gluconate provides superior protection against postischemic dysfunction and oxidative injury compared to Mg-sulfate. Mol. Cell. Biochem. 2003, 245, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Garcia, L.A.; Dejong, S.C.; Martin, S.M.; Smith, R.S.; Buettner, G.R.; Kerber, R.E. Magnesium reduces free radicals in an in vivo coronary occlusion model. J. Am. Coll. Cardiol. 1998, 32, 536–539. [Google Scholar] [CrossRef]
- Zhou, Q.; Olinescu, R.M.; Kummerow, F.A. Influence of low magnesium concentrations in the medium on antioxidant system in the cultured human arterial endothelial cell. Magnes. Res. 1999, 12, 19–29. [Google Scholar] [PubMed]
- Lebrecht, D.; Venhoff, A.C.; Kirschner, J.; Wiech, T.; Venhoff, N.; Walker, U.A. Mitochondrial tubulopathy in tenofovir disopoxil fumarate-treated rats. J. Acquir. Immune Defic. Syndr. 2009, 51, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Masho, S.W.; Wang, C.L.; Nixon, D.E. Review of tenofovir-emtricitabine (TDF-FTC) use. Ther. Clin. Risk. Manag. 2007, 3, 1097–1104. [Google Scholar] [PubMed]
- Abraham, P.; Ramamoorthy, H.; Isaac, B. Depletion of the cellular antioxidant system contributes to tenofovir disoproxil fumarate—Induced mitochondrial damage and increased oxido-nitrosative stress in the kidney. J. Biomed. Sci. 2013, 20, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Ustianowski, A.; Arends, J.E. Tenofovir: What we have learnt after 7.5 million person-years of use. Infect. Dis. Ther. 2015, 4, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Musso, C.G.; Belloso, W.H.; Glassock, R.J. Water, electrolytes, and acid-base alterations in HIV infected patients. World J. Nephrol. 2016, 5, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, C.M. Antiretroviral therapy and the kidney. Top. Antivir. Med. 2014, 22, 655–658. [Google Scholar] [PubMed]
- Kramer, J.H.; Spurney, C.; Iantorno, M.; Tziros, C.; Mak, I.T.; Tejero-Taldo, M.I.; Chmielinska, J.J.; Komarov, A.M.; Weglicki, W.B. Neurogenic inflammation and cardiac dysfunction due to hypomagnesemia. Am. J. Med. Sci. 2009, 338, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Mak, I.T.; Chmielinska, J.J.; Kramer, J.H.; Spurney, C.F.; Weglicki, W.B. Loss of neutral endopeptidase activity contributes to neutrophil activation and cardiac dysfunction during chronic hypomagnesemia: Protection by substance P receptor blockade. Exp. Clin. Cardiol. 2011, 16, 121–124. [Google Scholar] [PubMed]
- Weglicki, W.B.; Mak, I.T.; Chmielinska, J.J.; Tejero-Taldo, M.I.; Komarov, A.M.; Kramer, J.H. The role of magnesium deficiency in cardiovascular and intestinal inflammation. Magnes. Res. 2010, 23, 199–206. [Google Scholar]
- Tejero-Taldo, M.I.; Kramer, J.H.; Mak, I.T.; Komarov, A.M.; Chmielinska, J.J.; Weglicki, W.B. The nerve-heart connection in the pro-oxidant response to Mg-deficiency. Heart Fail. Rev. 2006, 11, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Mak, I.T.; Kramer, J.H.; Weglicki, W.B. Suppression of neutrophil and endothelial activation by substance P receptor blockade in the Mg-deficient rat. Magnes. Res. 2003, 16, 91–97. [Google Scholar] [PubMed]
- Mak, I.T.; Kramer, J.H.; Chmielinska, J.J.; Spurney, C.F.; Weglicki, W.B. EGFR-TKI, erlotinib, causes hypomagnesemia, oxidative stress, and cardiac dysfunction: Attenuation by NK-1 receptor blockade. J. Cardiovasc. Pharmacol. 2015, 65, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Slot, C. Plasma creatinine determination a new and specific Jaffe’s reaction method. Scand. J. Clin. Lab. Investig. 1965, 17, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A. Modeling transport in the kidney: Investigating function and dysfunction. Am. J. Physiol. Ren. Physiol. 2009, 298, F475–F484. [Google Scholar] [CrossRef] [PubMed]
- Rangan, G.K.; Tesch, G.H. Methods in renal research: Quantification of renal pathology by image analysis. Nephrology 2007, 12, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Douglas, S.D.; Ho, W.Z.; Gettes, D.R.; Cnaan, A.; Zhao, H.; Leserman, J.; Petitto, J.M.; Golden, R.N.; Evans, D.L. Elevated substance P levels in HIV-infected men. AIDS 2001, 15, 2043–2045. [Google Scholar] [CrossRef] [PubMed]
- Douglas, S.D.; Cnaan, A.; Lynch, K.G.; Benton, T.; Zhao, H.; Gettes, D.R.; Evans, D.L. Elevated substance P levels in HIV-infected women in comparison to HIV-negative women. AIDS Res. Hum. Retroviruses 2008, 24, 375–378. [Google Scholar] [CrossRef] [PubMed]










| Initial Wt | Wt Gain at 18 Weeks | Food Consumed | cART Dosage | |
|---|---|---|---|---|
| Rat Groups | (gm) | (% of Initial wt) | (gm/kg/day) | (mg/kg/day) |
| Normal Mg | ||||
| A-1 (Ctl) | 269 ± 8 | 38.1 ± 3.1 | 41.7 ± 2.1 | |
| A-2 (+cART) | 267 ± 6 | 38.4 ± 3.7 | 43.1 ± 2.1 | 47.5 ± 2.3 |
| A-3 (Tg) | 236 ± 4 | 38.9 ± 1.3 | 44.0 ± 1.8 | |
| A-4 (Tg + cART) | 261 ± 5 | 29.4 ± 0.8 ** | 42.7 ± 1.7 | 47.3 ± 1.9 |
| Mg Supplemented | ||||
| B-1 (Ctl) | 260 ± 8 | 39.2 ± 3.4 | 44.6 ± 6.0 | |
| B-2 (+cART) | 249 ± 7 | 45.1 ± 2.2 | 43.3 ± 2.7 | 47.7 ± 2.7 |
| B-3 (Tg) | 229 ± 9 | 49.0 ± 4.2 * | 46.6 ± 6.5 | |
| B-4 (Tg + cART) | 219 ± 6 | 48.2 ± 2.0 ++ | 43.8 ± 3.7 | 48.1 ± 3.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mak, I.T.; Chmielinska, J.J.; Spurney, C.F.; Weglicki, W.B.; Kramer, J.H. Combination ART-Induced Oxidative/Nitrosative Stress, Neurogenic Inflammation and Cardiac Dysfunction in HIV-1 Transgenic (Tg) Rats: Protection by Mg. Int. J. Mol. Sci. 2018, 19, 2409. https://doi.org/10.3390/ijms19082409
Mak IT, Chmielinska JJ, Spurney CF, Weglicki WB, Kramer JH. Combination ART-Induced Oxidative/Nitrosative Stress, Neurogenic Inflammation and Cardiac Dysfunction in HIV-1 Transgenic (Tg) Rats: Protection by Mg. International Journal of Molecular Sciences. 2018; 19(8):2409. https://doi.org/10.3390/ijms19082409
Chicago/Turabian StyleMak, I. Tong, Joanna J. Chmielinska, Christopher F. Spurney, William B. Weglicki, and Jay H. Kramer. 2018. "Combination ART-Induced Oxidative/Nitrosative Stress, Neurogenic Inflammation and Cardiac Dysfunction in HIV-1 Transgenic (Tg) Rats: Protection by Mg" International Journal of Molecular Sciences 19, no. 8: 2409. https://doi.org/10.3390/ijms19082409
APA StyleMak, I. T., Chmielinska, J. J., Spurney, C. F., Weglicki, W. B., & Kramer, J. H. (2018). Combination ART-Induced Oxidative/Nitrosative Stress, Neurogenic Inflammation and Cardiac Dysfunction in HIV-1 Transgenic (Tg) Rats: Protection by Mg. International Journal of Molecular Sciences, 19(8), 2409. https://doi.org/10.3390/ijms19082409





