The Promise of Novel Biomarkers for Head and Neck Cancer from an Imaging Perspective
Abstract
1. Introduction
2. Biomarker Identification by PET and SPECT
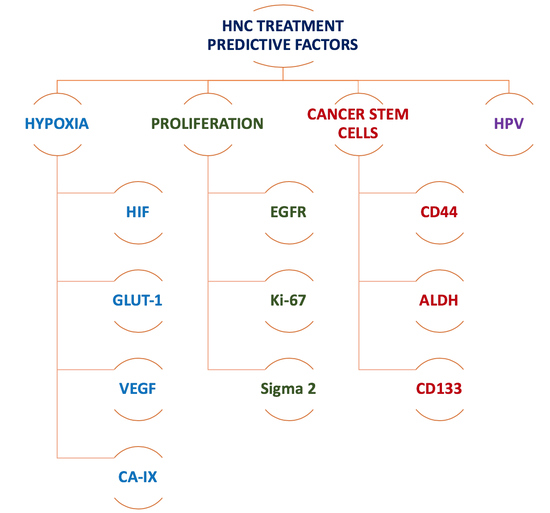
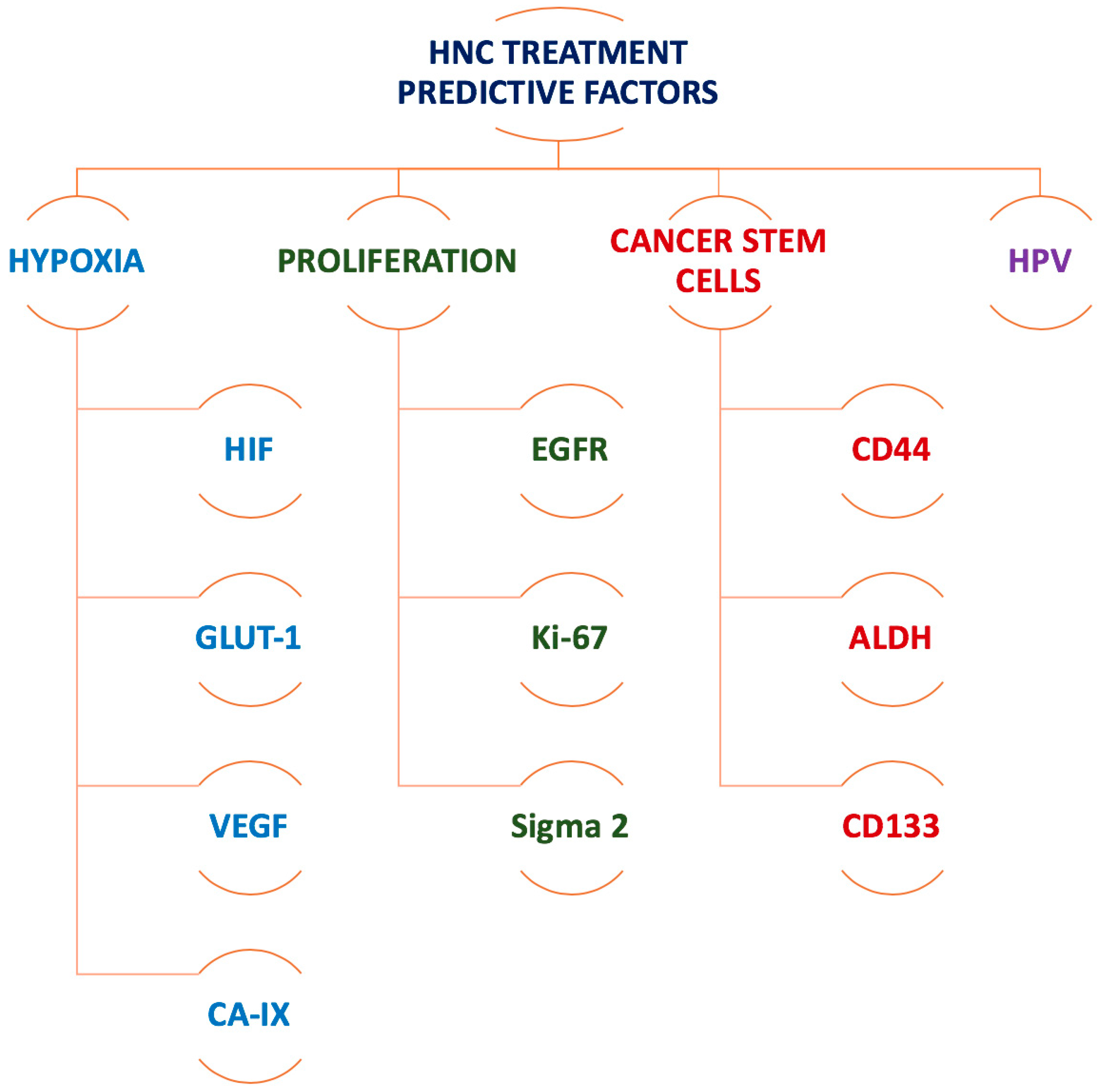
2.1. Imaging Biomarkers for Hypoxia
2.2. Imaging Biomarkers for Tumour Proliferation
3. Biomarker Identification by fMRI
4. The Road Ahead: Imaging Biomarkers for Cancer Stem Cells
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA: Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Janssen, H.L.; Haustermans, K.M.; Balm, A.J.; Begg, A.C. Hypoxia in head and neck cancer: How much, how important? Head Neck 2005, 27, 622–638. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.; Krause, M.; Hill, R. Exploring the role of cancer stem cells in radioresistance. Nat. Rev. Cancer 2008, 8, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.J. Radiobiology for the Radiologist, 5th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2000. [Google Scholar]
- Marcu, L.G.; Toma-Dasu, I.; Dasu, A.; Mercke, C. Radiotherapy and Clinical Radiobiology of Head and Neck Cancer, 1st ed.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Nordsmark, M.; Bentzen, S.M.; Rudat, V.; Brizel, D.; Lartigau, E.; Stadler, P.; Becker, A.; Adam, M.; Molls, M.; Dunst, J.; et al. Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiother. Oncol. 2005, 77, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Chirla, R.; Marcu, L. PET-based quantification of statistical properties of hypoxic tumor subvolumes in head and neck cancer. Phys. Med. 2016, 32, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Ljungkvist, A.S.; Bussink, J.; Kaanders, J.H.; van der Kogel, A.J. Dynamics of tumor hypoxia measured with bioreductive hypoxic cell markers. Radiat. Res. 2007, 167, 127–145. [Google Scholar] [CrossRef] [PubMed]
- Bittner, M.I.; Wiedenmann, N.; Bucher, S.; Hentschel, M.; Mix, M.; Rücker, G.; Weber, W.A.; Meyer, P.T.; Werner, M.; Grosu, A.L.; et al. Analysis of relation between hypoxia PET imaging and tissue-based biomarkers during head and neck radiochemotherapy. Acta Oncol. 2016, 55, 1299–1304. [Google Scholar] [CrossRef] [PubMed]
- Norikane, T.; Yamamoto, Y.; Maeda, Y.; Kudomi, N.; Matsunaga, T.; Haba, R.; Iwasaki, A.; Hoshikawa, H.; Nishiyama, Y. Correlation of 18F-fluoromisonidazole PET findings with HIF-1α and p53 expressions in head and neck cancer: comparison with 18F-FDG PET. Nucl. Med. Commun. 2014, 35, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Li, X.F.; Zou, H.; Sun, X.; Shen, B. 18F-Fluoromisonidazole in tumor hypoxia imaging. Oncotarget 2017, 8, 94969–94979. [Google Scholar] [PubMed]
- Yutani, K.; Kusuoka, H.; Fukuchi, K.; Tatsumi, M.; Nishimura, T. Applicability of 99mTc-HL91, a putative hypoxic tracer, to detection of tumor hypoxia. J. Nucl. Med. 1999, 40, 854–861. [Google Scholar] [PubMed]
- Sato, J.; Kitagawa, Y.; Yamazaki, Y.; Hata, H.; Okamoto, S.; Shiga, T.; Shindoh, M.; Kuge, Y.; Tamaki, N. 18F-fluoromisonidazole PET uptake is correlated with hypoxia-inducible factor-1a expression in oral squamous cell carcinoma. J. Nucl. Med. 2013, 54, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, L.S.; Buus, S.; Nordsmark, M.; Bentzen, L.; Munk, O.L.; Keiding, S.; Overgaard, J. Identifying hypoxia in human tumors: A correlation study between 18F-FMISO PET and the Eppendorf oxygen-sensitive electrode. Acta Oncol. 2010, 49, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Souvatzoglou, M.; Astner, S.T.; Vaupel, P.; Nüsslin, F.; Wilkens, J.J.; Ziegler, S.I. Quantitative assessment of hypoxia kinetic models by a cross-study of dynamic 18F-FAZA and 15O-H2O in patients with head and neck tumors. J. Nucl. Med. 2010, 51, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Lehtiö, K.; Eskola, O.; Viljanen, T.; Oikonen, V.; Groenroos, T.; Sillanmaeki, L.; Grénman, R.; Minn, H. Imaging perfusion and hypoxia with PET to predict radiotherapy response in head-and-neck cancer. Int. J. Radiat. 2004, 59, 971–982. [Google Scholar] [CrossRef] [PubMed]
- Grönroos, T.J.; Lehtiö, K.; Söderström, K.O.; Kronqvist, P.; Laine, J.; Eskola, O.; Viljanen, T.; Grénman, R.; Solin, O.; Minn, H. Hypoxia, blood flow and metabolism in squamous-cell carcinoma of the head and neck: Correlations between multiple immunohistochemical parameters and PET. BMC Cancer 2014, 14, 876. [Google Scholar] [CrossRef] [PubMed]
- Komar, G.; Lehtiö, K.; Seppänen, M.; Eskola, O.; Levola, H.; Lindholm, P.; Sipilä, H.; Seppälä, J.; Grénman, R.; Solin, O.; et al. Prognostic value of tumour blood flow, [18F]EF5 and [18F]FDG PET/CT imaging in patients with head and neck cancer treated with radiochemotherapy. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 2042–2050. [Google Scholar] [CrossRef] [PubMed]
- Silén, J.; Högel, H.; Kivinen, K.; Silvoniemi, A.; Forsback, S.; Löyttyniemi, E.; Solin, O.; Grénman, R.; Minn, H.; Jaakkola, P.M.; et al. Uptake of [18F]EF5 as a tracer for hypoxic and aggressive phenotype in experimental head and neck squamous cell carcinoma. Transl. Oncol. 2014, 7, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, Z.; Kolb, H.C.; Walsh, J.C.; Zhang, J.; Guan, Y. 18F-HX4 hypoxia imaging with PET/CT in head and neck cancer: a comparison with 18F-FMISO. Nucl. Med. Commun. 2012, 33, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Löck, S.; Perrin, R.; Seidlitz, A.; Bandurska-Luque, A.; Zschaeck, S.; Zöphel, K.; Krause, M.; Steinbach, J.; Kotzerke, J.; Zips, D.; et al. Residual tumour hypoxia in head-and-neck cancer patients undergoing primary radiochemotherapy, final results of a prospective trial on repeat FMISO-PET imaging. Radiother. Oncol. 2017, 124, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, L.S.; Johansen, J.; Kallehauge, J.; Primdahl, H.; Busk, M.; Lassen, P.; Alsner, J.; Sørensen, B.S.; Toustrup, K.; Jakobsen, S.; et al. FAZA PET/CT hypoxia imaging in patients with squamous cell carcinoma of the head and neck treated with radiotherapy: results from the DAHANCA 24 trial. Radiother. Oncol. 2012, 105, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Graves, E.E.; Hicks, R.J.; Binns, D.; Bressel, M.; Le, Q.T.; Peters, L.; Young, R.J.; Rischin, D. Quantitative and qualitative analysis of [(18)F]FDG and [(18)F]FAZA positron emission tomography of head and neck cancers and associations with HPV status and treatment outcome. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Lassen, P.; Eriksen, J.G.; Hamilton-Dutoit, S.; Tramm, T.; Alsner, J.; Overgaard, J. HPV-associated p16-expression and response to hypoxic modification of radiotherapy in head and neck cancer. Radiother. Oncol. 2010, 94, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Komar, G.; Seppanen, M.; Eskola, O.; Lindholm, P.; Grönroos, T.J.; Forsback, S.; Sipila, H.; Evans, S.M.; Solin, O.; Minn, H. F-18-EF5: A new PET tracer for imaging hypoxia in head and neck cancer. J. Nucl. Med. 2008, 49, 1944–1951. [Google Scholar] [CrossRef] [PubMed]
- Lopci, E.; Grassi, I.; Rubello, D.; Colletti, P.M.; Cambioli, S.; Gamboni, A.; Salvi, F.; Cicoria, G.; Lodi, F.; Dazzi, C.; et al. Prognostic evaluation of disease outcome in solid tumors investigated with 64Cu-ATSM PET/CT. Clin. Nucl. Med. 2016, 41, e87–e92. [Google Scholar] [CrossRef] [PubMed]
- Grassi, I.; Nanni, C.; Cicoria, G.; Blasi, C.; Bunkheila, F.; Lopci, E.; Colletti, P.M.; Rubello, D.; Fanti, S. Usefulness of 64Cu-ATSM in head and neck cancer: A preliminary prospective study. Clin. Nucl. Med. 2014, 39, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Colombié, M.; Gouard, S.; Frindel, M.; Vidal, A.; Chérel, M.; Kraeber-Bodéré, F.; Rousseau, C.; Bourgeois, M. Focus on the controversial aspects of (64)Cu-ATSM in tumoral hypoxia mapping by PET imaging. Front. Med. 2015, 2, 58. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, J.A.; Guillem, J.G.; Schöder, H.; Lee, N.Y.; Divgi, C.R.; Ruby, J.A.; Humm, J.L.; Lee-Kong, S.A.; Burnazi, E.M.; Cai, S.; et al. Pilot study of PET imaging of 124I-iodoazomycin galactopyranoside (IAZGP), a putative hypoxia imaging agent, in patients with colorectal cancer and head and neck cancer. EJNMMI Res. 2013, 3, 42. [Google Scholar] [CrossRef] [PubMed]
- Groshar, D.; McEwan, A.J.; Parliament, M.B.; Urtasun, R.C.; Golberg, L.E.; Hoskinson, M.; Mercer, J.R.; Mannan, R.H.; Wiebe, L.I.; Chapman, J.D. Imaging tumor hypoxia and tumor perfusion. J. Nucl. Med. 1993, 34, 885–888. [Google Scholar] [PubMed]
- Van De Wiele, C.; Versijpt, J.; Dierckx, R.A.; Moerman, M.; Lemmerling, M.; D’asseler, Y.; Vermeersch, H. 99Tc(m) labelled HL91 versus computed tomography and biopsy for the visualization of tumour recurrence of squamous head and neck carcinoma. Nucl. Med. Commun. 2001, 22, 269–275. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, L.K.; Yim, C.B.; Franssen, G.M.; Kaanders, J.H.; Rajander, J.; Solin, O.; Grönroos, T.J.; Boerman, O.C.; Bussink, J. PET of EGFR with (64) Cu-cetuximab-F(ab’)2 in mice with head and neck squamous cell carcinoma xenografts. Contrast Media Mol. Imaging 2016, 11, 65–70. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, L.K.; Hoeben, B.A.; Kaanders, J.H.; Franssen, G.M.; Boerman, O.C.; Bussink, J. Imaging of epidermal growth factor receptor expression in head and neck cancer with SPECT/CT and 111In-labeled cetuximab-F(ab’)2. J. Nucl. Med. 2013, 54, 2118–2124. [Google Scholar] [CrossRef] [PubMed]
- Song, I.H.; Noh, Y.; Kwon, J.; Jung, J.H.; Lee, B.C.; Kim, K.I.; Lee, Y.J.; Kang, J.H.; Rhee, C.S.; Lee, C.H.; et al. Immuno-PET imaging based radioimmunotherapy in head and neck squamous cell carcinoma model. Oncotarget 2017, 8, 92090–92105. [Google Scholar] [CrossRef] [PubMed]
- Menda, Y.; Boles Ponto, L.L.; Dornfeld, K.J.; Tewson, T.J.; Watkins, G.L.; Schultz, M.K.; Sunderland, J.J.; Graham, M.M.; Buatti, J.M. Kinetic analysis of 3’-deoxy-3’-(18)F-fluorothymidine ((18)F-FLT) in head and neck cancer patients before and early after initiation of chemoradiation therapy. J. Nucl. Med. 2009, 50, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Troost, E.G.; Bussink, J.; Hoffmann, A.L.; Boerman, O.C.; Oyen, W.J.; Kaanders, J.H. 18F-FLT PET/CT for early response monitoring and dose escalation in oropharyngeal tumors. J. Nucl. Med. 2010, 51, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Bollineni, V.R.; Kramer, G.M.; Jansma, E.P.; Liu, Y.; Oyen, W.J. A systematic review on [18F]FLT-PET uptake as a measure of treatment response in cancer patients. Eur. J. Cancer 2016, 55, 81–97. [Google Scholar] [CrossRef] [PubMed]
- Hoeben, B.A.; Troost, E.G.; Span, P.N.; van Herpen, C.M.; Bussink, J.; Oyen, W.J.; Kaanders, J.H. 18F-FLT PET during radiotherapy or chemoradiotherapy in head and neck squamous cell carcinoma is an early predictor of outcome. J. Nucl. Med. 2013, 54, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, K.T.; Wang, L.M.; Wallen, C.A.; Childers, S.R.; Cline, J.M.; Keng, P.C.; Mach, R.H. Sigma-2 receptors as a biomarker of proliferation in solid tumours. Br. J. Cancer 2000, 82, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Rowland, D.J.; Tu, Z.; Xu, J.; Ponde, D.; Mach, R.H.; Welch, M.J. Synthesis and in vivo evaluation of 2 high-affinity 76Br-labeled sigma2-receptor ligands. J. Nucl. Med. 2006, 47, 1041–1048. [Google Scholar] [PubMed]
- Mach, R.H.; Dehdashti, F.; Wheeler, K.T. PET radiotracers for imaging the proliferative status of solid tumors. PET Clin. 2009, 4, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Dehdashti, F.; Laforest, R.; Gao, F.; Shoghi, K.I.; Aft, R.L.; Nussenbaum, B.; Kreisel, F.H.; Bartlett, N.L.; Cashen, A.; Wagner-Johnson, N.; et al. Assessment of cellular proliferation in tumors by PET using 18F-ISO-1. J. Nucl. Med. 2013, 54, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, J.M.; Homer, J.J.; West, C.M. Dynamic contrast-enhanced magnetic resonance imaging biomarkers in head and neck cancer: Potential to guide treatment? A systematic review. Oral Oncol. 2014, 50, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Min, M.; Lin, P.; Liney, G.; Lee, M.; Forstner, D.; Fowler, A.; Holloway, L. A review of the predictive role of functional imaging in patients with mucosal primary head and neck cancer treated with radiation therapy. J. Med. Imaging Radiat. Oncol. 2017, 61, 99–123. [Google Scholar] [CrossRef] [PubMed]
- Padhani, A.R.; Krohn, K.A.; Lewis, J.S.; Alber, M. Imaging oxygenation of human tumours. Eur. Radiol. 2007, 17, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.F.A.; Parra, C.; Lu, Y.; Shukla-Dave, A. Evaluation of head and neck tumors with functional MR imaging. Magn. Reson. Imaging Clin. N. Am. 2016, 24, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, M.; Van Calster, B.; Vandecaveye, V.; De Keyzer, F.; Roebben, I.; Hermans, R.; Nuyts, S. Integrating pretreatment diffusion weighted MRI into a multivariable prognostic model for head and neck squamous cell carcinoma. Radiother. Oncol. 2014, 110, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Detre, J.A.; Rao, H.; Wang, D.J.; Chen, Y.F.; Wang, Z. Applications of arterial spin labeled MRI in the brain. J. Magn. Reson. Imaging 2012, 35, 1026–1037. [Google Scholar] [CrossRef] [PubMed]
- Heryanto, Y.D.; Achmad, A.; Taketomi-Takahashi, A.; Tsushima, Y. In vivo molecular imaging of cancer stem cells. Am. J. Nucl. Med. Mol. Imaging 2014, 5, 14–26. [Google Scholar] [PubMed]
- Lim, E.-K.; Kim, H.-O.; Jang, E.; Park, J.; Lee, K.; Suh, J.S.; Huh, Y.M.; Haam, S. Hyaluronan-modified magnetic nanoclusters for detection of CD44-overexpressing breast cancer by MR imaging. Biomaterials 2011, 32, 7941–7950. [Google Scholar] [CrossRef] [PubMed]
- King, A.D.; Yeung, D.K.; Yu, K.H.; Mo, F.K.; Hu, C.W.; Bhatia, K.S.; Gary, M.; Vlantis, A.C.; Wong, J.K.; Ahuja, A.T. Monitoring of treatment response after chemoradiotherapy for head and neck cancer using in vivo 1H MR spectroscopy. Eur. Radiol. 2010, 20, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Razek, A.A.K.A.; Poptani, H. MR spectrsocopy of head and neck cancer. Eur. J. Radiol. 2013, 82, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Horsman, M.R.; Mortensen, L.S.; Petersen, J.B.; Busk, M.; Overgaard, J. Imaging hypoxia to improve radiotherapy outcome. Nat. Rev. Clin. Oncol. 2012, 9, 674–687. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.H.; Panek, R.; Bhide, S.A.; Nutting, C.M.; Harrington, K.J.; Newbold, K.L. The emerging potential of magnetic resonance imaging in personalizing radiotherapy for head and neck cancer: An oncologist’s perspective. Br. J. Radiol. 2017, 90, 20160768. [Google Scholar] [CrossRef] [PubMed]
- Vandecaveye, V.; De Keyzer, F.; Vander Poorten, V.; Dirix, P.; Verbeken, E.; Nuyts, S.; Hermans, R. Head and neck squamous cell carcinoma: value of diffusion-weighted MR imaging for nodal staging. Radiology 2009, 251, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Chawla, S.; Loevner, L.; Kim, S.; Hwang, W.T.; Wang, S.; Verma, G.; Mohan, S.; LiVolsi, V.; Quon, H.; Poptani, H. Dynamic contrast-enhanced MRI–derived intracellular water lifetime (τi): A prognostic marker for patients with head and neck squamous cell carcinomas. Am. J. Neuroradiol. 2018, 39, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Shukla-Dave, A.; Lee, N.Y.; Jansen, J.F.; Thaler, H.T.; Stambuk, H.E.; Fury, M.G.; Patel, S.G.; Moreira, A.L.; Sherman, E.; Karimi, S. Dynamic contrast-enhanced magnetic resonance imaging as a predictor of outcome in head-and-neck squamous cell carcinoma patients with nodal metastases. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 1837–1844. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, P.; Subesinghe, M.; Patel, C.; Prestwich, R.; Scarsbrook, A.F. Functional imaging for radiation treatment planning, response assessment, and adaptive therapy in head and neck cancer. Radiographics 2013, 33, 1909–1929. [Google Scholar] [CrossRef] [PubMed]
- Taylor, N.J.; Baddeley, H.; Goodchild, K.A.; Powell, M.E.; Thoumine, M.; Culver, L.A.; Stirling, J.J.; Saunders, M.I.; Hoskin, P.J.; Phillips, H.; et al. BOLD MRI of human tumor oxygenation during carbogen breathing. J. Magn. Reson. Imaging 2001, 14, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Moore, N.; Lyle, S. Quiescent, slow-cycling stem cell populations in cancer: A review of the evidence and discussion of significance. J. Oncol. 2011, 2011, 396076. [Google Scholar] [CrossRef] [PubMed]
- Prince, M.E.; Sivanandan, R.; Kaczorowski, A.; Wolf, G.T.; Kaplan, M.J.; Dalerba, P.; Weissman, I.L.; Clarke, M.F.; Ailles, L.E. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc. Natl. Acad. Sci. USA 2007, 104, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.L.; Owen, J.H.; Hauff, S.J.; Park, J.J.; Papagerakis, S.; Bradford, C.R.; Carey, T.E.; Prince, M.E. Head and neck cancer stem cells: The effect of HPV—an in vitro and mouse study. Otolaryngol. Head Neck Surg. 2013, 149, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Harper, L.J.; Piper, K.; Common, J.; Fortune, F.; Machenzie, I.C. Stem cell patterns in cell lines derived from head and neck squamous cell carcinoma. J. Oral Pathol. Med. 2007, 36, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Zöller, M. CD44: Can a cancer-initiating cell profit from an abundantly expressed molecule? Nat. Rev. Cancer 2011, 11, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Yüce, I.; Bayram, A.; Cağlı, S.; Canöz, Ö.; Bayram, S.; Güney, E. The role of CD44 and matrix metalloproteinase-9 expression in predicting neck metastasis of supraglottic laryngeal carcinoma. Am. J. Otolaryngol. 2011, 32, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Ilardi, G.; Zambrano, N.; Merolla, F.; Siano, M.; Varricchio, S.; Vecchione, M.; De Rosa, G.; Mascolo, M.; Staibano, S. Histopathological determinants of tumor resistance: A special look to the immunohistochemical expression of carbonic anhydrase IX in human cancers. Curr. Med. Chem. 2014, 21, 1569–1582. [Google Scholar] [CrossRef] [PubMed]
- Clay, M.R.; Tabor, M.; Owen, J.H.; Carey, T.E.; Bradford, C.R.; Wolf, G.T.; Wicha, M.S.; Prince, M.E. Single-marker identification of head and neck squamous cell carcinoma cancer stem cells with aldehyde dehydrogenase. Head Neck 2010, 32, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Kurth, I.; Hein, L.; Mäbert, K.; Peitzsch, C.; Koi, L.; Cojoc, M.; Kunz-Schughart, L.; Baumann, M.; Dubrovska, A. Cancer stem cell related markers of radioresistance in head and neck squamous cell carcinoma. Oncotarget 2015, 6, 34494–34509. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Wu, M.J.; Huang, C.Y.; Lin, S.C.; Chuang, T.H.; Yu, C.C.; Lo, J.F. CD133/Src axis mediates tumor initiating property and epithelial-mesenchymal transition of head and neck cancer. PLoS ONE 2011, 6, e28053. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, Y.; Furukawa, T.; Kiyono, Y.; Watanabe, R.; Waki, A.; Mori, T.; Yoshii, H.; Oh, M.; Asai, T.; Okazawa, H.; et al. Copper-64-diacetyl-bis (N4-methylthiosemicarbazone) accumulates in rich regions of CD133+ highly tumourigenic cells in mouse colon carcinoma. Nucl. Med. Biol. 2010, 37, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Gaedicke, S.; Braun, F.; Prasad, S.; Machein, M.; Firat, E.; Hettich, M.; Gudihal, R.; Zhu, X.; Klingner, K.; Schüler, J.; et al. Noninvasive positron emission tomography and fluorescence imaging of CD133+ tumour stem cells. Proc. Natl. Acad. Sci. USA 2014, 111, E692–E701. [Google Scholar] [CrossRef] [PubMed]
- Spiegelberg, D.; Mortensen, A.C.; Selvaraju, R.K.; Eriksson, O.; Stenerlöw, B.; Nestor, M. Molecular imaging of EGFR and CD44v6 for prediction and response monitoring of HSP90 inhibition in an in vivo squamous cell carcinoma model. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.J.; Kanwar, A.; Mohamed, A.S.; Fuller, C.D. Radiomics in head and neck cancer: From exploration to application. Transl. Cancer Res. 2016, 5, 371–382. [Google Scholar] [CrossRef]
| Predictive Assay | Oxygenation Status | Proliferative Potential | Intrinsic Radioresistance (Subpopulation of Cancer Stem Cells?) |
|---|---|---|---|
| Purpose | To identify the patient group that would benefit from hypoxic cell sensitisers. | To differentiate between tumours with slow and fast proliferation. | To correlate cell line radiosensitivity with tumour response to radiation. |
| Technique | Polarographic needle electrode Endogenous/exogenous markers; 3D models; microvessel density. | Kinetic parameter measurements: length of S phase, potential doubling time; labelling index; clonogenic survival. | Dose-response curves; Colony growth (MTT), micronucleus, chromosomal, DNA damage (Comet) assays; tumour control assay. |
| Limitation | Invasive; Unreliable (biopsies); Costly and time consuming; Require high level expertise. | No robust correlation between kinetic parameters and treatment outcome; Time consuming. | Highly time consuming. |
| Present/Future | Hypoxia-specific PET radiotracers: F-MISO; F-FAZA; Cu-ATSM; other radiotracers BOLD/TOLD (blood/tissue oxygen level-dependent) MRI | Proliferation-specific PET radiotracers: F-FLT; F-ISO-1; 11C-based radiotracers. | Cancer stem cell-specific PET radiotracers; MRI; HPV-status based identification of more radioresponsive tumours. |
| PET STUDIES | ||
|---|---|---|
| Tracer | Tumour Marker/Parameter for Hypoxia | Correlation between PET Tracer and Tumour Markers |
| 18F-FMISO | pO2 (Mortensen 2010) [14] | No correlation was observed between pO2 measurements (Eppendorf) and F-MISO. Tumours were more hypoxic based on pO2 measurements. |
| HIF-1α (Sato 2013) [11] | Strong correlation with HIF-1α was found. | |
| HIF-1α (Norikane 2014) [12] | Only a weak correlation of hypoxic volume with HIF-1α expression was observed. | |
| CA-IX (Bittner 2016) [10] | No correlation between CA-IX and tracer uptake was observed. | |
| 18F-FAZA | Blood flow via 15O-H2O (Shi 2010) (compartmental model analysis) [15] | Very similar distribution pattern between tracer accumulation and blood flow during early imaging and different pattern at later imaging times in line with tracer uptake by hypoxic regions. |
| 18F-FETNIM | pO2 (Lehtiö 2004) [16] | Correlation between the hypoxic volume as indicated by F-FETNIM and pO2 was only found in a limited number of patients. |
| HIF-1α, VEGF, CD31 Blood flow via 15O-H2O (Grönroos 2014) [17] | Immunohistochemical biomarkers for hypoxia and blood flow did not correlate with F-FETNIM uptake. | |
| 18F-EF5 | Blood flow via 15O-H2O (Komar 2014) [18] | No correlation between F-EF5 uptake and blood flow assessed with the perfusion tracer 15O-H2O |
| CA-IX, HIF-1α (cell line study) (Silén 2014) [19] | Very good correlation between F-EF5 uptake and CA-IX/HIF-1α expressions, indicative of a more aggressive phenotype. | |
| 18F-HX4 | CA-IX (Chen 2012) [20] | F-HX4 uptake is correlated with CA-IX expression. |
| 64Cu-ATSM | No correlation studies reported | |
| SPECT STUDIES | ||
| 123I-IAZA | No correlation studies reported | |
| 99mTc-HL91 | GLUT-1 (in rat tumour) (Yutani 1999) [21] | Strong expression of GLUT-1 in tumour sites with high tracer uptake, showing hypoxia-avid properties. |
| Name | Biomarker | Pulse Sequence | Contrast | Notes | Reference |
|---|---|---|---|---|---|
| BOLD Blood oxygen level dependent imaging | Acute hypoxia | Multi-echo GRE | None | Measured by R2* | Padhani et al. (2007) [46] |
| DWI Diffusion weighted imaging | Tissue diffusion Chronic hypoxia | Echo planar imaging Single shot spin echo | None | Measures diffusion restriction resulting from cellular density of tumour tissue | Jansen et al. (2016) [47], Lambrecht et al. (2014) [48] |
| DCE Dynamic contrast-enhanced imaging | Angiogenesis Tissue perfusion Hypoxia | Fast multiphase spoiled GRE | Gadolinium | Sequential imaging measures movement of contrast from tumour vasculature to interstitial space | Bernstein et al. (2014) [44] |
| ASL Arterial Spin Labelling | Tumour perfusion | Echo planar imaging Multi shot spin echo | None | Radiofrequency waves magnetically label arterial blood water for tracking | Detre et al. (2012) [49], Jansen et al. (2016) [47] |
| Cancer Stem Cell Imaging | CSCs (CD44+) | T2 | SPIO (USPIO) | CD44+ cells T2 signal decreased by magnetic nanoclusters | Heryanto et al. (2014) [50], Lim et al. (2011) [51] |
| MR Spectroscopy | Metabolite concentration | None | Biochemical rather than anatomical information | King et al. (2010) [52], Razek & Poptani (2013) [53] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcu, L.G.; Reid, P.; Bezak, E. The Promise of Novel Biomarkers for Head and Neck Cancer from an Imaging Perspective. Int. J. Mol. Sci. 2018, 19, 2511. https://doi.org/10.3390/ijms19092511
Marcu LG, Reid P, Bezak E. The Promise of Novel Biomarkers for Head and Neck Cancer from an Imaging Perspective. International Journal of Molecular Sciences. 2018; 19(9):2511. https://doi.org/10.3390/ijms19092511
Chicago/Turabian StyleMarcu, Loredana G., Paul Reid, and Eva Bezak. 2018. "The Promise of Novel Biomarkers for Head and Neck Cancer from an Imaging Perspective" International Journal of Molecular Sciences 19, no. 9: 2511. https://doi.org/10.3390/ijms19092511
APA StyleMarcu, L. G., Reid, P., & Bezak, E. (2018). The Promise of Novel Biomarkers for Head and Neck Cancer from an Imaging Perspective. International Journal of Molecular Sciences, 19(9), 2511. https://doi.org/10.3390/ijms19092511