Jasmonates: News on Occurrence, Biosynthesis, Metabolism and Action of an Ancient Group of Signaling Compounds
Abstract
1. Introduction
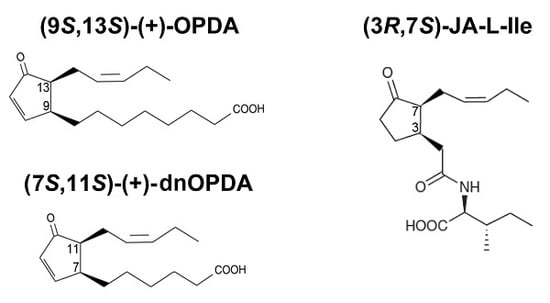
2. Occurrence of Jasmonic Acid (JA) Compounds
3. Biosynthesis of JA
3.1. Galactolipases Active in JA Biosynthesis
3.2. 13-Lipoxygenase (LOX)
3.3. Allene Oxide Synthase (AOS)
3.4. Allene Oxide Cyclase (AOC)
3.5. OPDA Reductase (OPR3)
3.6. β-Oxidation of the Carboxylic Acid Side Chain (ACX, MFP, KAT)
4. The Bypass in JA Formation-the COI1-Independent and OPR3-Independent Route
5. Regulation of JA Biosynthesis
6. Arabidopsides
7. Evolution of JA Biosynthesis
8. Homeostasis Among JA Compounds by Metabolism
8.1. Conjugation
8.2. Hydroxylation
8.3. Carboxylation
8.4. Decarboxylation
8.5. Methylation and Esterification
8.6. Sulfation
8.7. O-Glycosylation
9. Perception, Signaling and Expression in JA/JA-Ile Dependent Processes
9.1. COI1: The Critical Component of the Complex Perceiving JA-Ile
9.2. The Core Complex in JA-Induced Gene Expression
Acknowledgments
Funding
Conflicts of Interest
Abbreviations
| α-LeA | α-Linolenic Acid |
| 13-HPOT | (13S)-Hydroperoxyoctadecatrienoic Acid |
| cis-(+)-OPDA | cis-(+)-12-Oxophytodienoic Acid |
| OPDA-Ile | cis-(+)-12-Oxophytodienoic Acid Isoleucine Conjugate |
| JA | Jasmonic Acid |
| JA-Ile | Jasmonic Acid Isoleucine Conjugate |
| OPC-8 | 3-Oxo-2-(2-Pentenyl)-Cyclopentane-1-Octanoic Acid |
| acx1 | Acyl CoA-Oxidase1 |
| AOC | Allene Oxide Cyclase |
| AOS | Allene Oxide Synthase |
| coi1 | Coronatine Insensitive1 |
| dad1 | Delayed Anther Dehiscence1 |
| jai1 | Jasmonic Acid Insensitive1 |
| JAR1 | JA-Amino Acid Synthetase |
| JAT1 | Jasmonic Acid Transporter1 |
| 13-LOX | 13-Lipoxygenase |
| myc2 | bHLHzip Transcription Factor MYC2 |
| OPR3 | OPDA Reductase3 |
| PLA1 | Phospholipase A1 |
| PLIP2,3 | Plastid Lipase2,3 |
References
- Wasternack, C.; Feussner, I. The oxylipin pathways: Biochemistry and function. Annu. Rev. Entomol. 2018, 69, 363–386. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C. How jasmonates earned their laurels: Past and present. J. Plant Growth Regul. 2015, 34, 761–794. [Google Scholar] [CrossRef]
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in annals of botany. Ann. Bot. 2013, 111, 1021–1058. [Google Scholar] [CrossRef] [PubMed]
- Kazan, K.; Lyons, R. Intervention of phytohormone pathways by pathogen effectors. Plant Cell 2014, 26, 2285–2309. [Google Scholar] [CrossRef] [PubMed]
- Schuman, M.C.; Baldwin, I.T. The layers of plant responses to insect herbivores. Annu. Rev. Entomol. 2016, 61, 373–394. [Google Scholar] [CrossRef] [PubMed]
- Goossens, J.; Mertens, J.; Goossens, A. Role and functioning of bHLH transcription factors in jasmonate signalling. J. Exp. Bot. 2017, 68, 1333–1347. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C.; Song, S. Jasmonates: Biosynthesis, metabolism, and signaling by proteins activating and repressing transcription. J. Exp. Bot. 2017, 68, 1303–1321. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Yan, C.; Li, L.; Xie, D.; Li, C. Jasmonates; Elsevier: New York City, NY, USA, 2018; pp. 243–272. [Google Scholar]
- Howe, G.A.; Major, I.T.; Koo, A.J. Modularity in jasmonate signaling for multistress resilience. Annu. Rev. Entomol. 2018, 69, 387–415. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, T.; Sakayama, H.; de Vries, J.; Buschmann, H.; Saint-Marcoux, D.; Ullrich, K.K.; Haas, F.B.; Vanderstraeten, L.; Becker, D.; Lang, D.; et al. The chara genome: Secondary complexity and implications for plant terrestrialization. Cell 2018, 174, 448–464.e24. [Google Scholar] [CrossRef] [PubMed]
- Bowman, J.L.; Kohchi, T.; Yamato, K.T.; Jenkins, J.; Shu, S.; Ishizaki, K.; Yamaoka, S.; Nishihama, R.; Nakamura, Y.; Berger, F.; et al. Insights into land plant evolution garnered from the Marchantia polymorpha genome. Cell 2017, 171, 287–304.e15. [Google Scholar] [CrossRef] [PubMed]
- Han, G.-Z. Evolution of jasmonate biosynthesis and signaling mechanisms. J. Exp. Bot. 2017, 68, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Demole, E.; Lederer, E.; Mercier, D. Isolement et détermination de la structure du jasmonate de méthyle, constituant odorant charactéristique de lèssence de jasmin. Helv. Chim. Acta 1962, 45, 675–685. [Google Scholar] [CrossRef]
- Aldrige, D.; Galt, S.; Giles, D.; Turner, W. Metabolites of Lasiodiplodia theobromae. J. Chem. Soc. 1971, 1623–1627. [Google Scholar] [CrossRef]
- Miersch, O.; Preiss, A.; Sembdner, G.; Schreiber, K. (+)-7-iso-jasmonic acid and related compounds from Botryodiplodia theobromae. Phytochemistry 1987, 26, 1037–1039. [Google Scholar] [CrossRef]
- Miersch, O.; Schmidt, J.; Sembdner, G.; Schreiber, K. Jasmonic acid-like substances from the culture filtrate of Botryodiplodia theobromae. Phytochemistry 1989, 28, 1303–1305. [Google Scholar] [CrossRef]
- Andolfi, A.; Maddau, L.; Cimmino, A.; Linaldeddu, B.T.; Basso, S.; Deidda, A.; Serra, S.; Evidente, A. Lasiojasmonates A–C, three jasmonic acid esters produced by Lasiodiplodia sp., a grapevine pathogen. Phytochemistry 2014, 103, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Chini, A.; Cimmino, A.; Masi, M.; Reveglia, P.; Nocera, P.; Solano, R.; Evidente, A. The fungal phytotoxin lasiojasmonate a activates the plant jasmonic acid pathway. J. Exp. Bot. 2018, 69, 3095–3102. [Google Scholar] [CrossRef] [PubMed]
- Eng, F.; Zienkiewicz, K.; Gutiérrez-Rojas, M.; Favela-Torres, E.; Feussner, I. Jasmonic acid biosynthesis by microorganisms: Derivatives, first evidences on biochemical pathways and culture conditions for production. PeerJ Prepr. 2018, 6, e26655v1. [Google Scholar]
- Miersch, O.; Bohlmann, H.; Wasternack, C. Jasmonates and related compounds from Fusarium oxysporum. Phytochemistry 1999, 50, 517–523. [Google Scholar] [CrossRef]
- Oliw, E.; Hamberg, M. An allene oxide and 12-oxophytodienoic acid are key intermediates in jasmonic acid biosynthesis by Fusarium oxysporum. J. Lipid Res. 2017, 58, 1670–1680. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Ohshika, J.; Takahashi, T.; Ishizaki, K.; Kohchi, T.; Matusuura, H.; Takahashi, K. Functional analysis of allene oxide cyclase, MpAOC, in the liverwort Marchantia polymorpha. Phytochemistry 2015, 116, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Stumpe, M.; Göbel, C.; Faltin, B.; Beike, A.K.; Hause, B.; Himmelsbach, K.; Bode, J.; Kramell, R.; Wasternack, C.; Frank, W.; et al. The moss Physcomitrella patens contains cyclopentenones but no jasmonates: Mutations in allene oxide cyclase lead to reduced fertility and altered sporophyte morphology. New Phytol. 2010, 188, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Ogorodnikova, A.V.; Mukhitova, F.K.; Grechkin, A.N. Oxylipins in the spikemoss selaginella martensii: Detection of divinyl ethers, 12-oxophytodienoic acid and related cyclopentenones. Phytochemistry 2015, 118, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Pratiwi, P.; Tanaka, G.; Takahashi, T.; Xie, X.; Yoneyama, K.; Matsuura, H.; Takahashi, K. Identification of jasmonic acid and jasmonoyl-isoleucine, and characterization of AOS, AOC, OPR and JAR1 in the model lycophyte Selaginella moellendorffii. Plant Cell Physiol. 2017, 58, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Monte, I.; Ishida, S.; Zamarreño, A.M.; Hamberg, M.; Franco-Zorrilla, J.M.; García-Casado, G.; Gouhier-Darimont, C.; Reymond, P.; Takahashi, K.; García-Mina, J.M.; et al. Ligand-receptor co-evolution shaped the jasmonate pathway in land plants. Nat. Chem. Biol. 2018, 14, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Novák, O.; Napier, R.; Ljung, K. Zooming in on plant hormone analysis: Tissue- and cell-specific approaches. Annu. Rev. Entomol. 2017, 68, 323–348. [Google Scholar] [CrossRef] [PubMed]
- Šimura, J.; Antoniadi, I.; Široká, J.; Tarkowská, D.; Strnad, M.; Ljung, K.; Novák, O. Plant hormonomics: Multiple phytohormone profiling by targeted metabolomics. Plant Physiol. 2018, 177, 476–489. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.; Miersch, O.; Büttner, C.; Dathe, W.; Sembdner, G. Occurrence of the plant growth regulator jasmonic acid in plants. J. Plant Growth Regul. 1984, 3, 1–8. [Google Scholar] [CrossRef]
- Floková, K.; Feussner, K.; Herrfurth, C.; Miersch, O.; Tarkowská, D.; Strnad, M.; Feussner, I.; Wasternack, C.; Novák, O. A previously undescribed jasmonate compound in flowering Arabidopsis thaliana-The identification of cis-(+)-opda-ile. Phytochemistry 2015, 122, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Gruber, C.; Floková, K.; Miersch, O.; Strnad, M.; Novák, O.; Wasternack, C.; Hause, B. The recently identified isoleucine conjugate of cis-12-oxo-phytodienoic acid is partially active in cis-12-oxo-phytodienoic acid-specific gene expression of Arabidopsis thaliana. PLoS ONE 2016, 11, e0162829. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, A.; Yaguchi, T.; Nakagawa, H.; Sasaki, K.; Kuwata, N.; Matsuura, H.; Takahashi, K. Biosynthesis and in vitro enzymatic synthesis of the isoleucine conjugate of 12-oxo-phytodienoic acid from the isoleucine conjugate of α-linolenic acid. Bioorg. Med. Chem. Lett. 2018, 28, 1020–1023. [Google Scholar] [CrossRef] [PubMed]
- Vick, B.A.; Zimmerman, D.C. The biosynthesis of jasmonic acid: A physiological role for plant lipoxygenase. Biochem. Biophys. Res. Comm. 1983, 111, 470–477. [Google Scholar] [CrossRef]
- Chini, A.; Monte, I.; Zamarreño, A.M.; Hamberg, M.; Lassueur, S.; Reymond, P.; Weiss, S.; Stintzi, A.; Schaller, A.; Porzel, A.; et al. An opr3-independent pathway uses 4,5-didehydrojasmonate for jasmonate synthesis. Nat. Chem. Biol. 2018, 14, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Browse, J. Jasmonate passes muster: A receptor and targets for the defense hormone. Annu. Rev. Plant Biol. 2009, 60, 183–205. [Google Scholar] [CrossRef] [PubMed]
- Kombrink, E. Chemical and genetic exploration of jasmonate biosynthesis and signaling paths. Planta 2012, 236, 1351–1366. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, S.; Kwai-Oda, A.; Ueda, J.; Nishida, I.; Okada, K. The defective in anther dehiscence1 gene encodes a novel phospholipase a1 catalyzing the initial step of jasmonic acid biosynthesis, which synchronizes pollen maturation. Plant Cell 2001, 13, 2191–2209. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-T.; Chen, L.-J.; Herrfurth, C.; Feussner, I.; Li, H.-M. Reduced biosynthesis of digalactosyldiacylglycerol, a major chloroplast membrane lipid, leads to oxylipin overproduction and phloem cap lignification in arabidopsis. Plant Cell 2016, 28, 219–232. [Google Scholar] [PubMed]
- Li, H.-M.; Yu, C.-W. Chloroplast galactolipids: The link between photosynthesis, chloroplast shape, jasmonates, phosphate starvation and freezing tolerance. Plant Cell Physiol. 2018, 59, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Froehlich, J.E.; Zienkiewicz, A.; Hersh, H.L.; Benning, C. A plastid phosphatidylglycerol lipase contributes to the export of acyl groups from plastids for seed oil biosynthesis. Plant Cell 2017, 29, 1678–1696. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Guo, Q.; Froehlich, J.E.; Hersh, H.L.; Zienkiewicz, A.; Howe, G.A.; Benning, C. Two abscisic acid responsive plastid lipase genes involved in jasmonic acid biosynthesis in Arabidopsis thaliana. Plant Cell 2018, 30, 1006–1022. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, P.; Calvert, C.; Atzorn, R.; Wasternack, C.; Leyser, H.; Bowles, D. Ethylene as a signal mediating the wound response of tomato plants. Science 1996, 274, 1914–1917. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Tieman, D.; Jones, A.; Taylor, M.; Schmelz, E.; Huffaker, A.; Bies, D.; Chen, K.; Klee, H. A13-lipoxygenase, tomloxc, is essential for synthesis of c5 flavour volatiles in tomato. J. Exp. Bot. 2014, 65, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhai, Q.; Wei, J.; Li, S.; Wang, B.; Huang, T.; Du, M.; Sun, J.; Kang, L.; Li, C.-B.; et al. Role of tomato lipoxygenase d in wound-induced jasmonate biosynthesis and plant immunity to insect herbivores. PLoS Genet. 2013, 9, e1003964. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chen, X.; Yan, H.; Li, W.; Li, Y.; Cai, R.; Xiang, Y. The lipoxygenase gene family in poplar: Identification, classification, and expression in response to meja treatment. PLoS ONE 2015, 10, e0125526. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bao, Y.; Shan, D.; Wang, Z.; Song, X.; Wang, Z.; Wang, J.; He, L.; Wu, L.; Zhang, Z.; et al. Magnaporthe oryzae defeats rice defense by inducing miR319B and suppressing jasmonic acid signaling. Plant Physiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S.A.; Huffaker, A.; Kaplan, F.; Sims, J.; Ziemann, S.; Doehlemann, G.; Ji, L.; Schmitz, R.J.; Kolomiets, M.V.; Alborn, H.T.; et al. Maize death acids, 9-lipoxygenase–derived cyclopente(a)nones, display activity as cytotoxic phytoalexins and transcriptional mediators. Proc. Natl. Acad. Sci. USA 2015, 112, 11407–11412. [Google Scholar] [CrossRef] [PubMed]
- Grebner, W.; Stingl, N.E.; Oenel, A.; Mueller, M.J.; Berger, S. Lipoxygenase6-dependent oxylipin synthesis in roots is required for abiotic and biotic stress resistance of arabidopsis. Plant Physiol. 2013, 161, 2159–2170. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Lai, J.; Wu, Q.; Zhang, S.; Chen, L.; Dai, Y.-S.; Wang, C.; Du, J.; Xiao, S.; Yang, C. Jasmonate complements the function of arabidopsis lipoxygenase3 in salinity stress response. Plant Sci. 2016, 244, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ozalvo, R.; Cabrera, J.; Escobar, C.; Christensen, S.A.; Borrego, E.J.; Kolomiets, M.V.; Castresana, C.; Iberkleid, I.; Brown Horowitz, S. Two closely related members of arabidopsis 13-lipoxygenases (13-loxs), lox3 and lox4, reveal distinct functions in response to plant-parasitic nematode infection. Mol. Plant Pathol. 2014, 15, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, A.; Lenglet, A.; Wolfender, J.-L.; Farmer, E.E. Paired hierarchical organization of 13-lipoxygenases in arabidopsis. Plants 2016, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Schommer, C.; Palatnik, J.F.; Aggarwal, P.; Chételat, A.; Cubas, P.; Farmer, E.E.; Nath, U.; Weigel, D. Control of jasmonate biosynthesis and senescence by mir319 targets. PLoS Biol. 2008, 6, e230. [Google Scholar] [CrossRef] [PubMed]
- Freire, M.; Tourneur, C.; Granier, F.; Camonis, J.; Amrani, A.E.; Browning, K.; Robaglia, C. Plant lipoxygenase 2 is a translation initiation factor-4e-binding protein. Plant Mol. Biol. 2000, 44, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Ren, N.; Qi, J.; Lu, J.; Xiang, C.; Ju, H.; Cheng, J.; Lou, Y. The 9-lipoxygenase osr9-lox1 interacts with the 13-lipoxygenase-mediated pathway to regulate resistance to chewing and piercing-sucking herbivores in rice. Physiol. Plant. 2014, 152, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Brodhun, F.; Cristobal-Sarramian, A.; Zabel, S.; Newie, J.; Hamberg, M.; Feussner, I. An iron 13s-lipoxygenase with an α-linolenic acid specific hydroperoxidase activity from Fusarium oxysporum. PLoS ONE 2013, 8, e64919. [Google Scholar] [CrossRef] [PubMed]
- Newcomer, M.E.; Brash, A.R. The structural basis for specificity in lipoxygenase catalysis. Protein Sci. 2015, 24, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Newie, J.; Andreou, A.; Neumann, P.; Einsle, O.; Feussner, I.; Ficner, R. Crystal structure of a lipoxygenase from cyanothece sp. May reveal novel features for substrate acquisition. J. Lipid Res. 2016, 57, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Feussner, I.; Wasternack, C. The lipoxygenase pathway. Annu. Rev. Plant Biol. 2002, 53, 275–297. [Google Scholar] [CrossRef] [PubMed]
- Schaller, A.; Stintzi, A. Enzymes in jasmonate biosynthesis—Structure, function, regulation. Phytochemistry 2009, 70, 1532–1538. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Halitschke, R.; Kim, H.; Baldwin, I.; Feldmann, K.; Feyereisen, R. A knock-out mutation in allene oxide synthase results in male sterility and defective wound signal transduction in arabidopsis due to a block in jasmonic acid biosynthesis. Plant J. 2002, 31, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-S.; Nioche, P.; Hamberg, M.; Raman, C.S. Structural insights into the evolutionary paths of oxylipin biosynthetic enzymes. Nature 2008, 455, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Koeduka, T.; Ishizaki, K.; Mwenda, C.M.; Hori, K.; Sasaki-Sekimoto, Y.; Ohta, H.; Kohchi, T.; Matsui, K. Biochemical characterization of allene oxide synthases from the liverwort Marchantia polymorpha and green microalgae klebsormidium flaccidum provides insight into the evolutionary divergence of the plant CYP74 family. Planta 2015, 242, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Scholz, J.; Brodhun, F.; Hornung, E.; Herrfurth, C.; Stumpe, M.; Beike, A.; Faltin, B.; Frank, W.; Reski, R.; Feussner, I. Biosynthesis of allene oxides in Physcomitrella patens. BMC Plant Biol. 2012, 12, 228. [Google Scholar] [CrossRef] [PubMed]
- Toporkova, Y.Y.; Gorina, S.S.; Bessolitsyna, E.K.; Smirnova, E.O.; Fatykhova, V.S.; Brühlmann, F.; Ilyina, T.M.; Mukhtarova, L.S.; Grechkin, A.N. Double function hydroperoxide lyases/epoxyalcohol synthases (Cyp74c) of higher plants: Identification and conversion into allene oxide synthases by site-directed mutagenesis. Biochim. Biophys. Acta 2018, 1863, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jernerén, F.; Oliw, E.H. Purification and site-directed mutagenesis of linoleate 9s-dioxygenase-allene oxide synthase of Fusarium oxysporum confirms the oxygenation mechanism. Arch. Biochem. Biophys. 2017, 625–626, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Yoeun, S.; Rakwal, R.; Han, O. Dual positional substrate specificity of rice allene oxide synthase-1: Insight into mechanism of inhibition by type ii ligand imidazole. BMB Rep. 2013, 46, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Maucher, H.; Hause, B.; Feussner, I.; Ziegler, J.; Wasternack, C. Allene oxide synthases of barley (Hordeum vulgare cv. Salome): Tissue specific regulation in seedling development. Plant J. 2000, 21, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, F.; Tang, J.; Wang, W.; Zhang, F.; Wang, G.; Chu, J.; Yan, C.; Wang, T.; Chu, C.; et al. Activation of the jasmonic acid pathway by depletion of the hydroperoxide lyase oshpl3 reveals crosstalk between the hpl and aos branches of the oxylipin pathway in rice. PLoS ONE 2012, 7, e50089. [Google Scholar] [CrossRef] [PubMed]
- Hibara, K.-I.; Isono, M.; Mimura, M.; Sentoku, N.; Kojima, M.; Sakakibara, H.; Kitomi, Y.; Yoshikawa, T.; Itoh, J.-I.; Nagato, Y. Jasmonate regulates juvenile-to-adult phase transition in rice. Development 2016, 143, 3407–3416. [Google Scholar] [CrossRef] [PubMed]
- Grechkin, A.N.; Ogorodnikova, A.V.; Egorova, A.M.; Mukhitova, F.K.; Ilyina, T.M.; Khairutdinov, B. Allene oxide synthase pathway in cereal roots: Detection of novel oxylipin graminoxins. ChemistryOpen 2018, 7, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, E.; Pollmann, S. Molecular mechanism of enzymatic allene oxide cyclization in plants. Plant Physiol. Biochem. 2008, 46, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, E.; Zerbe, P.; Schaller, F. The crystal structure of Arabidopsis thaliana allene oxide cyclase: Insights into the oxylipin cyclization reaction. Plant Cell 2006, 18, 3201–3217. [Google Scholar] [CrossRef] [PubMed]
- Neumann, P.; Brodhun, F.; Sauer, K.; Herrfurth, C.; Hamberg, M.; Brinkmann, J.; Scholz, J.; Dickmanns, A.; Feussner, I.; Ficner, R. Crystal structures of Physcomitrella patens aoc1 and aoc2: Insights into the enzyme mechanism and differences in substrate specificity. Plant Physiol. 2012, 160, 1251–1266. [Google Scholar] [CrossRef] [PubMed]
- Stenzel, I.; Hause, B.; Maucher, H.; Pitzschke, A.; Miersch, O.; Ziegler, J.; Ryan, C.; Wasternack, C. Allene oxide cyclase dependence of the wound response and vascular bundle-specific generation of jasmonates in tomato-amplification in wound signaling. Plant J. 2003, 33, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.-C.; Chen, J.-F.; Xiao, Y.; Di, P.; Xuan, H.-J.; Zhou, X.; Zhang, L.; Chen, W.-S. Overexpression of allene oxide cyclase promoted tanshinone/phenolic acid production in salvia miltiorrhiza. Plant Cell Rep. 2012, 31, 2247–2259. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Dong, W.; Zhang, N.; Ai, X.; Wang, M.; Huang, Z.; Xiao, L.; Xia, G. A wheat allene oxide cyclase gene enhances salinity tolerance via jasmonate signaling. Plant Physiol. 2014, 164, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Riemann, M.; Haga, K.; Shimizu, T.; Okada, K.; Ando, S.; Mochizuki, S.; Nishizawa, Y.; Yamanouchi, U.; Nick, P.; Yano, M.; et al. Identification of rice Allene oxide Cyclase mutants and the function of jasmonate for defence against Magnaporthe oryzae. Plant J. 2013, 74, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Hazman, M.; Hause, B.; Eiche, E.; Nick, P.; Riemann, M. Increased tolerance to salt stress in OPDA-deficient rice allene oxide cyclase mutants is linked to an increased ROS-scavenging activity. J. Exp. Bot. 2015, 66, 3339–3352. [Google Scholar] [CrossRef] [PubMed]
- Dhakarey, R.; Raorane, M.L.; Treumann, A.; Peethambaran, P.K.; Schendel, R.R.; Sahi, V.P.; Hause, B.; Bunzel, M.; Henry, A.; Kohli, A.; et al. Physiological and proteomic analysis of the rice mutant cpm2 suggests a negative regulatory role of jasmonic acid in drought tolerance. Front. Plant Sci. 2017, 8, 1903. [Google Scholar] [CrossRef] [PubMed]
- Stenzel, I.; Otto, M.; Delker, C.; Kirmse, N.; Schmidt, D.; Miersch, O.; Hause, B.; Wasternack, C. ALLENE OXIDE CYCLASE (AOC) gene family members of Arabidopsis thaliana: Tissue- and organ-specific promoter activities and in vivo heteromerization. J. Exp. Bot. 2012, 63, 6125–6138. [Google Scholar] [CrossRef] [PubMed]
- Otto, M.; Naumann, C.; Brandt, W.; Wasternack, C.; Hause, B. Activity regulation by heteromerization of arabidopsis allene oxide cyclase family members. Plants 2016, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Le, T.B.; Han, C.S.; Cho, K.; Han, O. Covalent immobilization of oxylipin biosynthetic enzymes on nanoporous rice husk silica for production of cis (+)-12-oxophytodienoic acid. Artif. Cells Nanomed. Biotechnol. 2017, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Maynard, D.; Müller, S.M.; Hahmeier, M.; Löwe, J.; Feussner, I.; Gröger, H.; Viehhauser, A.; Dietz, K.-J. One-pot synthesis of bioactive cyclopentenones from α-linolenic acid and docosahexaenoic acid. Bioorg. Med. Chem. 2018, 26, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- De Marcos Lousa, C.; van Roermund, C.W.T.; Postis, V.L.G.; Dietrich, D.; Kerr, I.D.; Wanders, R.J.A.; Baldwin, S.A.; Baker, A.; Theodoulou, F.L. Intrinsic acyl-coa thioesterase activity of a peroxisomal atp binding cassette transporter is required for transport and metabolism of fatty acids. Proc. Natl. Acad. Sci. USA 2013, 110, 1279–1284. [Google Scholar] [CrossRef] [PubMed]
- Theodoulou, F.L.; Job, K.; Slocombe, S.P.; Footitt, S.; Holdsworth, M.; Baker, A.; Larson, T.R.; Graham, I.A. Jasmonic acid levels are reduced in comatose atp-binding cassette transporter mutants. Implications for transport of jasmonate precursors into peroxisomes. Plant Physiol. 2005, 137, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhou, F.; Liu, B.; Feng, D.; He, Y.; Qi, K.; Wang, H.; Wang, J. Comparative characterization, expression pattern and function analysis of the 12-oxo-phytodienoic acid reductase gene family in rice. Plant Cell Rep. 2011, 30, 981–995. [Google Scholar] [CrossRef] [PubMed]
- Sanders, P.; Lee, P.; Biesgen, C.; Boone, J.; Beals, T.; Weiler, E.; Goldberg, R. The arabidopsis DELAYED DEHISCENCE1 gene encodes an enzyme in the jasmonic acid synthesis pathway. Plant Cell 2000, 12, 1041–1061. [Google Scholar] [CrossRef] [PubMed]
- Stintzi, A.; Browse, J. The arabidopsis male-sterile mutant, opr3, lacks the 12-oxophytodienoic acid reductase required for jasmonate synthesis. Proc. Natl. Acad. Sci. USA 2000, 97, 10625–10630. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Qi, T.; Huang, H.; Ren, Q.; Wu, D.; Chang, C.; Peng, W.; Liu, Y.; Peng, J.; Xie, D. The jasmonate-zim domain proteins interact with the R2R3-MYB transcription factors MYB21 and MYB24 to affect jasmonate-regulated stamen development in arabidopsis. Plant Cell 2011, 23, 1000–1013. [Google Scholar] [CrossRef] [PubMed]
- Qi, T.; Huang, H.; Song, S.; Xie, D. Regulation of jasmonate-mediated stamen development and seed production by a bHLH-myb complex in arabidopsis. Plant Cell 2015, 27, 1620–1633. [Google Scholar] [CrossRef] [PubMed]
- Chehab, E.W.; Kim, S.; Savchenko, T.; Kliebenstein, D.; Dehesh, K.; Braam, J. Intronic t-DNA insertion renders arabidopsis opr3 a conditional jasmonic acid-producing mutant. Plant Physiol. 2011, 156, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Pan, X.; Deng, Y.; Wu, H.; Liu, P.; Li, X. Atopr3 specifically inhibits primary root growth in arabidopsis under phosphate deficiency. Sci. Rep. 2016, 6, 24778. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Baker, A.; Bartel, B.; Linka, N.; Mullen, R.T.; Reumann, S.; Zolman, B.K. Plant peroxisomes: Biogenesis and function. Plant Cell 2012, 24, 2279–2303. [Google Scholar] [CrossRef] [PubMed]
- Miersch, O.; Wasternack, C. Octadecanoid and jasmonate signaling in tomato (Lycopersicon esculentum mill.) leaves: Endogenous jasmonates do not induce jasmonate biosynthesis. Biol. Chem. 2000, 381, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Schilmiller, A.L.; Liu, G.; Lee, G.I.; Jayanty, S.; Sageman, C.; Vrebalov, J.; Giovannoni, J.J.; Yagi, K.; Kobayashi, Y.; et al. Role of b-oxidation in jasmonate biosynthesis and systemic wound signaling in tomato. Plant Cell 2005, 17, 971–986. [Google Scholar] [CrossRef] [PubMed]
- Schilmiller, A.L.; Koo, A.J.K.; Howe, G.A. Functional diversification of acyl-coenzyme a oxidases in jasmonic acid biosynthesis and action. Plant Physiol. 2007, 143, 812–824. [Google Scholar] [CrossRef] [PubMed]
- Richmond, T.; Bleecker, A. A defect in b-oxidation causes abnormal inflorescence development in arabidopsis. Plant Cell 1999, 11, 1911–1923. [Google Scholar] [CrossRef] [PubMed]
- Castillo, M.C.; Martinez, C.; Buchala, A.; Metraux, J.-P.; Leon, J. Gene-specific involvement of b-oxidation in wound-activated responses in arabidopsis. Plant Physiol. 2004, 135, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K.; Kienow, L.; Schmelzer, E.; Colby, T.; Bartsch, M.; Miersch, O.; Wasternack, C.; Kombrink, E.; Stuible, H.-P. A new type of peroxisomal acyl-coenzyme a synthetase from Arabidopsis thaliana has the catalytic capacity to activate biosynthetic precursors of jasmonic acid. J. Biol. Chem. 2005, 280, 13962–13972. [Google Scholar] [CrossRef] [PubMed]
- Wiszniewski, A.A.G.; Bussell, J.D.; Long, R.L.; Smith, S.M. Knockout of the two evolutionarily conserved peroxisomal 3-ketoacyl-coa thiolases in arabidopsis recapitulates the abnormal inflorescence meristem 1 phenotype. J. Exp. Bot. 2014, 65, 6723–6733. [Google Scholar] [CrossRef] [PubMed]
- Howe, G.A. Metabolic end run to jasmonate. Nat. Chem. Biol. 2018, 14, 109–110. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C.; Hause, B. A bypass in jasmonate biosynthesis—The OPR3-independent formation. Trends Plant Sci. 2018, 23, 276–279. [Google Scholar] [CrossRef] [PubMed]
- Scholz, S.S.; Reichelt, M.; Boland, W.; Mithöfer, A. Additional evidence against jasmonate-induced jasmonate induction hypothesis. Plant Sci. 2015, 239, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Ahammed, G.J.; Wang, G.T.; Xu, C.J.; Chen, K.S.; Zhou, Y.H.; Yu, J.Q. Tomato cry1a plays a critical role in the regulation of phytohormone homeostasis, plant development, and carotenoid metabolism in fruits. Plant Cell Environ. 2018, 41, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Bonaventure, G.; Gfeller, A.; Proebsting, W.M.; Hortensteiner, S.; Chetelat, A.; Martinoia, E.; Farmer, E.E. A gain-of-function allele of tpc1 activates oxylipin biogenesis after leaf wounding in arabidopsis. Plant J. 2007, 49, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Schütz, A.-L.; Galano, J.-M.; Herrfurth, C.; Feussner, K.; Durand, T.; Brodhun, F.; Feussner, I. The alphabet of galactolipids in Arabidopsis thaliana. Front. Plant Sci. 2011, 2, 95. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Shiva, S.; Roth, M.; Tamura, P.; Zheng, L.; Li, M.; Sarowar, S.; Honey, S.; McEllhiney, D.; Hinkes, P.; et al. Lipid changes after leaf wounding in Arabidopsis thaliana: Expanded lipidomic data form the basis for lipid co-occurrence analysis. Plant J. 2014, 80, 728–743. [Google Scholar]
- Göbel, C.; Feussner, I. Methods for the analysis of oxylipins in plants. Phytochemistry 2009, 70, 1485–1503. [Google Scholar] [CrossRef] [PubMed]
- Stelmach, B.; Müller, A.; Hennig, P.; Gebhardt, S.; Schubert-Zsilavecz, M.; Weiler, E. A novel class of oxylipins, sn1-o-(12-oxophytodienoyl)-sn2-o-(hexadecatrienoyl)-monogalactosyl diglyceride, from Arabidopsis thaliana. J. Biol. Chem. 2001, 276, 12832–12838. [Google Scholar] [CrossRef] [PubMed]
- Koo, A.J.K.; Gao, X.; Jones, A.D.; Howe, G.A. A rapid wound signal activates the systemic synthesis of bioactive jasmonates in arabidopsis. Plant J. 2009, 59, 974–986. [Google Scholar] [CrossRef] [PubMed]
- Kourtchenko, O.; Andersson, M.X.; Hamberg, M.; Brunnstrom, A.; Gobel, C.; McPhail, K.L.; Gerwick, W.H.; Feussner, I.; Ellerstrom, M. Oxo-phytodienoic acid-containing galactolipids in arabidopsis: Jasmonate signaling dependence. Plant Physiol. 2007, 145, 1658–1669. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.X.; Hamberg, M.; Kourtchenko, O.; Brunnstrom, A.; McPhail, K.L.; Gerwick, W.H.; Gobel, C.; Feussner, I.; Ellerstrom, M. Oxylipin profiling of the hypersensitive response in Arabidopsis thaliana: Formation of a novel oxo-phytodienoic acid-containing galactolipid, arabidopside e. J. Biol. Chem. 2006, 281, 31528–31537. [Google Scholar] [CrossRef] [PubMed]
- Glauser, G.; Dubugnon, L.; Mousavi, S.A.R.; Rudaz, S.; Wolfender, J.-L.; Farmer, E.E. Velocity estimates for signal propagation leading to systemic jasmonic acid accumulation in wounded arabidopsis. J. Biol. Chem. 2009, 284, 34506–34513. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, A.K.; Fahlberg, P.; Ellerström, M.; Andersson, M.X. Oxo-phytodienoic acid (opda) is formed on fatty acids esterified to galactolipids after tissue disruption in Arabidopsis thaliana. FEBS Lett. 2012, 586, 2483–2487. [Google Scholar] [CrossRef] [PubMed]
- Pedras, M.S.C.; To, Q.H. Defense and signalling metabolites of the crucifer erucastrum canariense: Synchronized abiotic induction of phytoalexins and galacto-oxylipins. Phytochemistry 2017, 139, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, A.K.; Fahlberg, P.; Johansson, O.N.; Hamberg, M.; Andersson, M.X.; Ellerström, M. The activity of hydroperoxide lyase 1 regulates accumulation of galactolipids containing 12-oxo-phytodienoic acid in arabidopsis. J. Exp. Bot. 2016, 67, 5133–5144. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, A.K.; Johansson, O.N.; Fahlberg, P.; Kommuri, M.; Töpel, M.; Bodin, L.J.; Sikora, P.; Modarres, M.; Ekengren, S.; Nguyen, C.T.; et al. Acylated monogalactosyl diacylglycerol: Prevalence in the plant kingdom and identification of an enzyme catalyzing galactolipid head group acylation in Arabidopsis thaliana. Plant J. 2015, 84, 1152–1166. [Google Scholar] [CrossRef] [PubMed]
- Böttcher, C.; Weiler, E. Cyclo-oxylipin-galactolipids in plants: Occurrence and dynamics. Planta 2007, 226, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Hartley, S.E.; Eschen, R.; Horwood, J.M.; Gange, A.C.; Hill, E.M. Infection by a foliar endophyte elicits novel arabidopside-based plant defence reactions in its host, cirsium arvense. New Phytol. 2015, 205, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Ueda, J.; Miyamoto, K.; Aoki, M.; Hirata, T.; Sato, T.; Momotani, Y. Identification of jasmonic acid in chlorella and spirulina. Bull. Univ. Osaka Pref. 1991, 23, 103–108. [Google Scholar]
- Qiu, Y.-L.; Li, L.; Wang, B.; Chen, Z.; Knoop, V.; Groth-Malonek, M.; Dombrovska, O.; Lee, J.; Kent, L.; Rest, J.; et al. The deepest divergences in land plants inferred from phylogenomic evidence. Proc. Natl. Acad. Sci. USA 2006, 103, 15511–15516. [Google Scholar] [CrossRef] [PubMed]
- Rensing, S.A.; Lang, D.; Zimmer, A.D.; Terry, A.; Salamov, A.; Shapiro, H.; Nishiyama, T.; Perroud, P.-F.; Lindquist, E.A.; Kamisugi, Y.; et al. The physcomitrella genome reveals evolutionary insights into the conquest of land by plants. Science 2008, 319, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Collén, J.; Porcel, B.; Carré, W.; Ball, S.G.; Chaparro, C.; Tonon, T.; Barbeyron, T.; Michel, G.; Noel, B.; Valentin, K.; et al. Genome structure and metabolic features in the red seaweed Chondrus crispus shed light on evolution of the archaeplastida. Proc. Natl. Acad. Sci. USA 2013, 110, 5247–5252. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, Y.; Li, S.-S.; Han, G.-Z. Insights into the origin and evolution of the plant hormone signaling machinery. Plant Physiol. 2015, 167, 872–886. [Google Scholar] [CrossRef] [PubMed]
- Banks, J.A.; Nishiyama, T.; Hasebe, M.; Bowman, J.L.; Gribskov, M.; dePamphilis, C.; Albert, V.A.; Aono, N.; Aoyama, T.; Ambrose, B.A.; et al. The compact selaginella genome identifies changes in gene content associated with the evolution of vascular plants. Science 2011, 332, 960–963. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, D.; Hwang, I. A sophisticated network of signaling pathways regulates stomatal defenses to bacterial pathogens. Mol. Plant 2015, 8, 566–581. [Google Scholar] [CrossRef] [PubMed]
- Bown, L.; Li, Y.; Berrué, F.; Verhoeven, J.T.P.; Dufour, S.C.; Bignell, D.R.D. Coronafacoyl phytotoxin biosynthesis and evolution in the common scab pathogen Streptomyces scabiei. Appl. Environ. Microbiol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Littleson, M.M.; Baker, C.M.; Dalençon, A.J.; Frye, E.C.; Jamieson, C.; Kennedy, A.R.; Ling, K.B.; McLachlan, M.M.; Montgomery, M.G.; Russell, C.J.; et al. Scalable total synthesis and comprehensive structure–activity relationship studies of the phytotoxin coronatine. Nat. Commun. 2018, 9, 1105. [Google Scholar] [CrossRef] [PubMed]
- Widemann, E.; Smirnova, E.; Aubert, Y.; Miesch, L.; Heitz, T. Dynamics of jasmonate metabolism upon flowering and across leaf stress responses in Arabidopsis thaliana. Plants 2016, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Koo, A.J.; Howe, G.A. Catabolism and deactivation of the lipid-derived hormone jasmonoyl-isoleucine. Front. Plant Sci. 2012, 3, 19. [Google Scholar] [CrossRef] [PubMed]
- Heitz, T.; Smirnova, E.; Widemann, E.; Aubert, Y.; Pinot, F.; Ménard, R. The rise and fall of jasmonate biological activities. In Lipids in Plant and Algae Development; Nakamura, Y., Li-Beisson, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 405–426. [Google Scholar]
- Wasternack, C.; Strnad, M. Jasmonate signaling in plant stress responses and development–active and inactive compounds. New Biotechnol. 2016, 33, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Koo, A.J. Metabolism of the plant hormone jasmonate: A sentinel for tissue damage and master regulator of stress response. Phytochem. Rev. 2018, 17, 51–80. [Google Scholar] [CrossRef]
- Fonseca, S.; Chini, A.; Hamberg, M.; Adie, B.; Porzel, A.; Kramell, R.; Miersch, O.; Wasternack, C.; Solano, R. (+)-7-iso-jasmonoyl-l-isoleucine is the endogenous bioactive jasmonate. Nat. Chem. Biol. 2009, 5, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Staswick, P.E.; Tiryaki, I. The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in arabidopsis. Plant Cell 2004, 16, 2117–2127. [Google Scholar] [CrossRef] [PubMed]
- Westfall, C.S.; Muehler, A.M.; Jez, J.M. Enzyme action in the regulation of plant hormone responses. J. Biol. Chem. 2013, 288, 19304–19311. [Google Scholar] [CrossRef] [PubMed]
- Sherp, A.M.; Westfall, C.S.; Alvarez, S.; Jez, J.M. Arabidopsis thaliana gh3.15 acyl acid amido synthetase has a highly specific substrate preference for the auxin precursor indole-3-butyric acid. J. Biol. Chem. 2018, 293, 4277–4288. [Google Scholar] [CrossRef] [PubMed]
- Monte, I.; Hamberg, M.; Chini, A.; Gimenez-Ibanez, S.; Garcia-Casado, G.; Porzel, A.; Pazos, F.; Boter, M.; Solano, R. Rational design of a ligand-based antagonism of jasmonate perception. Nat. Chem. Biol. 2014, 10, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Meesters, C.; Mönig, T.; Oeljeklaus, J.; Krahn, D.; Westfall, C.; Hause, B.; Jez, J.; Kaiser, M.; Kombrink, E. A chemical inhibitor of jasmonate signaling targets JAR1 in Arabidopsis thaliana. Nat. Chem. Biol. 2014, 10, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Westfall, C.S.; Zubieta, C.; Herrmann, J.; Kapp, U.; Nanao, M.H.; Jez, J.M. Structural basis for prereceptor modulation of plant hormones by gh3 proteins. Science 2012, 336, 1708–1711. [Google Scholar] [CrossRef] [PubMed]
- Suza, W.; Rowe, M.; Hamberg, M.; Staswick, P. A tomato enzyme synthesizes (+)-7-iso-jasmonoyl-l-isoleucine in wounded leaves. Planta 2010, 231, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, K.; Alamgir, K.M.; Yamashita, Y.; Mori, I.C.; Matsuura, H.; Galis, I. Response of rice to insect elicitors and the role of OSJAR1 in wound and herbivory-induced JA-ILE accumulation. J. Integr. Plant Biol. 2013, 55, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Svyatyna, K.; Jikumaru, Y.; Brendel, R.; Reichelt, M.; MithÖFer, A.; Takano, M.; Kamiya, Y.; Nick, P.; Riemann, M. Light induces jasmonate-isoleucine conjugation via OSJAR1-dependent and -independent pathways in rice. Plant Cell Environ. 2014, 37, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Chen, Y.; Charnikhova, T.; Mulder, P.P.J.; Heijmans, J.; Hoogenboom, A.; Agalou, A.; Michel, C.; Morel, J.-B.; Dreni, L.; et al. OSJAR1 is required for ja-regulated floret opening and anther dehiscence in rice. Plant Mol. Biol. 2014, 86, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Li, S.; Gu, M.; Yao, R.; Li, Y.; Chen, J.; Yang, M.; Nan, F.; Xi, D. Endogenous bioactive jasmonate is composed of a set of (+)-7-iso-ja-amino acid conjugates. Plant Physiol. 2016 2016, 172, 2154–2164. [Google Scholar] [CrossRef]
- Reveglia, P.; Chini, A.; Mandoli, A.; Masi, M.; Cimmino, A.; Pescitelli, G.; Evidente, A. Synthesis and mode of action studies of n-[(−)-jasmonyl]-s-tyrosin and ester seiridin jasmonate. Phytochemistry 2018, 147, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.-L.; Okamoto, H.; Wang, M.; Ang, L.-H.; Matsui, M.; Goodman, H.; Deng, X.W. FIN219, an auxin-regulated gene, defines a link between phytochrome a and the downstream regulator cop1 in light control of arabidopsis development. Genes Dev. 2000, 14, 1958–1970. [Google Scholar] [PubMed]
- Ballaré, C.L. Light regulation of plant defense. Annu. Rev. Entomol. 2014, 65, 335–363. [Google Scholar] [CrossRef] [PubMed]
- Robson, F.; Okamoto, H.; Patrick, E.; Harris, S.-R.; Wasternack, C.; Brearley, C.; Turner, J.G. Jasmonate and phytochrome a signaling in arabidopsis wound and shade responses are integrated through jaz1 stability. Plant Cell 2010, 22, 1143–1160. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.-L.; Okamoto, H. Molecular interaction of jasmonate and phytochrome a signalling. J. Exp. Bot. 2014, 65, 2847–2857. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.; Jiang, H.-W.; Hsieh, H.-L. FAR-RED insensitive 219/jar1 contributes to shade avoidance responses of arabidopsis seedlings by modulating key shade signaling components. Front. Plant Sci. 2017, 8, 1901. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Ho, S.-S.; Kuo, T.-Y.; Hsieh, H.-L.; Cheng, Y.-S. Structural basis of jasmonate-amido synthetase FIN219 in complex with glutathione s-transferase FIP1 during the ja signal regulation. Proc. Natl. Acad. Sci. USA 2017, 114, E1815–E1824. [Google Scholar] [CrossRef] [PubMed]
- Patkar, R.N.; Benke, P.I.; Qu, Z.; Constance Chen, Y.Y.; Yang, F.; Swarup, S.; Naqvi, N.I. A fungal monooxygenase-derived jasmonate attenuates host innate immunity. Nat. Chem. Biol. 2015, 11, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Caarls, L.; Elberse, J.; Awwanah, M.; Ludwig, N.R.; de Vries, M.; Zeilmaker, T.; Van Wees, S.C.M.; Schuurink, R.C.; Van den Ackerveken, G. Arabidopsis jasmonate-induced oxygenases down-regulate plant immunity by hydroxylation and inactivation of the hormone jasmonic acid. Proc. Natl. Acad. Sci. USA 2017, 114, 6388–6393. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, E.; Marquis, V.; Poirier, L.; Aubert, Y.; Zumsteg, J.; Ménard, R.; Miesch, L.; Heitz, T. Jasmonic acid oxidase 2 hydroxylates jasmonic acid and represses basal defense and resistance responses against botrytis cinerea infection. Mol. Plant 2017, 10, 1159–1173. [Google Scholar] [CrossRef] [PubMed]
- Bruckhoff, V.; Haroth, S.; Feussner, K.; König, S.; Brodhun, F.; Feussner, I. Functional characterization of CYP94-genes and identification of a novel jasmonate catabolite in flowers. PLoS ONE 2016, 11, e0159875. [Google Scholar] [CrossRef] [PubMed]
- Koo, A.J.; Thireault, C.; Zemelis, S.; Poudel, A.N.; Zhang, T.; Kitaoka, N.; Brandizzi, F.; Matsuura, H.; Howe, G.A. Endoplasmic reticulum-associated inactivation of the hormone jasmonoyl-l-isoleucine by multiple members of the cytochrome p450 94 family in arabidopsis. J. Biol. Chem. 2014, 289, 29728–29738. [Google Scholar] [CrossRef] [PubMed]
- Koo, A.J.K.; Cooke, T.F.; Howe, G.A. Cytochrome P450 CYP94B3 mediates catabolism and inactivation of the plant hormone jasmonoyl-l-isoleucine. Proc. Nat. Acad. Sci. USA 2011, 108, 9298–9303. [Google Scholar] [CrossRef] [PubMed]
- Heitz, T.; Widemann, E.; Lugan, R.; Miesch, L.; Ullmann, P.; Désaubry, L.; Holder, E.; Grausem, B.; Kandel, S.; Miesch, M.; et al. Cytochromes P450 CYP94C1 and CYP94B3 catalyze two successive oxidation steps of plant hormone jasmonoyl-isoleucine for catabolic turnover. J. Biol. Chem. 2012, 287, 6296–6306. [Google Scholar] [CrossRef] [PubMed]
- Poudel, A.N.; Zhang, T.; Kwasniewski, M.; Nakabayashi, R.; Saito, K.; Koo, A.J. Mutations in jasmonoyl-l-isoleucine-12-hydroxylases suppress multiple ja-dependent wound responses in Arabidopsis thaliana. Biochim. Biophys. Acta 2016, 1861, 1396–1408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Poudel, A.N.; Jewell, J.B.; Kitaoka, N.; Staswick, P.; Matsuura, H.; Koo, A.J. Hormone crosstalk in wound stress response: Wound-inducible amidohydrolases can simultaneously regulate jasmonate and auxin homeostasis in Arabidopsis thaliana. J. Exp. Bot. 2016, 67, 2107–2120. [Google Scholar] [CrossRef] [PubMed]
- Sanchez Carranza, A.P.; Singh, A.; Steinberger, K.; Panigrahi, K.; Palme, K.; Dovzhenko, A.; Dal Bosco, C. Hydrolases of the ilr1-like family of Arabidopsis thaliana modulate auxin response by regulating auxin homeostasis in the endoplasmic reticulum. Sci. Rep. 2016, 6, 24212. [Google Scholar] [CrossRef] [PubMed]
- Woldemariam, M.; Ongokesung, N.; Baldwin, I.; Galis, I. Jasmonoyl-l-isoleucine hydrolase 1 (JIH1) regulates jasmonoyl-l-isoleucine levels and attenuates plant defenses against herbivores. Plant J. 2012, 72, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wei, K.; Wang, S.; Zhao, W.; Ma, C.; Hettenhausen, C.; Wu, J.; Cao, G.; Sun, G.; Baldwin, I.T.; et al. COI1-regulated hydroxylation of jasmonoyl-l-isoleucine impairs nicotiana attenuata’s resistance to the generalist herbivore spodoptera litura. J. Agric. Food Chem. 2016, 64, 2822–2831. [Google Scholar] [CrossRef] [PubMed]
- Acosta, I.F.; Laparra, H.; Romero, S.P.; Schmelz, E.; Hamberg, M.; Mottinger, J.P.; Moreno, M.A.; Dellaporta, S.L. TASSELSEED1 is a lipoxygenase affecting jasmonic acid signaling in sex determination of maize. Science 2009, 323, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Hayward, A.P.; Moreno, M.A.; Howard, T.P.; Hague, J.; Nelson, K.; Heffelfinger, C.; Romero, S.; Kausch, A.P.; Glauser, G.; Acosta, I.F.; et al. Control of sexuality by the sk1-encoded UDP-glycosyltransferase of maize. Sci. Adv. 2016, 2, e1600991. [Google Scholar] [CrossRef] [PubMed]
- Miersch, O.; Neumerkel, J.; Dippe, M.; Stenzel, I.; Wasternack, C. Hydroxylated jasmonates are commonly occurring metabolites of jasmonic acid and contribute to a partial switch-off in jasmonate signaling. New Phytol. 2008, 177, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Schuman, M.C.; Baldwin, I.T. Field studies reveal functions of chemical mediators in plant interactions. Chem. Soc. Rev. 2018, 47, 5338–5353. [Google Scholar] [CrossRef] [PubMed]
- Schuman, M.C.; Meldau, S.; Gaquerel, E.; Diezel, C.; McGale, E.; Greenfield, S.; Baldwin, I.T. The active jasmonate JA-ILE regulates a specific subset of plant jasmonate-mediated resistance to herbivores in nature. Front. Plant Sci. 2018, 9, 787. [Google Scholar] [CrossRef] [PubMed]
- Bruce, T.J.A.; Matthes, M.C.; Chamberlain, K.; Woodcock, C.M.; Mohib, A.; Webster, B.; Smart, L.E.; Birkett, M.A.; Pickett, J.A.; Napier, J.A. Cis-jasmone induces Arabidopsis genes that affect the chemical ecology of multitrophic interactions with aphids and their parasitoids. Proc. Natl. Acad. Sci. USA 2008, 105, 4553–4558. [Google Scholar] [CrossRef] [PubMed]
- Matthes, M.; Bruce, T.; Ton, J.; Verrier, P.; Pickett, J.; Napier, J. The transcriptome of cis-jasmone-induced resistance in Arabidopsis thaliana and its role in indirect defence. Planta 2010, 232, 1163–1180. [Google Scholar] [CrossRef] [PubMed]
- Oluwafemi, S.; Dewhirst, S.Y.; Veyrat, N.; Powers, S.; Bruce, T.J.A.; Caulfield, J.C.; Pickett, J.A.; Birkett, M.A. Priming of production in maize of volatile organic defence compounds by the natural plant activator cis-jasmone. PLoS ONE 2013, 8, e62299. [Google Scholar] [CrossRef] [PubMed]
- Etl, F.; Berger, A.; Weber, A.; Schönenberger, J.; Dötterl, S. Nocturnal plant bugs use cis-jasmone to locate inflorescences of an araceae as feeding and mating site. J. Chem. Ecol. 2016, 42, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Schulze, B.; Dabrowska, P.; Boland, W. Rapid enzymatic isomerization of 12-oxophytodienoic acid in the gut of lepidopteran larvae. ChemBioChem 2007, 8, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Guo, X.; Liu, G.; Song, Y.; Ho, C.-T.; Hou, R.; Zhang, L.; Wan, X. A comparative analysis for the volatile compounds of various chinese dark teas using combinatory metabolomics and fungal solid-state fermentation. J. Food Drug Anal. 2018, 26, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Matsui, R.; Amano, N.; Takahashi, K.; Taguchi, Y.; Saburi, W.; Mori, H.; Kondo, N.; Matsuda, K.; Matsuura, H. Elucidation of the biosynthetic pathway of cis-jasmone in Lasiodiplodia theobromae. Sci. Rep. 2017, 7, 6688. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhou, F.; Pichersky, E. Jasmone hydroxylase, a key enzyme in the synthesis of the alcohol moiety of pyrethrin insecticides. Plant Physiol. 2018, 177, 1498–1509. [Google Scholar] [CrossRef] [PubMed]
- Stitz, M.; Gase, K.; Baldwin, I.T.; Gaquerel, E. Ectopic expression of AtJMT in Nicotiana attenuata: Creating a metabolic sink has tissue-specific consequences for the jasmonate metabolic network and silences downstream gene expression. Plant Physiol. 2011, 157, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Sheard, L.B.; Tan, X.; Mao, H.; Withers, J.; Ben-Nissan, G.; Hinds, T.R.; Kobayashi, Y.; Hsu, F.-F.; Sharon, M.; Browse, J.; et al. Jasmonate perception by inositol-phosphate-potentiated COI1-JAZ co-receptor. Nature 2010, 468, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Chaiprasongsuk, M.; Zhang, C.; Qian, P.; Chen, X.; Li, G.; Trigiano, R.N.; Guo, H.; Chen, F. Biochemical characterization in norway spruce (Picea abies) of sabath methyltransferases that methylate phytohormones. Phytochemistry 2018, 149, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Egea, I.; Albaladejo, I.; Meco, V.; Morales, B.; Sevilla, A.; Bolarin, M.C.; Flores, F.B. The drought-tolerant solanum pennellii regulates leaf water loss and induces genes involved in amino acid and ethylene/jasmonate metabolism under dehydration. Sci. Rep. 2018, 8, 2791. [Google Scholar] [CrossRef] [PubMed]
- Hirschmann, F.; Krause, F.; Papenbrock, J. The multi-protein family of sulfotransferases in plants: Composition, occurrence, substrate specificity and functions. Front. Plant Sci. 2014, 5, 556. [Google Scholar] [CrossRef] [PubMed]
- Koprivova, A.; Kopriva, S. Sulfation pathways in plants. Chem. Biol. Interact. 2016, 259, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Gidda, S.; Miersch, O.; Levitin, A.; Schmidt, J.; Wasternack, C.; Varin, L. Biochemical and molecular characterization of a hydroxyjasmonate sulfotransferase from Arabidopsis thaliana. J. Biol. Chem. 2003, 278, 17895–17900. [Google Scholar] [CrossRef] [PubMed]
- Mugford, S.G.; Yoshimoto, N.; Reichelt, M.; Wirtz, M.; Hill, L.; Mugford, S.T.; Nakazato, Y.; Noji, M.; Takahashi, H.; Kramell, R.; et al. Disruption of adenosine-5′-phosphosulfate kinase in Arabidopsis reduces levels of sulfated secondary metabolites. Plant Cell 2009, 21, 910–927. [Google Scholar] [CrossRef] [PubMed]
- Hannapel, D.J. A model system of development regulated by the long-distance transport of mRNA. J. Integr. Plant Biol. 2010, 52, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Glauser, G.; Grata, E.; Dubugnon, L.; Rudaz, S.; Farmer, E.E.; Wolfender, J.-L. Spatial and temporal dynamics of jasmonate synthesis and accumulation in arabidopsis in response to wounding. J. Biol. Chem. 2008, 283, 16400–16407. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Partz, C.; Brandt, W.; David, A.; Rendon-Anaya, M.; Herrera-Estrella, A.; Mithöfer, A.; Boland, W. Synthesis of 6-substituted 1-oxoindanoyl isoleucine conjugates and modeling studies with the COI1-JAZ coreceptor complex of lima bean. J. Chem. Ecol. 2014, 40, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Mithofer, A.; Kombrink, E.; Boland, W.; Hamamoto, S.; Uozumi, N.; Tohma, K.; Ueda, M. 12-hydroxyjasmonic acid glucoside is a COI1-JAZ-independent activator of leaf-closing movement in Samanea saman. Plant Physiol. 2011, 155, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; Yang, G.; Nukadzuka, Y.; Ishimaru, Y.; Tamura, S.; Manabe, Y. Functional importance of the sugar moiety of jasmonic acid glucoside for bioactivity and target affinity. Org. Biomol. Chem. 2015, 13, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Aleman, G.H.; Machado, R.A.R.; Gorls, H.; Baldwin, I.T.; Boland, W. Synthesis, structural characterization and biological activity of two diastereomeric JA-ILE macrolactones. Org. Biomol. Chem. 2015, 13, 5885–5893. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.-X.; Feys, B.; James, S.; Nieto-Rostro, M.; Turner, J. COI1: An arabidopsis gene required for jasmonate-regulated defense and fertility. Science 1998, 280, 1091–1094. [Google Scholar] [CrossRef] [PubMed]
- Katsir, L.; Schilmiller, A.L.; Staswick, P.E.; He, S.Y.; Howe, G.A. COI1 is a critical component of a receptor for jasmonate and the bacterial virulence factor coronatine. Proc. Natl. Acad. Sci. USA 2008, 105, 7100–7105. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zhang, C.; Gu, M.; Bai, Z.; Zhang, W.; Qi, T.; Cheng, Z.; Peng, W.; Luo, H.; Nan, F.; et al. The arabidopsis coronatine insensitive1 protein is a jasmonate receptor. Plant Cell 2009, 21, 2220–2236. [Google Scholar] [CrossRef] [PubMed]
- Mosblech, A.; Thurow, C.; Gatz, C.; Feussner, I.; Heilmann, I. Jasmonic acid perception by coi1 involves inositol polyphosphates in Arabidopsis thaliana. Plant J. 2011, 65, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Thines, B.; Katsir, L.; Melotto, M.; Niu, Y.; Mandaokar, A.; Liu, G.; Nomura, K.; He, S.Y.; Howe, G.A.; Browse, J. Jaz repressor proteins are targets of the scfcoi1 complex during jasmonate signalling. Nature 2007, 448, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Melotto, M.; Mecey, C.; Niu, Y.; Chung, H.S.; Katsir, L.; Yao, J.; Zeng, W.; Thines, B.; Staswick, P.; Browse, J.; et al. A critical role of two positively charged amino acids in the JAS motif of arabidopsis JAZ proteins in mediating coronatine- and jasmonoyl isoleucine-dependent interactions with the COI1 F-box protein. Plant J. 2008, 55, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, L.; Goossens, A. The JAZ proteins: A crucial interface in the jasmonate signaling cascade. Plant Cell 2011, 23, 3089–3100. [Google Scholar] [CrossRef] [PubMed]
- Nagels Durand, A.; Pauwels, L.; Goossens, A. The ubiquitin system and jasmonate signaling. Plants 2016, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Goossens, J.; Fernández-Calvo, P.; Schweizer, F.; Goossens, A. Jasmonates: Signal transduction components and their roles in environmental stress responses. Plant Mol. Biol. 2016, 91, 673–689. [Google Scholar] [CrossRef] [PubMed]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wasternack, C.; Strnad, M. Jasmonates: News on Occurrence, Biosynthesis, Metabolism and Action of an Ancient Group of Signaling Compounds. Int. J. Mol. Sci. 2018, 19, 2539. https://doi.org/10.3390/ijms19092539
Wasternack C, Strnad M. Jasmonates: News on Occurrence, Biosynthesis, Metabolism and Action of an Ancient Group of Signaling Compounds. International Journal of Molecular Sciences. 2018; 19(9):2539. https://doi.org/10.3390/ijms19092539
Chicago/Turabian StyleWasternack, Claus, and Miroslav Strnad. 2018. "Jasmonates: News on Occurrence, Biosynthesis, Metabolism and Action of an Ancient Group of Signaling Compounds" International Journal of Molecular Sciences 19, no. 9: 2539. https://doi.org/10.3390/ijms19092539
APA StyleWasternack, C., & Strnad, M. (2018). Jasmonates: News on Occurrence, Biosynthesis, Metabolism and Action of an Ancient Group of Signaling Compounds. International Journal of Molecular Sciences, 19(9), 2539. https://doi.org/10.3390/ijms19092539