Synthesis, Characterization, and Antimicrobial Properties of Peptides Mimicking Copolymers of Maleic Anhydride and 4-Methyl-1-pentene
Abstract
:1. Introduction
2. Results and Discussion
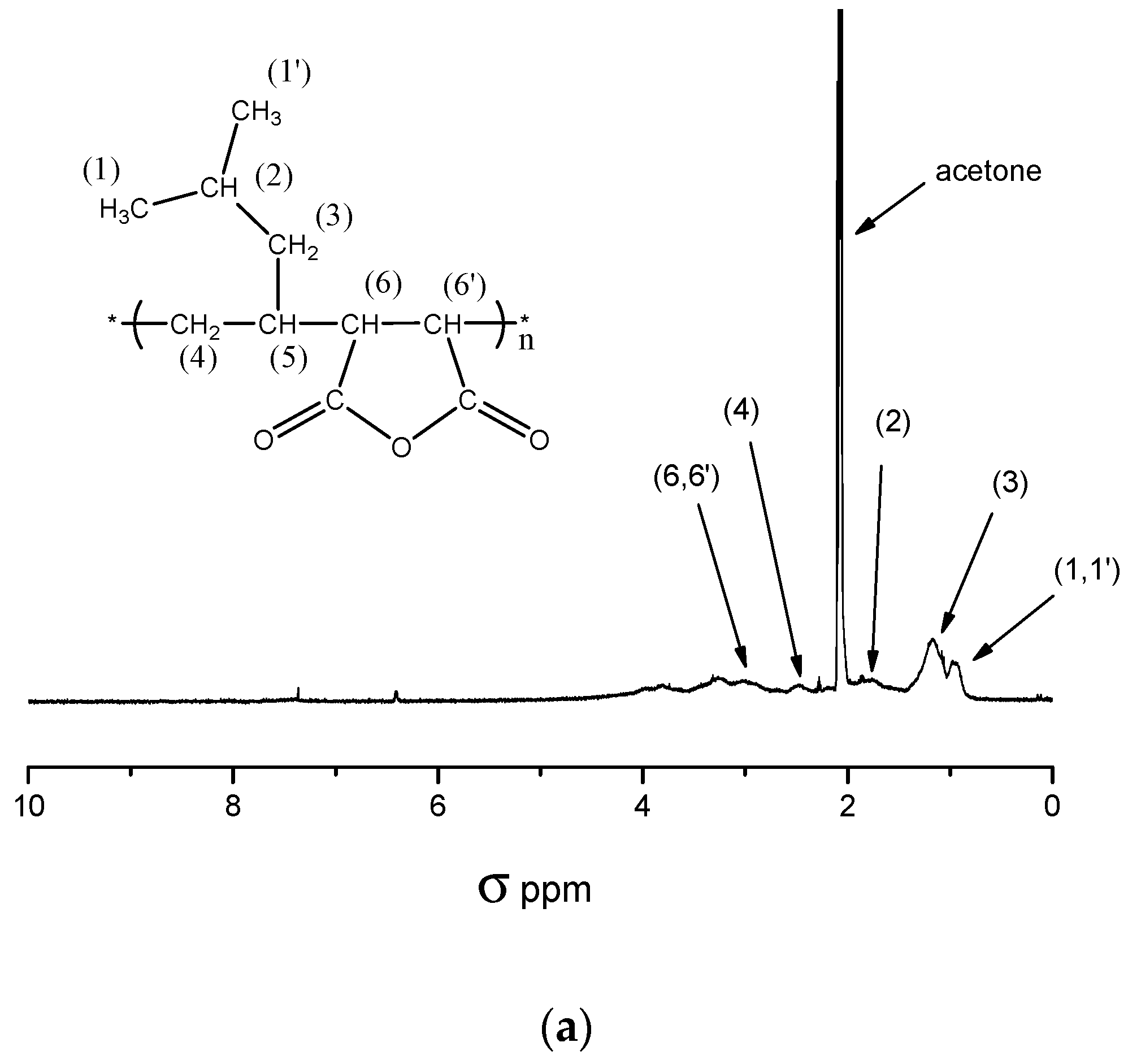
2.1. Copolymerization of Maleic Anhydride with 4-Methyl-1-pentene
- A—integration value of benzylic proton signal (σ = 4.90–5.30 ppm),
- m—number of benzylic protons = 2,
- B—integration value of polymer backbone and side chains proton signal (σ = 0.70–4.2),
- n—number of protons in signal mentioned in B = 14.
2.2. Grafting of DMAPA onto P[MP-alt-MSA] Copolymer
2.2.1. Model Reaction of Amidoacidification
2.2.2. Amidoacidification and Imidization of Poly[(4-methyl-1-pentene)-alt-maleic Anhydride
2.2.3. Chemical Imidization
2.2.4. Thermal Imidization
2.3. Quaternization of C3
2.4. Solubility in Selected Solvents
2.5. Thermal Properties
2.6. Investigation of Antimicrobial Properties of the Cationic Copolymer
3. Materials and Methods
3.1. Materials
3.2. Measurements/Apparatus
3.3. Syntheses
3.3.1. Poly[(4-methyl-1-pentene)-alt-maleic anhydride] (Copolymer 1, C1)
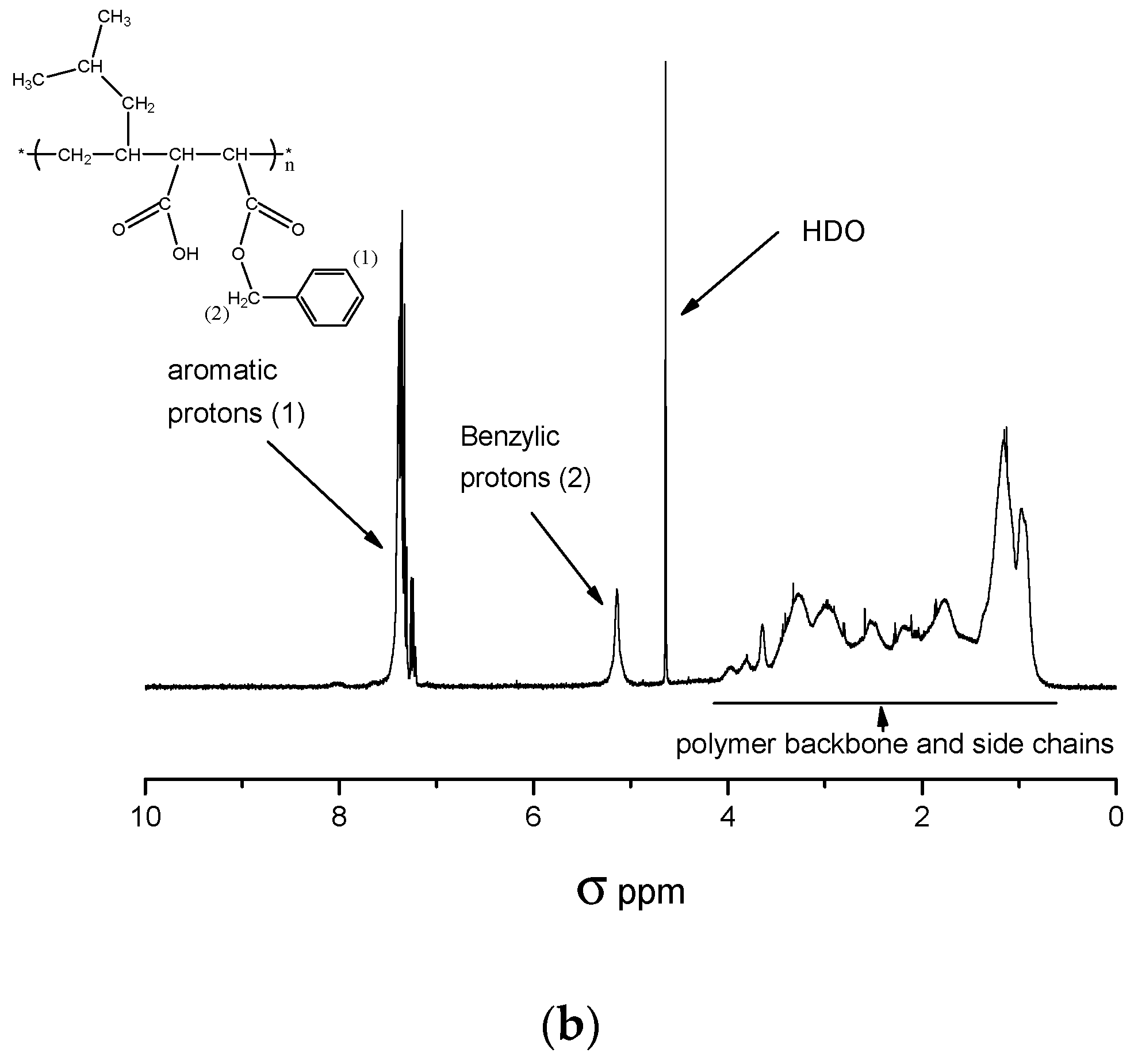
3.3.2. Determination of the Maleic Anhydride Content of C1 by 1H-NMR
3.3.3. Amidoacidification of C1 to Poly[(4-methyl-1-pentene)-alt-(1-(3-N,N-dimethylaminopropyl)malemic acid)] (Copolymer 2, C2)
3.3.4. Thermal Imidization of C2 to Poly[(4-methyl-1-pentene)-alt-(1-(3-N,N-dimethylaminopropyl)maleimide)] (Copolymer 3a, C3a)
3.3.5. Chemical Imidization of Amidoacidified C2 (Copolymer 3b, C3b)
3.3.6. Preparation of Imide C3 in a One-Pot Reaction (Copolymer 3c, C3c)
3.3.7. Synthesis of Poly[(4-methyl-1-pentene)-alt-(1-(3-N,N,N-trimethylammonium-propyl)-maleimidoiodide)] by Quaternization of Imidized C3 with Methyl Iodide (Copolymer 4, C4)
3.3.8. Sequential Quaternization of Imidized C3 with Methyl and Dodecyl Iodide (Copolymer 5, C5)
3.3.9. Synthesis of Poly[(4-methyl-1-pentene)-alt-(1-(3-N,N-dimethyl-N-dodecylammoniumpropyl)-maleimidoiodide)] (Copolymer 6, C6)
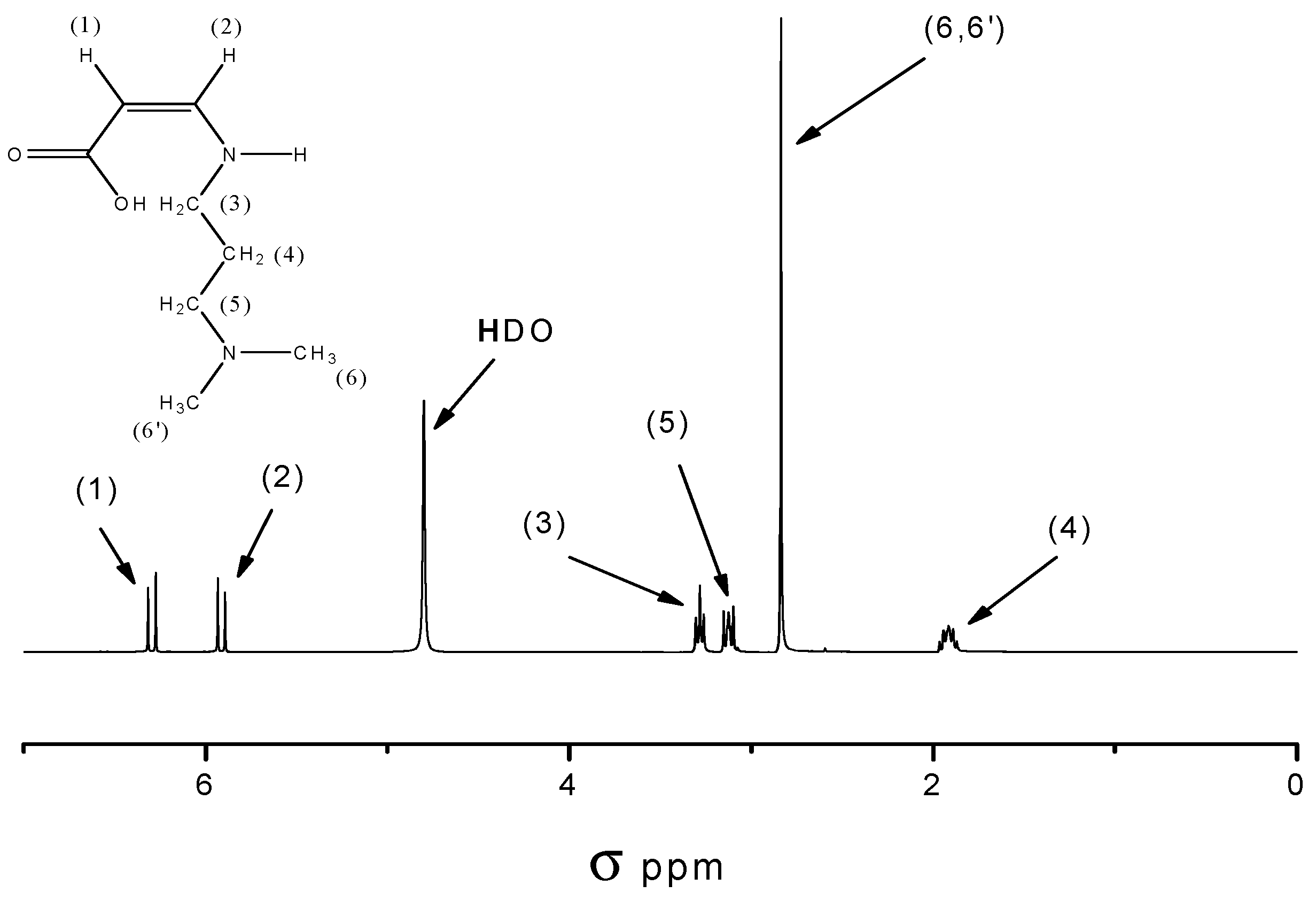
3.3.10. Synthesis of 3-(N,N-dimethylamino)propyl Maleic Acid Amide (M1)
3.3.11. Chemical Imidization (Z)-4-(N,N-dimethylamino)propylamino-4-oxobut-2-enoic Acid (M2)
3.3.12. Synthesis of 3-(N,N-dimethylamino)propyl Succinamic Acid (M3)
3.3.13. Thermal Imidization of (Z)-4-(N,N-dimethylamino)propylamino-4-oxobutanoic Acid (M4)
3.4. Antimicrobial Tests
3.5. Hemolytic Activity
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Cornell, R.J.; Donaruma, L.G. 2-Methacryloxytropones. Intermediates for synthesis of biologically active polymers. J. Med. Chem. 1965, 8, 388–390. [Google Scholar] [PubMed]
- Tashiro, T. Antibacterial and bacterium adsorbing macromolecules. Macromol. Mater. Eng. 2001, 286, 63–87. [Google Scholar] [CrossRef]
- Kenawy, E.R.; Mahmoud, Y.A.G. Biologically active polymers, 6-synthesis and antimicrobial activity of some linear copolymers with quaternary ammonium and phosphonium groups. Macromol. Biosc. 2003, 3, 107–116. [Google Scholar] [CrossRef]
- Sanda, F.; Endo, T. Syntheses and functions of polymers based on amino acids. Macromol. Chem. Phys. 1999, 200, 2651–2661. [Google Scholar] [CrossRef]
- Ilker, M.F.; Nusslein, K.; Tew, G.N.; Coughlin, E.B. Tuning the hemolytic and antibacterial activities of amphiphilic polynorbornene derivatives. J. Am. Chem. Soc. 2004, 126, 15870–15875. [Google Scholar] [CrossRef] [PubMed]
- Rivas, B.L.; Pereira, E.D.; Mondaca, M.A. Biostatic behavior of side chain charged-polycations and polymer-ag complexes. Polym. Bull. 2003, 50, 327–333. [Google Scholar] [CrossRef]
- Tashiro, T. Removal of bacteria from water by systems based on insoluble polystyrene-polyethyleneimine. J. Appl. Polym. Sci. 1992, 46, 899–907. [Google Scholar] [CrossRef]
- Hazzizalaskar, J.; Nurdin, N.; Helary, G.; Sauvet, G. Biocidal polymers active by contact.1. Synthesis of polybutadiene with pendant quaternary ammonium groups. J. Appl. Polym. Sci. 1993, 50, 651–662. [Google Scholar] [CrossRef]
- Nurdin, N.; Helary, G.; Sauvet, G. Biocidal polymers active by contact. II. Biological evaluation of polyurethane coatings with pendant quaternary ammonium-salts. J. Appl. Polym. Sci. 1993, 50, 663–670. [Google Scholar] [CrossRef]
- Nurdin, N.; Helary, G.; Sauvet, G. Biocidal polymers active by contact. III. Aging of biocidal polyurethane coatings in water. J. Appl. Polym. Sci. 1993, 50, 671–678. [Google Scholar] [CrossRef]
- Hazzizalaskar, J.; Helary, G.; Sauvet, G. Biocidal polymers active by contact. IV. Polyurethanes based on polysiloxanes with pendant primary alcohols and quaternary ammonium groups. J. Appl. Polym. Sci. 1995, 58, 77–84. [Google Scholar] [CrossRef]
- Sauvet, G.; Fortuniak, W.; Kazmierski, K.; Chojnowski, J. Amphiphilic block and statistical siloxane copolymers with antimicrobial activity. J. Appl. Polym. Sci. 2003, 41, 2939–2948. [Google Scholar] [CrossRef]
- Ikeda, T.; Hirayama, H.; Suzuki, K.; Yamaguchi, H.; Tazuke, S. Biologically-active polycations. 6. Polymeric pyridinium salts with well-defined main chain structure. Macromol. Chem. 1986, 187, 333–340. [Google Scholar] [CrossRef]
- Li, G.J.; Shen, J.R.; Zhu, Y.L. Study of pyridinium-type functional polymers. II. Antibacterial activity of soluble pyridinium-type polymers. J. Appl. Polym. Sci. 1998, 67, 1761–1768. [Google Scholar] [CrossRef]
- Sauvet, G.; Dupond, S.; Kazmierski, K.; Chojnowski, J. Biocidal polymers active by contact. V. Synthesis of polysiloxanes with biocidal activity. J. Appl. Polym. Sci. 2000, 75, 1005–1012. [Google Scholar] [CrossRef]
- Tashiro, T. Removal of Escherichia-coli from water by systems based on insoluble polystyrene-poly(ethylene glycol)s, polystyrene-polyethylenimines, and polystyrene-polyethylenepolyamines quaternized. J. Appl. Polym. Sci. 1991, 43, 1369–1377. [Google Scholar] [CrossRef]
- Augusta, S.; Gruber, H.F.; Streichsbier, F. Synthesis and antibacterial activity of immobilized quaternary ammonium-salts. J. Appl. Polym. Sci. 1994, 53, 1149–1163. [Google Scholar] [CrossRef]
- Fields, J.E.; Johnson, J.H. Air Purification Process. U.S. Patent 3,340,680, 1 February 1967. [Google Scholar]
- Destais, N.; Ades, D.; Sauvet, G. Synthesis, characterization and biocidal properties of epoxy resins containing quaternary ammonium salts. Polym. Bull. 2000, 44, 401–408. [Google Scholar] [CrossRef]
- Kenawy, E.R.; Abdel-Hay, F.I.; El-Raheem, A.; El-Shanshoury, R.; El-Newehy, M.H. Biologically active polymers: Synthesis and antimicrobial activity of modified glycidyl methacrylate polymers having a quaternary ammonium and phosphonium groups. J. Control. Release 1998, 50, 145–152. [Google Scholar] [CrossRef]
- Kenawy, E.R.; Abdel-Hay, F.I.; El-Shanshoury, A.E.R.R.; El-Newehy, M.H. Biologically active polymers. V. Synthesis and antimicrobial activity of modified poly(glycidyl methacrylate-co-2-hydroxyethyl methacrylate) derivatives with quaternary ammonium and phosphonium salts. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 2384–2393. [Google Scholar] [CrossRef]
- Kanazawa, A.; Ikeda, T.; Endo, T. Polymeric phosphonium salts as a novel class of cationic biocides. 8. Synergistic effect on antibacterial activity of polymeric phosphonium and ammonium-salts. J. Appl. Polym. Sci. 1994, 53, 1245–1249. [Google Scholar] [CrossRef]
- Kanazawa, A.; Ikeda, T.; Endo, T. Novel polycationic biocides-synthesis and antibacterial activity of polymeric phosphonium salts. J. Polym. Sci. Part A Polym. Chem. 1993, 31, 335–343. [Google Scholar] [CrossRef]
- Kanazawa, A.; Ikeda, T.; Endo, T. Polymeric phosphonium salts as a novel class of cationic biocides. 2. Effects of couter anion and molecular-weight on antibacterial activity of polymeric phosphonium salts. J. Polym. Sci. Part A Polym. Chem. 1993, 31, 1441–1447. [Google Scholar] [CrossRef]
- Kanazawa, A.; Ikeda, T.; Endo, T. Polymeric phosphonium salts as a novel class of cationic biocides. 9. Effect of side-chain length between main-chain and active group on antibacterial activity. J. Polym. Sci. Part A Polym. Chem. 1994, 32, 1997–2001. [Google Scholar] [CrossRef]
- Kanazawa, A.; Ikeda, T.; Endo, T. Polymeric phosphonium salts as a novel class of cationic biocides. 10. Antibacterial activity of filters incorporating phosphonium biocides. J. Appl. Polym. Sci. 1994, 54, 1305–1310. [Google Scholar] [CrossRef]
- Kanazawa, A.; Ikeda, T.; Endo, T. Polymeric phosphonium salts as a novel class of cationic biocides. 7. Synthesis and antibacterial activity of polymeric phosphonium salts and their model compounds containing long alkyl chains. J. Appl. Polym. Sci. 1994, 53, 1237–1244. [Google Scholar] [CrossRef]
- Timofeeva, L.; Kleshcheva, N. Antimicrobial polymers: Mechanism of action, factors of activity, and applications. Appl. Microbiol. Biotechnol. 2011, 89, 475–492. [Google Scholar] [CrossRef] [PubMed]
- Monsanto Company. Pharmaceutical Compositions. GB Patent 1,260,451, 19 January 1972. [Google Scholar]
- Patel, H.; Raval, D.A.; Madamwar, D.; Patel, S.R. Polymeric prodrug: Synthesis, release study and antimicrobial property of poly(styrene-co-maleic anhydride)-bound acriflavine. Angew. Macrom. Chem. 1998, 263, 25–30. [Google Scholar] [CrossRef]
- Jeong, J.H.; Byoun, Y.S.; Ko, S.B.; Lee, Y.S. Chemical modification of poly(styrene-alt-maleic anhydride) with antimicrobial 4-aminobenzoic acid and 4-hydroxybenzoic acid. J. Ind. Eng. Chem. 2001, 7, 310–315. [Google Scholar]
- Jeong, J.H.; Byoun, Y.S.; Lee, Y.S. Poly(styrene-alt-maleic anhydride)-4-aminophenol conjugate: Synthesis and antibacterial activity. React. Funcct. Polym. 2002, 50, 257–263. [Google Scholar] [CrossRef]
- Bierbaum, G. Antibiotische Peptide-Lantibiotika. Chemother. J. 1999, 8, 204–209. [Google Scholar]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Zasloff, M. Magainins, a class of antimicrobial peptides from xenopus skin-isolation, characterization of 2 active forms, and partial cdna sequence of a precursor. Proc. Natl. Acad. Sci. USA 1987, 84, 5449–5453. [Google Scholar] [CrossRef] [PubMed]
- Broekaert, W.F.; Terras, F.R.; Cammue, B.P.; Osborn, R.W. Plant Defensins: Novel Antimicrobial Peptides as Components of the Host Defense System. Plant Physiol. 1995, 108, 1353–1358. [Google Scholar] [CrossRef] [PubMed]
- Cornut, I.; Büttner, K.; Dasseux, J.L.; Dufourcq, J. The amphipathic α-helix concept: Application to the de novo design of ideally amphipathic Leu, Lys peptides with hemolytic activity higher than that of melittin. FEBS Lett. 1994, 349, 29–33. [Google Scholar] [CrossRef]
- Beven, L.; Castano, S.; Dufourcq, J.; Wieslander, A.; Wroblewski, H. The antibiotic activity of cationic linear amphipathic peptides: Lessons from the action of leucine/lysine copolymers on bacteria of the class Mollicutes. Eur. J. Biochem. 2003, 270, 2207–2217. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, K.; Caputo, G.A. Antimicrobial polymers as synthetic mimics of host-defense peptides. WIRE Nanomed. Nanobiotechnol. 2013, 5, 49–66. [Google Scholar] [CrossRef] [PubMed]
- Lienkamp, K.; Tew, G.N. Synthetic mimics of antimicrobial peptides (SMAMPs). Chem. Eur. J. 2009, 15, 11784–11800. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Rätzsch, M. The copolymerization of maleic anhydride with propene and isobutene. Macromol. Chem. 1986, 187, 1593–1596. [Google Scholar] [CrossRef]
- Nash, J.F.; Lin, T.-M. Resin Compositions and Method for Controlling Diarrhea. U.S. Patent 3,224,941, 21 December 1965. [Google Scholar]
- Komber, H. The 1H and 13C NMR spectra of alternating isobutene/maleic anhydride copolymer and the corresponding acid and sodium salt—A stereochemical analysis. Macromol. Chem. Phys. 1996, 197, 343–353. [Google Scholar] [CrossRef]
- Bortel, E.; Styslo, M. On the chemical communications of poly(maleic anhydride-co-isobutene) by means of hydrolysis, ammoniation or aminations. Macromol. Chem. Phys. 1990, 191, 2653–2662. [Google Scholar] [CrossRef]
- Bortel, E.; Styslo, M. On the structure of radically obtained maleic anhydride/C4-alkene copolymers. Macromol. Chem. Phys. 1988, 189, 1155–1165. [Google Scholar] [CrossRef]
- Kenawy, E.R. Biologically active polymers. Iv. Synthesis and antimicrobial activity of polymers containing 8-hydroxyquinoline moiety. J. Appl. Polym. Sci. 2001, 82, 1364–1374. [Google Scholar] [CrossRef]
- Iwabuchi, S.; Watanabe, Y.; Nakahira, T.; Kojima, K. Vinylhydroquinone. V. Tri-n-butylborane-initiated copolymerization of maleic anhydride and redox property of copolymers. J. Polym. Sci. Polym. Chem. Ed. 1979, 17, 1721–1726. [Google Scholar] [CrossRef]
- Szkudlarek, M.; Beginn, U.; Keul, H.; Möller, M. Synthesis of Terpolymers with Homogeneous Composition by Free Radical Copolymerization of Maleic Anhydride, Perfluorooctyl and Butyl or Dodecyl Methacrylates: Application of the Continuous Flow Monomer Addition Technique. Polymers 2017, 11, 610. [Google Scholar] [CrossRef]
- Kenawy, E.-R.; Sakran, M.A. Controlled release of polymer conjugated agrochemicals. System based on poly(methyl vinyl ether-alt-maleic anhydride). J. Appl. Polym. Sci. 2001, 80, 415–421. [Google Scholar] [CrossRef]
- Lee, W.-F.; Chen, Y.-M. Poly(sulfobetaine)s and corresponding cationic polymers. VIII. Synthesis and aqueous solution properties of a cationic poly(methyl iodide quaternized styrene-N,N-dimethylaminopropyl maleamidic acid)copolymer. J. Appl. Polym. Sci. 2000, 80, 1619–1626. [Google Scholar] [CrossRef]
- Kang, Y.; Seo, Y.-H.; Lee, C. Synthesis and Conductivity of PEGME Braneded Poly(ethylene-alt-maleimide) Based Solid Polymer Electrolyte. Bull. Korean Chem. Soc. 2000, 21, 241–244. [Google Scholar]
- Lee, S.S.; Ahn, T.O. Direct Polymer reaction of poly(styrene-co-maleic anhydride): Polymeric imidization. J. Appl. Polym. Sci. 1998, 71, 1187–1196. [Google Scholar] [CrossRef]
- Lee, W.-F.; Hwong, G.-Y. Polysulfobetaines and corresponding cationic polymers. IV. Synthesis and aqueous solution properties of cationic poly(MIQSDMAPM). J. Appl. Polym. Sci. 1996, 59, 599–608. [Google Scholar] [CrossRef]
- Lee, W.-F.; Huang, G.-Y. Polysulfobetaines and corresponding cationic polymers. VI. Synthesis and aqueous solution properties of cationic poly(methyl iodide quaternized acrylamide-N,N-dimthylaminopropylmaleimide copolymer) [poly(MIQADMAPM)]. J. Appl. Polym. Sci. 1996, 60, 187–199. [Google Scholar] [CrossRef]
- Lee, W.-F.; Chen, C.-F. Poly(sulfobetaine)s and corresponding cationic polymers. VII. Thermal degradation of copolymers derived from poly(acrylamide co-N,N-dimethylaminopropylmaleimide). J. Appl. Polym. Sci. 1997, 66, 95–103. [Google Scholar] [CrossRef]
- Rice, L.M.; Grogan, C.H.; Reid, E.E. Hypotensive Agents. III. Dialkylaminoalkyl Pyrrolydine Derivatives. J. Am. Chem. Soc. 1953, 75, 2261–2262. [Google Scholar] [CrossRef]
- Meng, Y.Z.; Tjong, S.C.; Hay, A.S.; Wang, S.J. Synthesis and proton conductivities of phosphonic acid containing poly-(arylene ether)s. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 3218–3226. [Google Scholar] [CrossRef]
- Sung, P.-H.; Chen, C.-Y.; Wu, S.-Y.; Huang, J.Y. Styrene/maleimide copolymer with stable second-order optical nonlinearity. J. Polym. Sci. Part A Polym. Chem. 1996, 34, 2189–2194. [Google Scholar] [CrossRef]
- Liu, Y.L.; Liu, Y.L.; Jeng, R.J.; Chiu, Y.-S. Triphenylphosphine oxide-based bismaleimide and poly(bismaleimide): Synthesis, characterization, and properties. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 1716–1725. [Google Scholar] [CrossRef]
- Wang, C.-S.; Hwang, H.-J. Investigation of bismaleimide containing naphthalene unit. II. Thermal behavior and properties of polymer. J. Polym. Sci. Part A Polym. Chem. 1996, 34, 1493–1500. [Google Scholar] [CrossRef]
- Alfaia, A.J.I.; Calado, A.R.T.; Reis, J.C.R. Quaternization reaction of aromatic heterocyclic imines in methanol—A case of strong anti-reactivity selectivity principle with isoselective temperature. Eur. J. Org. Chem. 2000, 2000, 3627–3631. [Google Scholar] [CrossRef]
- Johnson, T.W.; Klotz, I.M. Preparation and characterization of some derivatives of poly(ethyleneimine). Macromolecules 1974, 7, 149–153. [Google Scholar] [CrossRef]
- Bütün, V.; Armes, S.P.; Billingham, N.C. Selective quaternization of 2-(dimethylamino)ethyl methacrylate residues in tertiary amine methacrylate diblock copolymers. Macromolecules 2001, 34, 1148–1159. [Google Scholar] [CrossRef]
- Vogl, O.; Tirrell, D. Functional polymers with biologically active groups. J. Macromol. Sci. Chem. 1979, A13, 415–439. [Google Scholar] [CrossRef]
- Worley, S.D.; Sun, G. Biocidal Polymers. Trends Polym. Sci. 1996, 4, 364–370. [Google Scholar]
- Exley, E.E.; Pasley, L.C.; Sahukhal, G.S.; Abel, B.A.; Brown, T.D.; McCormick, C.L.; Heinhorst, S.; Koul, V.; Choudhary, V.; Elasri, M.O.; et al. Antimicrobial Peptide Mimicking Primary Amine and Guanidine Containing Methacrylamide Copolymers Prepared by Raft Polymerization. Biomacromolecules 2015, 16, 3845–3852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.; Heine, E.; Keusgen, N.; Keul, H.; Moeller, M. Synthesis and Characterization of Amphiphilic Monodisperse Compounds and Poly(ethylene imine)s: Influence of Their Microstructures on the Antimicrobial Properties. Biomacromolecules 2012, 13, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Kiss, E.; Heine, E.T.; Hill, K.; He, Y.C.; Keusgen, N.; Penzes, C.B.; Schnoller, D.; Gyulai, G.; Mendrek, A.; Keul, H.; et al. Membrane Affinity and Antibacterial Properties of Cationic Polyelectrolytes with Different Hydrophobicity. Macromol. Biosci. 2012, 12, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Roier, S.; Zingl, F.G.; Cakar, F.; Schild, S. Bacterial outer membrane vesicle biogenesis: A new mechanism and its implications. Microb. Cell 2016, 3, 257–259. [Google Scholar] [CrossRef] [PubMed]
- Manning, A.J.; Kuehn, M.J. Contribution of bacterial outer membrane vesicles to innate bacterial defense. BMC Microbiol. 2011, 11, 258–272. [Google Scholar] [CrossRef] [PubMed]
- Heredia, R.M.; Boeris, P.S.; Liffourrena, A.S.; Bergero, M.F.; López, G.A.; Lucchesi, G.I. Release of outer membrane vesicles in Pseudomonas putida as a response to stress caused by cationic surfactants. Microbiology 2016, 162, 813–822. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, I.A.; Kuehn, M.J. Stress-induced outer membrane vesicle production by Pseudomonas aeruginosa. J. Bacteriol. 2013, 195, 2971–2981. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, F.; Stöcker, C.; Gartzen, R.; Heine, E.; Keul, H.; Möller, M. Novel Antibacterial Polyglycidols: Relationship between Structure and Properties. Polymers 2018, 10, 96. [Google Scholar] [CrossRef]
- Sahl, H.-G.; Jack, R.W.; Bierbaum, G. Biosynthesis and biological activities of lantibiotics with unique post-translational modifications. Eur. J. Biochem. 1995, 230, 827–853. [Google Scholar] [CrossRef] [PubMed]
- Breukink, E.; de Kruijff, B. The lantibiotic Nisin, a special case or not? Biochim. Biophys. Acta 1999, 1462, 223–234. [Google Scholar] [CrossRef]
- Deutsches Institut für Normung. Medical Microbiology—Susceptibility Testing of Microbial Pathogens to Antimicrobial Agents—Part 7: Determination of the Minimum Bactericidal Concentration (MBC) with the Method of Microbouillondilution; Deutsches Institut für Normung: Berlin, Germany, 2009. [Google Scholar]
- Wang, Y.; Xu, J.; Zhang, Y.; Yan, H.; Liu, K. Antimicrobial and hemolytic activities of copolymers with cationic and hydrophobic groups: A comparison of block and random copolymers. Macromol. Biosci. 2011, 11, 1499–1504. [Google Scholar] [CrossRef] [PubMed]








| Composition | Carbon | Hydrogen | Oxygen |
|---|---|---|---|
| Calculated for C,H,O | 65.90 | 7.70 | 26.40 |
| Found | 65.54 | 7.85 | 26.61 |
| # | Composition | Carbon | Hydrogen | Nitrogen |
|---|---|---|---|---|
| #1 | Calculated for C15H28N2O3 | 64.30 | 9.80 | 9.80 |
| #2 | Calculated for C15H28N2O3·2H2O | 56.23 | 10.07 | 8.74 |
| #3 | Found | 56.18 | 9.73 | 9.78 |
| Solvent | C3 | C4 | C5 | C6 |
|---|---|---|---|---|
| Water | + | + | + | − |
| Methanol | + | − | + | − |
| Ethanol | + | − | + | − |
| Acetone | + | − | + | + |
| 2-Butanone | + | − | + | + |
| Ethyl acetate | + | − | − | − |
| THF | + | − | + | + |
| DMSO | + | + | + | − |
| DMF | + | + | + | − |
| Chloroform | + | − | − | + |
| # | Transition 1 (°C) | Transition 2 (°C) | Functional Group |
|---|---|---|---|
| C3 | 18 | 88 | -N(CH3)2 |
| C4 | 17 | 85 | -N(CH3)3+ |
| C5 | 17 | 104 | -N(CH3)3+ + -N(CH3)2 C12H25+ |
| C6 | 18 | 101 | -N(CH3)2 C12H25+ |
| Polymer | MIC100 in µg/mL | HC50 in µg/mL | |||
|---|---|---|---|---|---|
| E. coli ATCC 23716 | P. aeruginosa ATCC27853 | S. aureus ATCC 6538 | S. epidermidis ATCC 12228 | ||
| C3 | 20 | 100 | >1000 | 20 | 100 * |
| C4 | 200 | >>1000 | 1000 | 20 | >>1000 ** |
| C5 | 10 | 200 | 100 | 20 | 60 # |
| NISIN | 100 | >200 | 3 | 3 | >>340 + |
| # | DMF (g) | Ac2O (g) | MEK (g) | Et3N (g) | CH3COONa (g) | T (°C) | t (h) | Yield % | Appearance |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 15 | 15 | - | 0.1 | - | RT | 12 | 88 | brown powder |
| 2 | 15 | 15 | - | 0.1 | - | 80 | 18 | 70 | brown powder |
| 3 | 20 | 1.07 | - | 1.41 | - | 80 | 24 | 62 | brown, tarry |
| 4 | - | 1.0 | 15 | 0.8 | - | 90 | 18 | 87 | grey powder |
| 5 | - | 0.55 | 15 | 0.73 | - | 90 | 12 | 90 | grey powder |
| 6 | - | 7 | - | 1 | - | 80 | 20 | 78 | grey powder |
| 7 | - | 10 | - | - | 0.1 | 80 | 18 | 69 | brown powder |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szkudlarek, M.; Heine, E.; Keul, H.; Beginn, U.; Möller, M. Synthesis, Characterization, and Antimicrobial Properties of Peptides Mimicking Copolymers of Maleic Anhydride and 4-Methyl-1-pentene. Int. J. Mol. Sci. 2018, 19, 2617. https://doi.org/10.3390/ijms19092617
Szkudlarek M, Heine E, Keul H, Beginn U, Möller M. Synthesis, Characterization, and Antimicrobial Properties of Peptides Mimicking Copolymers of Maleic Anhydride and 4-Methyl-1-pentene. International Journal of Molecular Sciences. 2018; 19(9):2617. https://doi.org/10.3390/ijms19092617
Chicago/Turabian StyleSzkudlarek, Marian, Elisabeth Heine, Helmut Keul, Uwe Beginn, and Martin Möller. 2018. "Synthesis, Characterization, and Antimicrobial Properties of Peptides Mimicking Copolymers of Maleic Anhydride and 4-Methyl-1-pentene" International Journal of Molecular Sciences 19, no. 9: 2617. https://doi.org/10.3390/ijms19092617
APA StyleSzkudlarek, M., Heine, E., Keul, H., Beginn, U., & Möller, M. (2018). Synthesis, Characterization, and Antimicrobial Properties of Peptides Mimicking Copolymers of Maleic Anhydride and 4-Methyl-1-pentene. International Journal of Molecular Sciences, 19(9), 2617. https://doi.org/10.3390/ijms19092617