From Implantation to Birth: Insight into Molecular Melatonin Functions
Abstract
:1. Introduction
2. Molecular Mechanisms of Embryo Implantation
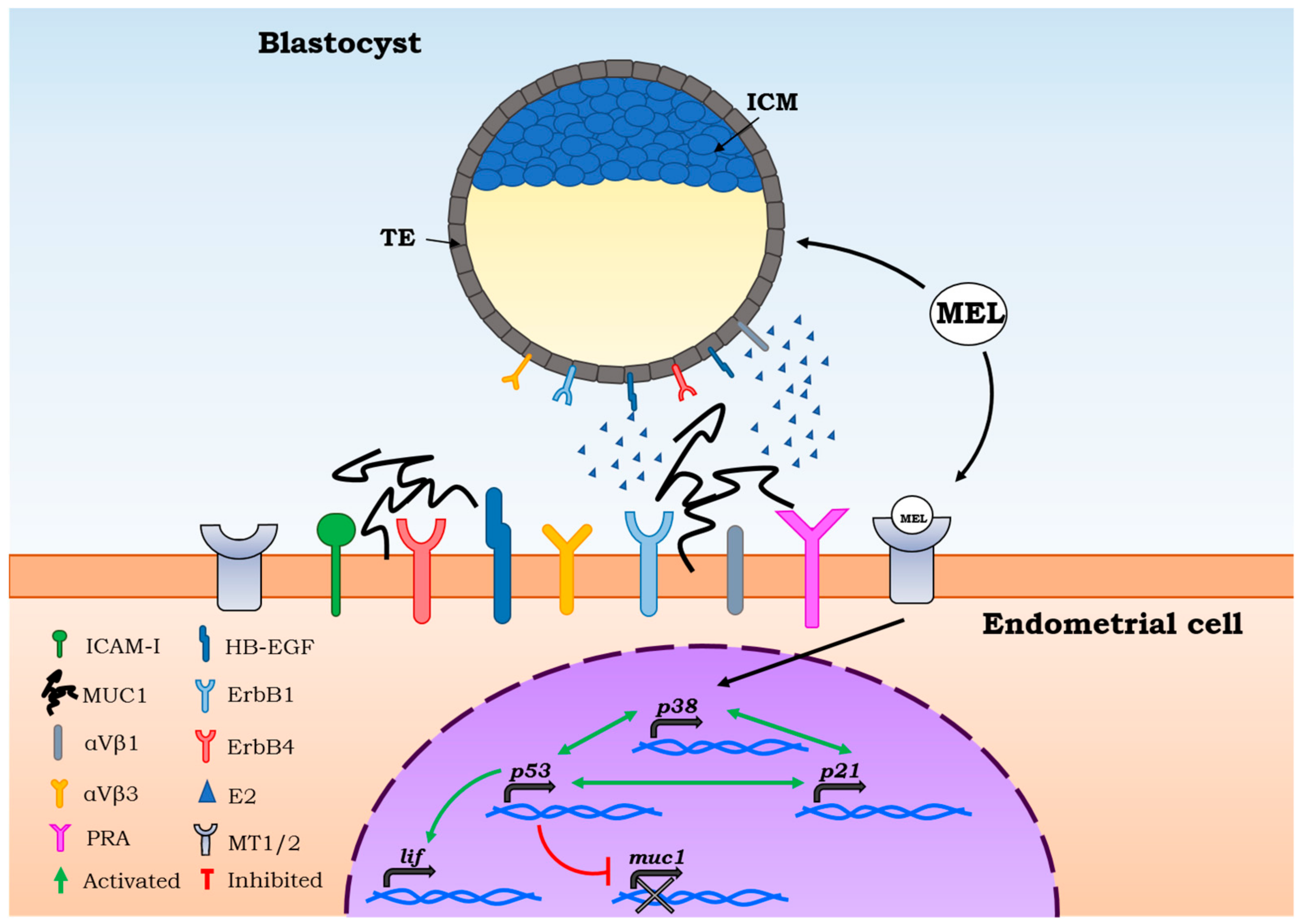
3. Melatonin Functions on Oocyte Quality and Embryo Implantation
4. Melatonin Functions on Pregnancy Outcomes
5. Conclusions
Author Contributions
Conflicts of Interest
Abbreviations
| CAT | Catalase |
| CL | Corpus luteum |
| CNS | central nervous system |
| E2 | Oestradiol |
| GnRH | Gonadotropin-releasing hormone |
| GSH | Glutathione |
| HB-EGF | Heparin binding epidermal growth factor |
| ICAM-I | Intracellular adhesion molecule |
| ICM | Internal cell mass |
| LH | Luteinizing hormone |
| MUC1 | Mucin |
| NaF | Sodium fluoride |
| P4 | Progesterone |
| ROS | Reactive oxygen species |
| SIRT | Sirtuin |
| SOD | Superoxide dismutase |
| TE | Trophectoderm |
References
- Reiter, R.J. The melatonin rhythm: Both a clock and a calendar. Experientia 1993, 49, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J. Melatonin and human reproduction. Ann. Med. 1998, 30, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Mahal, H.S.; Sharma, H.S.; Mukherjee, T. Antioxidant properties of melatonin: A pulse radiolysis study. Free Radic. Biol. Med. 1999, 26, 557–565. [Google Scholar] [CrossRef]
- Garcia, J.J.; Lopez-Pingarron, L.; Almeida-Souza, P.; Tres, A.; Escudero, P.; Garcia-Gil, F.A.; Tan, D.X.; Reiter, R.J.; Ramirez, J.M.; Bernal-Perez, M. Protective effects of melatonin in reducing oxidative stress and in preserving the fluidity of biological membranes: A review. J. Pineal Res. 2014, 56, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Ramis, M.R.; Esteban, S.; Miralles, A.; Tan, D.X.; Reiter, R.J. Protective effects of melatonin and mitochondria-targeted antioxidants against oxidative stress: A review. Curr. Med. Chem. 2015, 22, 2690–2711. [Google Scholar] [CrossRef] [PubMed]
- Barrett, P.; Bolborea, M. Molecular pathways involved in seasonal body weight and reproductive responses governed by melatonin. J. Pineal Res. 2012, 52, 376–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dijk, D.J.; Duffy, J.F. Circadian regulation of human sleep and age-related changes in its timing, consolidation and eeg characteristics. Ann. Med. 1999, 31, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Van Someren, E.J. Circadian rhythms and sleep in human aging. Chronobiol. Int. 2000, 17, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Copinschi, G.; Van Cauter, E. Effects of ageing on modulation of hormonal secretions by sleep and circadian rhythmicity. Horm. Res. 1995, 43, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, F.J.; Torres-Farfan, C.; Richter, H.G.; Mendez, N.; Campino, C.; Torrealba, F.; Valenzuela, G.J.; Seron-Ferre, M. Clock gene expression in adult primate suprachiasmatic nuclei and adrenal: Is the adrenal a peripheral clock responsive to melatonin? Endocrinology 2008, 149, 1454–1461. [Google Scholar] [CrossRef] [PubMed]
- Silver, A.C.; Arjona, A.; Walker, W.E.; Fikrig, E. The circadian clock controls toll-like receptor 9-mediated innate and adaptive immunity. Immunity 2012, 36, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tamura, H.; Tan, D.X.; Xu, X.Y. Melatonin and the circadian system: Contributions to successful female reproduction. Fertil. Steril. 2014, 102, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Summa, K.C.; Vitaterna, M.H.; Turek, F.W. Environmental perturbation of the circadian clock disrupts pregnancy in the mouse. PLoS ONE 2012, 7, e37668. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Korkmaz, A.; Rosales-Corral, S.A. Melatonin and stable circadian rhythms optimize maternal, placental and fetal physiology. Hum. Reprod. Update 2014, 20, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Okatani, Y.; Okamoto, K.; Hayashi, K.; Wakatsuki, A.; Tamura, S.; Sagara, Y. Maternal-fetal transfer of melatonin in pregnant women near term. J. Pineal Res. 1998, 25, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, D.K.; Ge, Y.W.; Sharman, E.H.; Bondy, S.C. Age-related changes in serum melatonin in mice: Higher levels of combined melatonin and 6-hydroxymelatonin sulfate in the cerebral cortex than serum, heart, liver and kidney tissues. J. Pineal Res. 2004, 36, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Handyside, A.H.; Montag, M.; Magli, M.C.; Repping, S.; Harper, J.; Schmutzler, A.; Vesela, K.; Gianaroli, L.; Geraedts, J. Multiple meiotic errors caused by predivision of chromatids in women of advanced maternal age undergoing in vitro fertilisation. Eur. J. Hum. Genet. 2012, 20, 742–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Geng, X.; Zheng, W.; Tang, J.; Xu, B.; Shi, Q. Current understanding of ovarian aging. Sci. China Life Sci. 2012, 55, 659–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grondahl, M.L.; Yding Andersen, C.; Bogstad, J.; Nielsen, F.C.; Meinertz, H.; Borup, R. Gene expression profiles of single human mature oocytes in relation to age. Hum. Reprod. 2010, 25, 957–968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jasmin; Peters, V.M.; Spray, D.C.; Mendez-Otero, R. Effect of mesenchymal stem cells and mouse embryonic fibroblasts on the development of preimplantation mouse embryos. In Vitro Cell Dev. Biol. Anim. 2016, 52, 497–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moniruzzaman, M.; Hasan, K.N.; Maitra, S.K. Melatonin actions on ovaprim (synthetic gnrh and domperidone)-induced oocyte maturation in carp. Reproduction 2016, 151, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Xie, L.; Ma, T.; Lv, D.; Jing, W.; Tian, X.; Song, Y.; Liu, Z.; Xiao, X.; Liu, G. Effects of melatonin on early pregnancy in mouse: Involving the regulation of star, cyp11a1, and ihh expression. Int. J. Mol. Sci. 2017, 18, 1637. [Google Scholar] [CrossRef] [PubMed]
- Paria, B.C.; Reese, J.; Das, S.K.; Dey, S.K. Deciphering the cross-talk of implantation: Advances and challenges. Science 2002, 296, 2185–2188. [Google Scholar] [CrossRef] [PubMed]
- Bergh, P.A.; Navot, D. The impact of embryonic development and endometrial maturity on the timing of implantation. Fertil. Steril. 1992, 58, 537–542. [Google Scholar] [CrossRef]
- Hertig, A.T.; Rock, J.; Adams, E.C. A description of 34 human ova within the first 17 days of development. Am. J. Anat. 1956, 98, 435–493. [Google Scholar] [CrossRef] [PubMed]
- Psychoyos, A. Uterine receptivity for nidation. Ann. N. Y. Acad. Sci. 1986, 476, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.J.; Tomasini, R.; McKeon, F.D.; Mak, T.W.; Melino, G. The p53 family: Guardians of maternal reproduction. Nat. Rev. Mol. Cell Biol. 2011, 12, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Aplin, J.D. The cell biological basis of human implantation. Baillieres Best Pract. Res. Clin. Obstet. Gynaecol. 2000, 14, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Aplin, J.D.; Hey, N.A.; Graham, R.A. Human endometrial muc1 carries keratan sulfate: Characteristic glycoforms in the luminal epithelium at receptivity. Glycobiology 1998, 8, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, L.H.; Olson, G.E.; Carson, D.D.; Chilton, B.S. Progesterone and implanting blastocysts regulate muc1 expression in rabbit uterine epithelium. Endocrinology 1998, 139, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Qu, C.; Sun, Q.; Wu, L.; Liu, Y.; Yang, Z.; Zhang, J. Sophoricoside fails the embryo implantation by compromising the uterine endometrial receptivity at implantation “window” of pregnant mice. Chem. Biol. Interact. 2014, 219, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Kimber, S.J. Carbohydrates and implantation of the mammalian embryo. In Endocrinology of Embryo-Endometrium Interactions; Glasser, S.R., Mulholland, J., Psychoyos, A., Eds.; Springer: Boston, MA, USA, 1994; pp. 279–296. [Google Scholar]
- Tabibzadeh, S.; Babaknia, A. The signals and molecular pathways involved in implantation, a symbiotic interaction between blastocyst and endometrium involving adhesion and tissue invasion. Hum. Reprod. 1995, 10, 1579–1602. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.; Swann, H.R.; Seif, M.W.; Kimber, S.J.; Aplin, J.D. Cell adhesion molecules on the oocyte and preimplantation human embryo. Hum. Reprod. 1995, 10, 1571–1578. [Google Scholar] [CrossRef] [PubMed]
- Schultz, J.F.; Mayernik, L.; Rout, U.K.; Armant, D.R. Integrin trafficking regulates adhesion to fibronectin during differentiation of mouse peri-implantation blastocysts. Dev. Genet. 1997, 21, 31–43. [Google Scholar] [CrossRef]
- Sharkey, A.M.; Smith, S.K. The endometrium as a cause of implantation failure. Best Pract. Res. Clin. Obstet. Gynaecol. 2003, 17, 289–307. [Google Scholar] [CrossRef]
- De Mouzon, J.; Rossin-Amar, B.; Bachelot, A.; Renon, C.; Devecchi, A. Fivnat. Influence of attempt rank in in vitro fertilization. Contracept. Fertil. Sex. 1998, 26, 466–472. [Google Scholar] [PubMed]
- Favetta, L.A.; St John, E.J.; King, W.A.; Betts, D.H. High levels of p66shc and intracellular ros in permanently arrested early embryos. Free Radic. Biol. Med. 2007, 42, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Lysiak, J.J.; Zheng, S.; Woodson, R.; Turner, T.T. Caspase-9-dependent pathway to murine germ cell apoptosis: Mediation by oxidative stress, bax, and caspase 2. Cell Tissue Res. 2007, 328, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Belsham, D.D. Melatonin receptor activation regulates gnrh gene expression and secretion in gt1-7 gnrh neurons. Signal transduction mechanisms. J. Biol. Chem. 2002, 277, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.A.; Silavin, S.L.; Wentworth, R.A.; Figueroa, J.P.; Honnebier, B.O.; Fishburne, J.I., Jr.; Nathanielsz, P.W. Different patterns of myometrial activity and 24-h rhythms in myometrial contractility in the gravid baboon during the second half of pregnancy. Biol. Reprod. 1992, 46, 1158–1164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, H.; Lee, O.H.; Lee, Y.; Yoon, H.; Chang, E.M.; Park, M.; Lee, J.W.; Hong, K.; Kim, J.O.; Kim, N.K.; et al. Melatonin prevents cisplatin-induced primordial follicle loss via suppression of pten/akt/foxo3a pathway activation in the mouse ovary. J. Pineal Res. 2016, 60, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Na, Y.; Hong, K.; Lee, S.; Moon, S.; Cho, M.; Park, M.; Lee, O.H.; Chang, E.M.; Lee, D.R.; et al. Synergistic effect of melatonin and ghrelin in preventing cisplatin-induced ovarian damage via regulation of foxo3a phosphorylation and binding to the p27(kip1) promoter in primordial follicles. J. Pineal Res. 2017, 63, e12432. [Google Scholar] [CrossRef] [PubMed]
- Brannstrom, M.; Norman, R.J. Involvement of leukocytes and cytokines in the ovulatory process and corpus luteum function. Hum. Reprod. 1993, 8, 1762–1775. [Google Scholar] [CrossRef] [PubMed]
- Ronnberg, L.; Kauppila, A.; Leppaluoto, J.; Martikainen, H.; Vakkuri, O. Circadian and seasonal variation in human preovulatory follicular fluid melatonin concentration. J. Clin. Endocrinol. MeTable 1990, 71, 492–496. [Google Scholar] [CrossRef]
- Tamura, H.; Takasaki, A.; Miwa, I.; Taniguchi, K.; Maekawa, R.; Asada, H.; Taketani, T.; Matsuoka, A.; Yamagata, Y.; Shimamura, K.; et al. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J. Pineal Res. 2008, 44, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Salhab, M.; Dhorne-Pollet, S.; Auclair, S.; Guyader-Joly, C.; Brisard, D.; Dalbies-Tran, R.; Dupont, J.; Ponsart, C.; Mermillod, P.; Uzbekova, S. In vitro maturation of oocytes alters gene expression and signaling pathways in bovine cumulus cells. Mol. Reprod. Dev. 2013, 80, 166–182. [Google Scholar] [CrossRef] [PubMed]
- Adriaens, I.; Jacquet, P.; Cortvrindt, R.; Janssen, K.; Smitz, J. Melatonin has dose-dependent effects on folliculogenesis, oocyte maturation capacity and steroidogenesis. Toxicology 2006, 228, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Bronson, F.H. Seasonal variation in human reproduction: Environmental factors. Q. Rev. Biol. 1995, 70, 141–164. [Google Scholar] [CrossRef] [PubMed]
- Partonen, T. Short note: Melatonin-dependent infertility. Med. Hypotheses 1999, 52, 487–488. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M. Oxidative stress and redox regulation on in vitro development of mammalian embryos. J. Reprod. Dev. 2012, 58, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Han, H.B.; Tian, X.Z.; Tan, D.X.; Wang, L.; Zhou, G.B.; Zhu, S.E.; Liu, G.S. Melatonin promotes embryonic development and reduces reactive oxygen species in vitrified mouse 2-cell embryos. J. Pineal Res. 2012, 52, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Chuffa, L.G.; Seiva, F.R.; Favaro, W.J.; Teixeira, G.R.; Amorim, J.P.; Mendes, L.O.; Fioruci, B.A.; Pinheiro, P.F.; Fernandes, A.A.; Franci, J.A.; et al. Melatonin reduces lh, 17 beta-estradiol and induces differential regulation of sex steroid receptors in reproductive tissues during rat ovulation. Reprod. Biol. Endocrinol. 2011, 9, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Z.; Wang, F.; Tian, X.; Ji, P.; Liu, G. Effects of melatonin administration on embryo implantation and offspring growth in mice under different schedules of photoperiodic exposure. Reprod. Biol. Endocrinol. 2017, 15, 78. [Google Scholar] [CrossRef] [PubMed]
- Asgari, Z.; Ghasemian, F.; Ramezani, M.; Bahadori, M.H. The effect of melatonin on the developmental potential and implantation rate of mouse embryos. Cell J. 2012, 14, 203–208. [Google Scholar] [PubMed]
- Ma, W.G.; Song, H.; Das, S.K.; Paria, B.C.; Dey, S.K. Estrogen is a critical determinant that specifies the duration of the window of uterine receptivity for implantation. Proc. Natl. Acad. Sci. USA 2003, 100, 2963–2968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richter, H.G.; Hansell, J.A.; Raut, S.; Giussani, D.A. Melatonin improves placental efficiency and birth weight and increases the placental expression of antioxidant enzymes in undernourished pregnancy. J. Pineal Res. 2009, 46, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Mediavilla, M.D.; Cos, S.; Sanchez-Barcelo, E.J. Melatonin increases p53 and p21waf1 expression in mcf-7 human breast cancer cells in vitro. Life Sci. 1999, 65, 415–420. [Google Scholar] [CrossRef]
- Santoro, R.; Mori, F.; Marani, M.; Grasso, G.; Cambria, M.A.; Blandino, G.; Muti, P.; Strano, S. Blockage of melatonin receptors impairs p53-mediated prevention of DNA damage accumulation. Carcinogenesis 2013, 34, 1051–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proietti, S.; Cucina, A.; Dobrowolny, G.; D’Anselmi, F.; Dinicola, S.; Masiello, M.G.; Pasqualato, A.; Palombo, A.; Morini, V.; Reiter, R.J.; et al. Melatonin down-regulates mdm2 gene expression and enhances p53 acetylation in mcf-7 cells. J. Pineal Res. 2014, 57, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Fu, B.; Peng, W.; Mao, T.; Wu, H.; Zhang, Y. Melatonin protect the development of preimplantation mouse embryos from sodium fluoride-induced oxidative injury. Environ. Toxicol. Pharmacol. 2017, 54, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Dey, S.K. Roadmap to embryo implantation: Clues from mouse models. Nat. Rev. Genet. 2006, 7, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Ishizuka, B.; Kuribayashi, Y.; Murai, K.; Amemiya, A.; Itoh, M.T. The effect of melatonin on in vitro fertilization and embryo development in mice. J. Pineal Res. 2000, 28, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Moshkdanian, G.; Moghani-Ghoroghi, F.; Pasbakhsh, P.; Nematollahi-Mahani, S.N.; Najafi, A.; Kashani, S.R. Melatonin upregulates erbb1 and erbb4, two primary implantation receptors, in pre-implantation mouse embryos. Iran. J. Basic. Med. Sci. 2017, 20, 655–661. [Google Scholar] [PubMed]
- Choi, J.; Park, S.M.; Lee, E.; Kim, J.H.; Jeong, Y.I.; Lee, J.Y.; Park, S.W.; Kim, H.S.; Hossein, M.S.; Jeong, Y.W.; et al. Anti-apoptotic effect of melatonin on preimplantation development of porcine parthenogenetic embryos. Mol. Reprod. Dev. 2008, 75, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Sturmey, R.G.; Reis, A.; Leese, H.J.; McEvoy, T.G. Role of fatty acids in energy provision during oocyte maturation and early embryo development. Reprod. Domest. Anim. 2009, 44, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Takasaki, A.; Taketani, T.; Tanabe, M.; Kizuka, F.; Lee, L.; Tamura, I.; Maekawa, R.; Aasada, H.; Yamagata, Y.; et al. The role of melatonin as an antioxidant in the follicle. J. Ovarian Res. 2012, 5, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bharti, V.K.; Srivastava, R.S.; Kumar, H.; Bag, S.; Majumdar, A.C.; Singh, G.; Pandi-Perumal, S.R.; Brown, G.M. Effects of melatonin and epiphyseal proteins on fluoride-induced adverse changes in antioxidant status of heart, liver, and kidney of rats. Adv. Pharmacol. Sci. 2014, 2014, 532969. [Google Scholar] [CrossRef] [PubMed]
- Mohseni, M.; Mihandoost, E.; Shirazi, A.; Sepehrizadeh, Z.; Bazzaz, J.T.; Ghazi-khansari, M. Melatonin may play a role in modulation of bax and bcl-2 expression levels to protect rat peripheral blood lymphocytes from gamma irradiation-induced apoptosis. Mutat. Res. 2012, 738, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Buyukavci, M.; Ozdemir, O.; Buck, S.; Stout, M.; Ravindranath, Y.; Savasan, S. Melatonin cytotoxicity in human leukemia cells: Relation with its pro-oxidant effect. Fundam. Clin. Pharmacol. 2006, 20, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Macias, M.; Escames, G.; Leon, J.; Acuna-Castroviejo, D. Melatonin but not vitamins c and e maintains glutathione homeostasis in t-butyl hydroperoxide-induced mitochondrial oxidative stress. FASEB J. 2000, 14, 1677–1679. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Melatonin and the theories of aging: A critical appraisal of melatonin’s role in antiaging mechanisms. J. Pineal Res. 2013, 55, 325–356. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, S.; Sozen, B.; Demir, N. Telomere length and telomerase activity during oocyte maturation and early embryo development in mammalian species. Mol. Hum. Reprod. 2014, 20, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Tatone, C.; Di Emidio, G.; Vitti, M.; Di Carlo, M.; Santini, S., Jr.; D’Alessandro, A.M.; Falone, S.; Amicarelli, F. Sirtuin functions in female fertility: Possible role in oxidative stress and aging. Oxid. Med. Cell Longev. 2015, 2015, 659687. [Google Scholar] [CrossRef] [PubMed]
- Watroba, M.; Szukiewicz, D. The role of sirtuins in aging and age-related diseases. Adv. Med. Sci. 2016, 61, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fang, L.; Lu, Z.; Xiong, J.; Wu, M.; Shi, L.; Luo, A.; Wang, S. Are sirtuins markers of ovarian aging? Gene 2016, 575, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, H.S.; Reizis, B.; Robbins, P.D. Sirt1 associates with eif2-alpha and regulates the cellular stress response. Sci. Rep. 2011, 1, 150. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.C.; Yu, M.S.; Lai, C.S. Significance of molecular signaling for protein translation control in neurodegenerative diseases. Neurosignals 2006, 15, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Wang, F.; Zhang, L.; Ji, P.; Wang, J.; Lv, D.; Li, G.; Chai, M.; Lian, Z.; Liu, G. Melatonin promotes the in vitro development of microinjected pronuclear mouse embryos via its anti-oxidative and anti-apoptotic effects. Int. J. Mol. Sci. 2017, 18, 988. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.M.; Robaire, B.; Hales, B.F. Paternal cyclophosphamide treatment causes postimplantation loss via inner cell mass-specific cell death. Teratology 1992, 45, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Shiao, N.H.; Chan, W.H. Injury effects of ginkgolide b on maturation of mouse oocytes, fertilization, and fetal development in vitro and in vivo. Toxicol. Lett. 2009, 188, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.; Jung, S.; Bazer, F.W.; Song, G.; Kim, J. Epidermal growth factor: Porcine uterine luminal epithelial cell migratory signal during the peri-implantation period of pregnancy. Mol. Cell Endocrinol. 2016, 420, 66–74. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Wang, J.; Li, Y.; Zhu, K.; Xu, Z.; Song, Y.; Song, Y.; Liu, G. Melatonin-related genes expressed in the mouse uterus during early gestation promote embryo implantation. J. Pineal Res. 2015, 58, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Tian, X.; Zhou, Y.; Tan, D.; Zhu, S.; Dai, Y.; Liu, G. Melatonin improves the quality of in vitro produced (ivp) bovine embryos: Implications for blastocyst development, cryotolerance, and modifications of relevant gene expression. PLoS ONE 2014, 9, e93641. [Google Scholar] [CrossRef] [PubMed]
- Moghani-Ghoroghi, F.; Moshkdanian, G.; Sehat, M.; Nematollahi-Mahani, S.N.; Ragerdi-Kashani, I.; Pasbakhsh, P. Melatonin pretreated blastocysts along with calcitonin administration improved implantation by upregulation of heparin binding-epidermal growth factor expression in murine endometrium. Cell J. 2018, 19, 599–606. [Google Scholar] [PubMed]
- Wang, F.; Tian, X.; Zhang, L.; Tan, D.; Reiter, R.J.; Liu, G. Melatonin promotes the in vitro development of pronuclear embryos and increases the efficiency of blastocyst implantation in murine. J. Pineal Res. 2013, 55, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, P.; Raffone, E.; Benedetto, V. Effect of the treatment with myo-inositol plus folic acid plus melatonin in comparison with a treatment with myo-inositol plus folic acid on oocyte quality and pregnancy outcome in ivf cycles. A prospective, clinical trial. Eur. Rev. Med. Pharmacol. Sci. 2010, 14, 555–561. [Google Scholar] [PubMed]
- Unfer, V.; Raffone, E.; Rizzo, P.; Buffo, S. Effect of a supplementation with myo-inositol plus melatonin on oocyte quality in women who failed to conceive in previous in vitro fertilization cycles for poor oocyte quality: A prospective, longitudinal, cohort study. Gynecol. Endocrinol. 2011, 27, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Pacchiarotti, A.; Carlomagno, G.; Antonini, G.; Pacchiarotti, A. Effect of myo-inositol and melatonin versus myo-inositol, in a randomized controlled trial, for improving in vitro fertilization of patients with polycystic ovarian syndrome. Gynecol. Endocrinol. 2016, 32, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Seron-Ferre, M.; Torres-Farfan, C.; Forcelledo, M.L.; Valenzuela, G.J. The development of circadian rhythms in the fetus and neonate. Semin. Perinatol. 2001, 25, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Kivela, A.; Kauppila, A.; Leppaluoto, J.; Vakkuri, O. Serum and amniotic fluid melatonin during human labor. J. Clin. Endocrinol. MeTable 1989, 69, 1065–1068. [Google Scholar] [CrossRef] [PubMed]
- Mirmiran, M.; Maas, Y.G.; Ariagno, R.L. Development of fetal and neonatal sleep and circadian rhythms. Sleep Med. Rev. 2003, 7, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Mendez, N.; Abarzua-Catalan, L.; Vilches, N.; Galdames, H.A.; Spichiger, C.; Richter, H.G.; Valenzuela, G.J.; Seron-Ferre, M.; Torres-Farfan, C. Timed maternal melatonin treatment reverses circadian disruption of the fetal adrenal clock imposed by exposure to constant light. PLoS ONE 2012, 7, e42713. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.; Purvis, C.C.; Drew, J.E.; Abramovich, D.R.; Williams, L.M. Melatonin receptors in human fetal brain: 2-[(125)i]iodomelatonin binding and mt1 gene expression. J. Pineal Res. 2002, 33, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Torres-Farfan, C.; Rocco, V.; Monso, C.; Valenzuela, F.J.; Campino, C.; Germain, A.; Torrealba, F.; Valenzuela, G.J.; Seron-Ferre, M. Maternal melatonin effects on clock gene expression in a nonhuman primate fetus. Endocrinology 2006, 147, 4618–4626. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, G.A.; Meyers, D.A.; Bleecker, E.R.; Pack, A.I. Identification of coding polymorphisms in human circadian rhythm genes per1, per2, per3, clock, arntl, cry1, cry2 and timeless in a multi-ethnic screening panel. DNA Seq. 2008, 19, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Hastings, M.H. Circadian clockwork: Two loops are better than one. Nat. Rev. Neurosci. 2000, 1, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Reppert, S.M.; Weaver, D.R. Coordination of circadian timing in mammals. Nature 2002, 418, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Drew, J.E.; Williams, L.M.; Hannah, L.T.; Barrett, P.; Abramovich, D.R. Melatonin receptors in the human fetal kidney: 2-[125i]iodomelatonin binding sites correlated with expression of mel1a and mel1b receptor genes. J. Endocrinol. 1998, 156, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Torres-Farfan, C.; Richter, H.G.; Germain, A.M.; Valenzuela, G.J.; Campino, C.; Rojas-Garcia, P.; Forcelledo, M.L.; Torrealba, F.; Seron-Ferre, M. Maternal melatonin selectively inhibits cortisol production in the primate fetal adrenal gland. J. Physiol. 2004, 554, 841–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunduz, B.; Stetson, M.H. Effects of photoperiod, pinealectomy, and melatonin implants on testicular development in juvenile siberian hamsters (phodopus sungorus). Biol. Reprod. 1994, 51, 1181–1187. [Google Scholar] [CrossRef] [PubMed]
- Shaw, D.; Goldman, B.D. Developmental changes in male siberian hamsters (phodopus sungorus) exposed to different gestational and postnatal photoperiods. J. Pineal Res. 2007, 43, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Waddell, B.J.; Wharfe, M.D.; Crew, R.C.; Mark, P.J. A rhythmic placenta? Circadian variation, clock genes and placental function. Placenta 2012, 33, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, S.; Nakazawa, K.; Sakai, J.; Kometani, K.; Iwashita, M.; Yoshimura, Y.; Maruyama, T. Melatonin as a local regulator of human placental function. J. Pineal Res. 2005, 39, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Lanoix, D.; Beghdadi, H.; Lafond, J.; Vaillancourt, C. Human placental trophoblasts synthesize melatonin and express its receptors. J. Pineal Res. 2008, 45, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Sainz, R.M.; Mayo, J.C.; Rodriguez, C.; Tan, D.X.; Lopez-Burillo, S.; Reiter, R.J. Melatonin and cell death: Differential actions on apoptosis in normal and cancer cells. Cell Mol. Life Sci. 2003, 60, 1407–1426. [Google Scholar] [CrossRef] [PubMed]
- Lanoix, D.; Lacasse, A.A.; Reiter, R.J.; Vaillancourt, C. Melatonin: The smart killer: The human trophoblast as a model. Mol. Cell Endocrinol. 2012, 348, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gitto, E.; Marseglia, L.; Manti, S.; D’Angelo, G.; Barberi, I.; Salpietro, C.; Reiter, R.J. Protective role of melatonin in neonatal diseases. Oxid. Med. Cell Longev. 2013, 2013, 980374. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, M.J.; Duntley, S.P.; Anch, A.M.; Nonneman, R. Active sleep and its role in the prevention of apoptosis in the developing brain. Med. Hypotheses 2004, 62, 876–879. [Google Scholar] [CrossRef] [PubMed]
- Cajochen, C.; Krauchi, K.; Mori, D.; Graw, P.; Wirz-Justice, A. Melatonin and s-20098 increase rem sleep and wake-up propensity without modifying nrem sleep homeostasis. Am. J. Physiol. 1997, 272, R1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Supramaniam, V.G.; Jenkin, G.; Loose, J.; Wallace, E.M.; Miller, S.L. Chronic fetal hypoxia increases activin a concentrations in the late-pregnant sheep. BJOG 2006, 113, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Nakamura, Y.; Terron, M.P.; Flores, L.J.; Manchester, L.C.; Tan, D.X.; Sugino, N.; Reiter, R.J. Melatonin and pregnancy in the human. Reprod. Toxicol. 2008, 25, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Kawamoto, M.; Sato, S.; Tamura, I.; Maekawa, R.; Taketani, T.; Aasada, H.; Takaki, E.; Nakai, A.; Reiter, R.J.; et al. Long-term melatonin treatment delays ovarian aging. J. Pineal Res. 2017, 62, e12381. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.; Winter, P.D.; Osmond, C.; Margetts, B.; Simmonds, S.J. Weight in infancy and death from ischaemic heart disease. Lancet 1989, 2, 577–580. [Google Scholar] [CrossRef]
- Ribatti, D.; Nico, B.; Crivellato, E. Morphological and molecular aspects of physiological vascular morphogenesis. Angiogenesis 2009, 12, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, A.; Sanchez-Barcelo, E.J.; Tan, D.X.; Reiter, R.J. Role of melatonin in the epigenetic regulation of breast cancer. Breast Cancer Res. Treat. 2009, 115, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Irmak, M.K.; Topal, T.; Oter, S. Melatonin seems to be a mediator that transfers the environmental stimuli to oocytes for inheritance of adaptive changes through epigenetic inheritance system. Med. Hypotheses 2005, 64, 1138–1143. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, A.; Rosales-Corral, S.; Reiter, R.J. Gene regulation by melatonin linked to epigenetic phenomena. Gene 2012, 503, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hobson, S.R.; Gurusinghe, S.; Lim, R.; Alers, N.O.; Miller, S.L.; Kingdom, J.C.; Wallace, E.M. Melatonin improves endothelial function in vitro and prolongs pregnancy in women with early-onset preeclampsia. J. Pineal Res. 2018, e12508. [Google Scholar] [CrossRef] [PubMed]
- Bouchlariotou, S.; Liakopoulos, V.; Giannopoulou, M.; Arampatzis, S.; Eleftheriadis, T.; Mertens, P.R.; Zintzaras, E.; Messinis, I.E.; Stefanidis, I. Melatonin secretion is impaired in women with preeclampsia and an abnormal circadian blood pressure rhythm. Ren. Fail. 2014, 36, 1001–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kivela, A. Serum melatonin during human pregnancy. Acta Endocrinol. 1991, 124, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Tamura, H.; Kashida, S.; Takayama, H.; Yamagata, Y.; Karube, A.; Sugino, N.; Kato, H. Changes of serum melatonin level and its relationship to feto-placental unit during pregnancy. J. Pineal Res. 2001, 30, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Anderka, M.; Declercq, E.R.; Smith, W. A time to be born. Am. J. Public Health 2000, 90, 124–126. [Google Scholar] [PubMed]
- Tamura, H.; Takayama, H.; Nakamura, Y.; Reiter, R.J.; Sugino, N. Fetal/placental regulation of maternal melatonin in rats. J. Pineal Res. 2008, 44, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.D.; Sayers, L.; Keirse, M.J.; Anderson, A.B.; Turnbull, A.C. Melatonin in amniotic fluid during human parturition. Br. J. Obstet. Gynaecol. 1978, 85, 684–686. [Google Scholar] [CrossRef] [PubMed]
- Man, G.C.W.; Zhang, T.; Chen, X.; Wang, J.; Wu, F.; Liu, Y.; Wang, C.C.; Cheong, Y.; Li, T.C. The regulations and role of circadian clock and melatonin in uterine receptivity and pregnancy-an immunological perspective. Am. J. Reprod. Immunol. 2017, 78, e12715. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carlomagno, G.; Minini, M.; Tilotta, M.; Unfer, V. From Implantation to Birth: Insight into Molecular Melatonin Functions. Int. J. Mol. Sci. 2018, 19, 2802. https://doi.org/10.3390/ijms19092802
Carlomagno G, Minini M, Tilotta M, Unfer V. From Implantation to Birth: Insight into Molecular Melatonin Functions. International Journal of Molecular Sciences. 2018; 19(9):2802. https://doi.org/10.3390/ijms19092802
Chicago/Turabian StyleCarlomagno, Gianfranco, Mirko Minini, Marco Tilotta, and Vittorio Unfer. 2018. "From Implantation to Birth: Insight into Molecular Melatonin Functions" International Journal of Molecular Sciences 19, no. 9: 2802. https://doi.org/10.3390/ijms19092802
APA StyleCarlomagno, G., Minini, M., Tilotta, M., & Unfer, V. (2018). From Implantation to Birth: Insight into Molecular Melatonin Functions. International Journal of Molecular Sciences, 19(9), 2802. https://doi.org/10.3390/ijms19092802






