From Tumor Immunology to Immunotherapy in Gastric and Esophageal Cancer
Abstract
:1. Introduction
2. Molecular Subtypes of Esophageal and Gastric Cancer
3. Immune Checkpoints PD-1/PD-L1/PD-L2 and Clinical Significance in Gastric and Esophageal Cancer
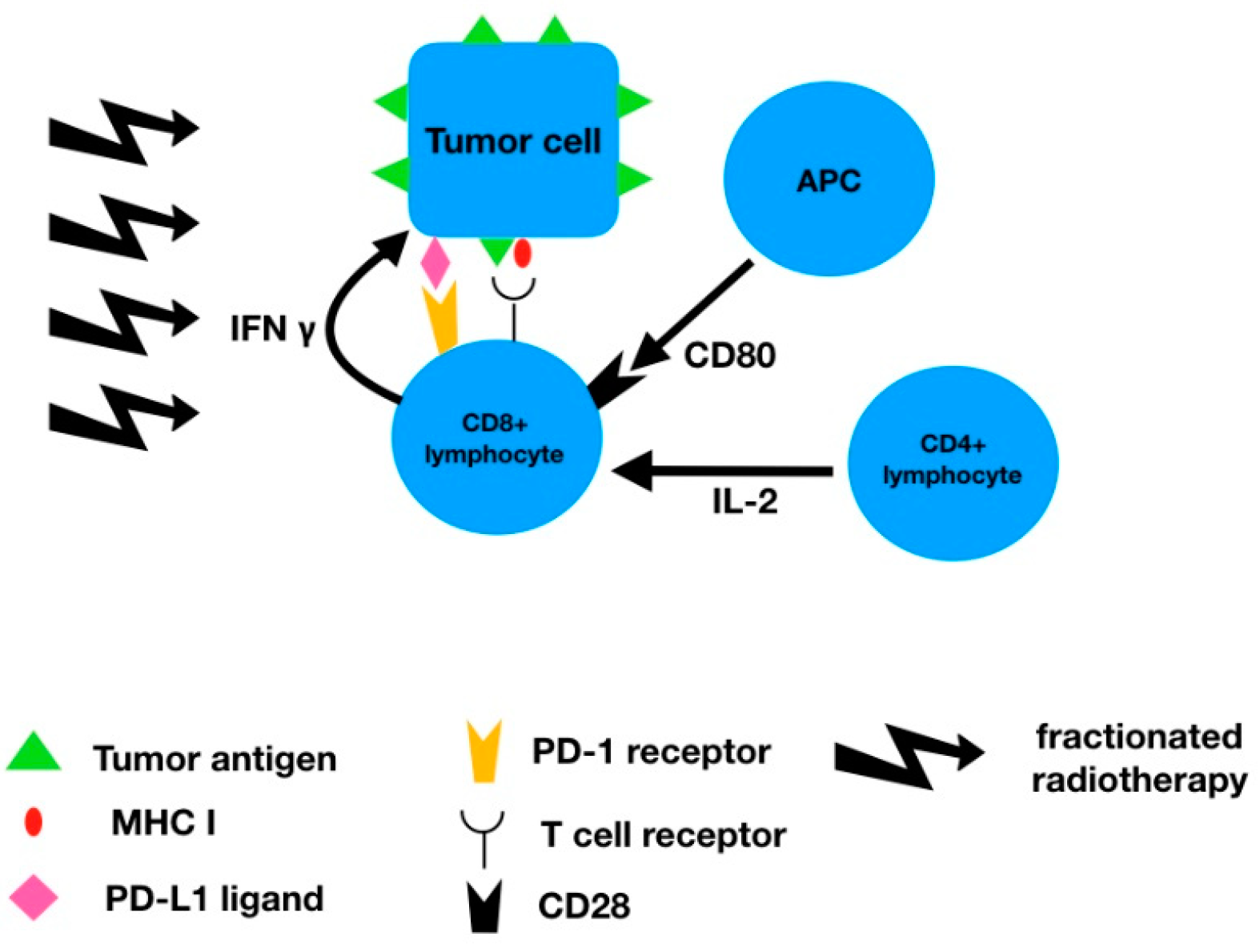
3.1. PD-L1 Expression and Radiotherapy
3.2. PD-L1 Expression and Systemic Treatment
3.3. PD-L1 Expression and EBV Infection
3.4. PD-L1 Expression and MSI Status
3.5. PD-L1 Expression and Epithelial-Mesenchymal Transition Phenotype
3.6. PD-L1 Expression and EGFR
4. Significance of Microsatellite Instability
5. Immunotherapy
6. Conclusions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Matzenauer, M.; Vrána, D.; Vlachová, Z.; Aujesky, R.; Vrba, R.; Neoral, C.; Melichar, B. Stereotactic radiotherapy in the treatment of local recurrences of esophageal cancer. Oncol. Lett. 2017, 13, 1807–1810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vrana, D.; Matzenauer, M.; Aujesky, R.; Vrba, R.; Neoral, C.; Melichar, B.; Souček, P. Potential Predictive Role of MicroRNAs in the Neoadjuvant Treatment of Esophageal Cancer. Anticancer Res. 2017, 37, 403–412. [Google Scholar] [CrossRef]
- Vrana, D.; Hlavac, V.; Brynychova, V.; Vaclavikova, R.; Neoral, C.; Vrba, J.; Aujesky, R.; Matzenauer, M.; Melichar, B.; Soucek, P. ABC Transporters and Their Role in the Neoadjuvant Treatment of Esophageal Cancer. Int. J. Mol. Sci. 2018, 19, 868. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Marconcini, R.; Spagnolo, F.; Stucci, L.S.; Ribero, S.; Marra, E.; Rosa, F.; Picasso, V.; Di Guardo, L.; Cimminiello, C.; Cavalieri, S.; et al. Current status and perspectives in immunotherapy for metastatic melanoma. Oncotarget 2018, 9, 12452–12470. [Google Scholar] [CrossRef]
- Santoni, M.; Massari, F.; Di Nunno, V.; Conti, A.; Cimadamore, A.; Scarpelli, M.; Montironi, R.; Cheng, L.; Battelli, N.; Lopez-Beltran, A. Immunotherapy in renal cell carcinoma: Latest evidence and clinical implications. Drugs Context 2018, 7, 212528. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Integrated genomic characterization of oesophageal carcinoma. Nature 2017, 541, 169–175. [Google Scholar] [CrossRef] [Green Version]
- Secrier, M.; Li, X.; de Silva, N.; Eldridge, M.D.; Contino, G.; Bornschein, J.; MacRae, S.; Grehan, N.; O’Donovan, M.; Miremadi, A.; et al. Oesophageal Cancer Clinical and Molecular Stratification (OCCAMS) Consortium. Mutational signatures in esophageal adenocarcinoma define etiologically distinct subgroups with therapeutic relevance. Nat. Genet. 2016, 48, 1131–1141. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar] [CrossRef] [Green Version]
- Alsaab, H.O.; Sau, S.; Alzhrani, R.; Tatiparti, K.; Bhise, K.; Kashaw, S.K.; Iyer, A.K. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front. Pharmacol. 2017, 8, 561. [Google Scholar] [CrossRef] [PubMed]
- Böger, C.; Behrens, H.M.; Mathiak, M.; Krüger, S.; Kalthoff, H.; Röcken, C. PD-L1 is an independent prognostic predictor in gastric cancer of Western patients. Oncotarget 2016, 7, 24269–24283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, M.A.; Ramos, M.F.K.P.; Faraj, S.F.; Dias, A.R.; Yagi, O.K.; Zilberstein, B.; Cecconello, I.; Alves, V.A.F.; de Mello, E.S.; Ribeiro, U., Jr. Clinicopathological and prognostic features of Epstein-Barr virus infection, microsatellite instability, and PD-L1 expression in gastric cancer. J. Surg. Oncol. 2018, 117, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Ohira, M.; Tanaka, H.; Muguruma, K.; Toyokawa, T.; Kubo, N.; Sakurai, K.; Amano, R.; Kimura, K.; Shibutani, M.; et al. Programmed Death-1 Ligand-1 (PDL1) Expression Is Associated with the Prognosis of Patients with Stage II/III Gastric Cancer. Anticancer Res. 2015, 35, 5369–5376. [Google Scholar] [PubMed]
- Zhang, L.; Qiu, M.; Jin, Y.; Ji, J.; Li, B.; Wang, X.; Yan, S.; Xu, R.; Yang, D. Programmed cell death ligand 1 (PD-L1) expression on gastric cancer and its relationship with clinicopathologic factors. Int. J. Clin. Exp. Pathol. 2015, 8, 11084–11091. [Google Scholar] [PubMed]
- Eto, S.; Yoshikawa, K. Programmed cell death protein 1 expression is an independent prognostic factor in gastric cancer after curative resection. Gastric Cancer 2016, 19, 466–471. [Google Scholar] [CrossRef]
- Tsutsumi, S.; Saeki, H.; Nakashima, Y.; Ito, S.; Oki, E.; Morita, M.; Oda, Y.; Okano, S.; Maehara, Y. Programmed death-ligand 1 expression at tumor invasive front is associated with epithelial-mesenchymal transition and poor prognosis in esophageal squamous cell carcinoma. Cancer Sci. 2017, 108, 1119–1127. [Google Scholar] [CrossRef] [Green Version]
- Kim, R.; Keam, B.; Kwon, D.; Ock, C.Y.; Kim, M.; Kim, T.M.; Kim, H.J.; Jeon, Y.K.; Park, I.K.; Kang, C.H.; et al. Programmed death ligand-1 expression and its prognostic role in esophageal squamous cell carcinoma. World J. Gastroenterol. 2016, 22, 8389–8397. [Google Scholar] [CrossRef]
- Zhang, W.; Pang, Q.; Zhang, X.; Yan, C.; Wang, Q.; Yang, J.; Yu, S.; Liu, X.; Pan, Y.; Yuan, Z.; et al. Programmed death-ligand 1 is prognostic factor in esophageal squamous cell carcinoma and is associated with epidermal growth factor receptor. Cancer Sci. 2017, 108, 590–597. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, M.; Mu, D.; Kong, L.; Zhang, J.; Zhao, F.; Li, Z.; Liu, X.; Bo, C.; Yu, J. CD8+/FOXP3+ ratio and PD-L1 expression associated with survival in pT3N0M0 stage esophageal squamous cell cancer. Oncotarget 2016, 7, 71455–71465. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Lo, A.W.I.; Wong, A.; Chen, W.; Wang, Y.; Lin, L.; Xu, J. Prognostic significance of tumor-infiltrating immune cells and PD-L1 expression in esophageal squamous cell carcinoma. Oncotarget 2017, 8, 30175–30189. [Google Scholar] [CrossRef] [PubMed]
- Jesinghaus, M.; Steiger, K.; Slotta-Huspenina, J.; Drecoll, E.; Pfarr, N.; Meyer, P.; Konukiewitz, B.; Bettstetter, M.; Wieczorek, K.; Ott, K.; et al. Increased intraepithelial CD3+ T-lymphocytes and high PD-L1 expression on tumor cells are associated with a favorable prognosis in esophageal squamous cell carcinoma and allow prognostic immunogenic subgrouping. Oncotarget 2017, 8, 46756–46768. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.F.; Chen, P.T.; Chen, W.C.; Lu, M.S.; Lin, P.Y.; Lee, KD. The role of PD-L1 in the radiation response and prognosis for esophageal squamous cell carcinoma related to IL-6 and T-cell immunosuppression. Oncotarget 2016, 7, 7913–7924. [Google Scholar] [CrossRef] [Green Version]
- Kawazoe, A.; Kuwata, T.; Kuboki, Y.; Shitara, K.; Nagatsuma, A.K.; Aizawa, M.; Yoshino, T.; Doi, T.; Ohtsu, A.; Ochiai, A. Clinicopathological features of programmed death ligand 1 expression with tumor-infiltrating lymphocyte, mismatch repair, and Epstein-Barr virus status in a large cohort of gastric cancer patients. Gastric Cancer 2017, 20, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Thompson, E.D.; Zahurak, M.; Murphy, A.; Cornish, T.; Cuka, N.; Abdelfatah, E.; Yang, S.; Duncan, M.; Ahuja, N.; Taube, J.M.; et al. Patterns of PD-L1 expression and CD8 T cell infiltration in gastric adenocarcinomas and associated immune stroma. Gut 2017, 66, 794–801. [Google Scholar] [CrossRef]
- Yang, J.H.; Kim, H.; Roh, S.Y.; Lee, M.A.; Park, J.M.; Lee, H.H.; Park, C.H.; Lee, H.H.; Jung, E.S.; Lee, S.H.; et al. Discordancy and changes in the pattern of programmed death ligand 1 expression before and after platinum-based chemotherapy in metastatic gastric cancer. Gastric Cancer 2018, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Seo, A.N.; Kang, B.W.; Kwon, O.K.; Park, K.B.; Lee, S.S.; Chung, H.Y.; Yu, W.; Bae, H.I.; Jeon, S.W.; Kang, H.; et al. Intratumoural PD-L1 expression is associated with worse survival of patients with Epstein-Barr virus-associated gastric cancer. Br. J. Cancer 2017, 117, 1753–1760. [Google Scholar] [CrossRef]
- Ito, S.; Okano, S.; Morita, M.; Saeki, H.; Tsutsumi, S.; Tsukihara, H.; Nakashima, Y.; Ando, K.; Imamura, Y.; Ohgaki, K.; et al. Expression of PD-L1 and HLA Class I in Esophageal Squamous Cell Carcinoma: Prognostic Factors for Patient Outcome. Ann. Surg. Oncol. 2016, 23 (Suppl. S4), 508–515. [Google Scholar] [CrossRef]
- Hsieh, C.C.; Hsu, H.S.; Li, A.F.; Chen, Y.J. Clinical relevance of PD-L1 and PD-L2 overexpression in patients with esophageal squamous cell carcinoma. J. Thorac. Dis. 2018, 10, 4433–4444. [Google Scholar] [CrossRef]
- Kollmann, D.; Ignatova, D.; Jedamzik, J.; Chang, Y.T.; Jomrich, G.; Baierl, A.; Kazakov, D.; Michal, M.; French, L.E.; Hoetzenecker, W.; et al. PD-L1 expression is an independent predictor of favorable outcome in patients with localized esophageal adenocarcinoma. Oncoimmunology 2018, 7, e1435226. [Google Scholar] [CrossRef]
- Tanaka, K.; Miyata, H.; Sugimura, K.; Kanemura, T.; Hamada-Uematsu, M.; Mizote, Y.; Yamasaki, M.; Wada, H.; Nakajima, K.; Takiguchi, S.; et al. Negative influence of programmed death-1-ligands on the survival of esophageal cancer patients treated with chemotherapy. Cancer Sci. 2016, 107, 726–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Lai, Y.; Sun, L.; Zhang, X.; Liu, R.; Feng, G.; Zhou, L.; Jia, L.; Huang, X.; Kang, Q.; et al. PD-L1 expression is associated with massive lymphocyte infiltration and histology in gastric cancer. Hum. Pathol. 2016, 55, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Q.; Ni, S.; Tan, C.; Cai, X.; Huang, D.; Sheng, W. Programmed death-ligand 1 expression in gastric cancer: Correlation with mismatch repair deficiency and HER2-negative status. Cancer Med. 2018, 7, 2612–2620. [Google Scholar] [CrossRef] [PubMed]
- Saito, R.; Abe, H.; Kunita, A.; Yamashita, H.; Seto, Y.; Fukayama, M. Overexpression and gene amplification of PD-L1 in cancer cells and PD-L1+ immune cells in Epstein-Barr virus-associated gastric cancer: The prognostic implications. Mod. Pathol. 2017, 30, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Lee, J.; Bang, H.; Kim, S.T.; Park, S.H.; An, J.Y.; Choi, M.G.; Lee, J.H.; Sohn, T.S.; Bae, J.M.; et al. cell death-ligand 1 expression predicts survival in patients with gastric carcinoma with microsatellite instability. Oncotarget 2017, 8, 13320–13328. [Google Scholar] [CrossRef]
- Ma, C.; Patel, K.; Singhi, A.D.; Ren, B.; Zhu, B.; Shaikh, F.; Sun, W. Programmed Death-Ligand 1 Expression Is Common in Gastric Cancer Associated with Epstein-Barr Virus or Microsatellite Instability. Am. J. Surg. Pathol. 2016, 40, 1496–1506. [Google Scholar] [CrossRef]
- Koh, J.; Ock, C.Y.; Kim, J.W.; Nam, S.K.; Kwak, Y.; Yun, S.; Ahn, S.H.; Park, D.J.; Kim, H.H.; Kim, W.H.; et al. Clinicopathologic implications of immune classification by PD-L1 expression and CD8-positive tumor-infiltrating lymphocytes in stage II and III gastric cancer patients. Oncotarget 2017, 8, 26356–26367. [Google Scholar] [CrossRef]
- An Investigational Immuno-therapy Study of Nivolumab or Placebo in Patients With Resected Esophageal or Gastroesophageal Junction Cancer (CheckMate 577). Available online: https://clinicaltrials.gov/ct2/show/NCT02743494 (accessed on 13 November 2018).
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. PACIFIC Investigators. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N. Engl. J. Med. 2018, 379, 2342–2350. [Google Scholar] [CrossRef]
- Tang, Y.; Li, G.; Wu, S.; Tang, L.; Zhang, N.; Liu, J.; Zhang, S.; Yao, L. Programmed death ligand 1 expression in esophageal cancer following definitive chemoradiotherapy: Prognostic significance and association with inflammatory biomarkers. Oncol. Lett. 2018, 15, 4988–4996. [Google Scholar] [CrossRef] [Green Version]
- Minsky, B.D.; Pajak, T.F.; Ginsberg, R.J.; Pisansky, T.M.; Martenson, J.; Komaki, R.; Okawara, G.; Rosenthal, S.A.; Kelsen, D.P. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: High-dose versus standard-dose radiation therapy. J. Clin. Oncol. 2002, 20, 1167–1174. [Google Scholar] [CrossRef]
- Dovedi, S.J.; Adlard, A.L.; Lipowska-Bhalla, G.; McKenna, C.; Jones, S.; Cheadle, E.J.; Stratford, I.J.; Poon, E.; Morrow, M.; Stewart, R.; et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014, 74, 5458–5468. [Google Scholar] [CrossRef] [PubMed]
- Mimura, K.; Teh, J.L.; Okayama, H.; Shiraishi, K.; Kua, L.F.; Koh, V.; Smoot, D.T.; Ashktorab, H.; Oike, T.; Suzuki, Y.; et al. PD-L1 expression is mainly regulated by interferon gamma associated with JAK-STAT pathway in gastric cancer. Cancer Sci. 2018, 109, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Ghebeh, H.; Lehe, C.; Barhoush, E.; Al-Romaih, K.; Tulbah, A.; Al-Alwan, M.; Hendrayani, S.F.; Manogaran, P.; Alaiya, A.; Al-Tweigeri, T.; et al. Doxorubicin downregulates cell surface B7-H1 expression and upregulates its nuclear expression in breast cancer cells: Role of B7-H1 as an anti-apoptotic molecule. Breast Cancer Res. 2010, 12, R48. [Google Scholar] [CrossRef] [PubMed]
- Rom-Jurek, E.M.; Kirchhammer, N.; Ugocsai, P.; Ortmann, O.; Wege, A.K.; Brockhoff, G. Regulation of Programmed Death Ligand 1 (PD-L1) Expression in Breast Cancer Cell Lines In Vitro and in Immunodeficient and Humanized Tumor Mice. Int. J. Mol. Sci. 2018, 19, 563. [Google Scholar] [CrossRef]
- Min, Z.; Yibo, F.; Xiaofang, C.; Kezuo, H.; Xiujuan, Q. 5-Fluorouracil induced up-regulation of exosomal PD-L1 causing immunosuppression in gastric cancer patients. Ann. Oncol. 2018, 29. [Google Scholar] [CrossRef]
- Fang, W.; Zhang, J.; Hong, S.; Zhan, J.; Chen, N.; Qin, T.; Tang, Y.; Zhang, Y.; Kang, S.; Zhou, T.; et al. EBV-driven LMP1 and IFN-γ up-regulate PD-L1 in nasopharyngeal carcinoma: Implications for oncotargeted therapy. Oncotarget 2014, 5, 12189–12202. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Park, H.E.; Cho, N.Y.; Lee, H.S.; Kang, G.H. Characterisation of PD-L1-positive subsets of microsatellite-unstable colorectal cancers. Br. J. Cancer 2016, 115, 490–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Xiong, Y.; Li, J.; Zheng, X.; Zhou, Q.; Turner, A.; Wu, C.; Lu, B.; Jiang, J. PD-L1 Expression Promotes Epithelial to Mesenchymal Transition in Human Esophageal Cancer. Cell Physiol. Biochem. 2017, 42, 2267–2280. [Google Scholar] [CrossRef] [Green Version]
- Ng, H.Y.; Li, J.; Tao, L.; Lam, A.K.; Chan, K.W.; Ko, J.M.Y.; Yu, V.Z.; Wong, M.; Li, B.; Lung, M.L. Chemotherapeutic Treatments Increase PD-L1 Expression in Esophageal Squamous Cell Carcinoma through EGFR/ERK Activation. Transl. Oncol. 2018, 11, 1323–1333. [Google Scholar] [CrossRef]
- Chen, K.; Cheng, G.; Zhang, F.; Zhang, N.; Li, D.; Jin, J.; Wu, J.; Ying, L.; Mao, W.; Su, D. Prognostic significance of programmed death-1 and programmed death-ligand 1 expression in patients with esophageal squamous cell carcinoma. Oncotarget 2016, 7, 30772–30780. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Cao, D.; Qu, L.; Cao, X.; Jia, Z.; Zhao, T.; Wang, Q.; Jiang, J. PD-1 and PD-L1 co-expression predicts favorable prognosis in gastric cancer. Oncotarget 2017, 8, 64066–64082. [Google Scholar] [CrossRef] [PubMed]
- Vanderwalde, A.; Spetzler, D.; Xiao, N.; Gatalica, Z.; Marshall, J. Microsatellite instability status determined by next-generation sequencing and compared with PD-L1 and tumor mutational burden in 11,348 patients. Cancer Med. 2018, 7, 746–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.J.; Lee, K.S.; Cho, H.J.; Kim, Y.H.; Yang, H.K.; Kim, W.H.; Kang, G.H. Prognostic implications of tumor-infiltrating FoxP3+ regulatory T cells and CD8+ cytotoxic T cells in microsatellite-unstable gastric cancers. Hum. Pathol. 2014, 45, 285–293. [Google Scholar] [CrossRef]
- Boissière-Michot, F.; Lazennec, G.; Frugier, H.; Jarlier, M.; Roca, L.; Duffour, J.; Du Paty, E.; Laune, D.; Blanchard, F.; Le Pessot, F.; et al. Characterization of an adaptive immune response in microsatellite-instable colorectal cancer. Oncoimmunology 2014, 3, e29256. [Google Scholar] [CrossRef]
- Polom, K.; Böger, C.; Smyth, E.; Marrelli, D.; Behrens, H.M.; Marano, L.; Becker, T.; Lordick, F.; Röcken, C.; Roviello, F. Synchronous metastatic gastric cancer-molecular background and clinical implications with special attention to mismatch repair deficiency. Eur. J. Surg. Oncol. 2018, 44, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Corso, G.; Pedrazzani, C.; Marrelli, D.; Pascale, V.; Pinto, E.; Roviello, F. Correlation of microsatellite instability at multiple loci with long-term survival in advanced gastric carcinoma. Arch. Surg. 2009, 144, 722–727. [Google Scholar] [CrossRef]
- Muzeau, F.; Fléjou, J.F.; Belghiti, J.; Thomas, G.; Hamelin, R. Infrequent microsatellite instability in oesophageal cancers. Br. J. Cancer 1997, 75, 1336–1339. [Google Scholar] [CrossRef] [Green Version]
- Smyth, E.C.; Wotherspoon, A.; Peckitt, C.; Gonzalez, D.; Hulkki-Wilson, S.; Eltahir, Z.; Fassan, M.; Rugge, M.; Valeri, N.; Okines, A.; et al. Mismatch Repair Deficiency, Microsatellite Instability, and Survival: An Exploratory Analysis of the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) Trial. JAMA Oncol. 2017, 3, 1197–1203. [Google Scholar] [CrossRef]
- Pembrolizumab in MSI-H or dMMR Solid Tumors: ‘First Tissue/Site-Agnostic’ Approval by FDA. Available online: http://www.ascopost.com/issues/february-10-2018/pembrolizumab-in-msi-h-or-dmmr-solid-tumors-first-tissuesite-agnostic-approval-by-fda/ (accessed on 13 November 2018).
- Kim, H.; An, J.Y.; Noh, S.H.; Shin, S.K.; Lee, Y.C.; Kim, H. High microsatellite instability predicts good prognosis in intestinal-type gastric cancers. J. Gastroenterol. Hepatol. 2011, 26, 585–592. [Google Scholar] [CrossRef]
- An, J.Y.; Kim, H.; Cheong, J.H.; Hyung, W.J.; Kim, H.; Noh, S.H. Microsatellite instability in sporadic gastric cancer: Its prognostic role and guidance for 5-FU based chemotherapy after R0 resection. Int. J. Cancer 2012, 131, 505–511. [Google Scholar] [CrossRef]
- Fang, W.L.; Chang, S.C.; Lan, Y.T.; Huang, K.H.; Chen, J.H.; Lo, S.S.; Hsieh, M.C.; Li, A.F.; Wu, C.W.; Chiou, S.H. Microsatellite instability is associated with a better prognosis for gastric cancer patients after curative surgery. World J. Surg. 2012, 36, 2131–2138. [Google Scholar] [CrossRef] [PubMed]
- Beghelli, S.; de Manzoni, G.; Barbi, S.; Tomezzoli, A.; Roviello, F.; Di Gregorio, C.; Vindigni, C.; Bortesi, L.; Parisi, A.; Saragoni, L.; et al. Microsatellite instability in gastric cancer is associated with better prognosis in only stage II cancers. Surgery 2006, 139, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Choi, Y.Y.; An, J.Y.; Shin, H.B.; Jo, A.; Choi, H.; Seo, S.H.; Bang, H.J.; Cheong, J.H.; Hyung, W.J.; et al. The benefit of microsatellite instability is attenuated by chemotherapy in stage II and stage III gastric cancer: Results from a large cohort with subgroup analyses. Int. J. Cancer 2015, 137, 819–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giampieri, R.; Maccaroni, E.; Mandolesi, A.; Del Prete, M.; Andrikou, K.; Faloppi, L.; Bittoni, A.; Bianconi, M.; Scarpelli, M.; Bracci, R.; et al. Mismatch repair deficiency may affect clinical outcome through immune response activation in metastatic gastric cancer patients receiving first-line chemotherapy. Gastric Cancer 2017, 20, 156–163. [Google Scholar] [CrossRef]
- Oki, E.; Kakeji, Y.; Zhao, Y.; Yoshida, R.; Ando, K.; Masuda, T.; Ohgaki, K.; Morita, M.; Maehara, Y. Chemosensitivity and survival in gastric cancer patients with microsatellite instability. Ann. Surg. Oncol. 2009, 16, 2510–2515. [Google Scholar] [CrossRef] [PubMed]
- Falchetti, M.; Saieva, C.; Lupi, R.; Masala, G.; Rizzolo, P.; Zanna, I.; Ceccarelli, K.; Sera, F.; Mariani-Costantini, R.; Nesi, G.; et al. Gastric cancer with high-level microsatellite instability: Target gene mutations, clinicopathologic features, and long-term survival. Hum Pathol. 2008, 39, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, D.; Polom, K.; Pascale, V.; Vindigni, C.; Piagnerelli, R.; De Franco, L.; Ferrara, F.; Roviello, G.; Garosi, L.; Petrioli, R.; et al. Strong Prognostic Value of Microsatellite Instability in Intestinal Type Non-cardia Gastric Cancer. Ann. Surg. Oncol. 2016, 23, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Muro, K.; Chung, H.C.; Shankaran, V.; Geva, R.; Catenacci, D.; Gupta, S.; Eder, J.P.; Golan, T.; Le, D.T.; Burtness, B.; et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): A multicentre, open-label, phase 1b trial. Lancet Oncol. 2016, 17, 717–726. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Doi, T.; Jang, R.W.; Muro, K.; Satoh, T.; Machado, M.; Sun, W.; Jalal, S.I.; Shah, M.A.; Metges, J.P.; et al. Safety and Efficacy of Pembrolizumab Monotherapy in Patients with Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol. 2018, 4, e180013. [Google Scholar] [CrossRef]
- Shitara, K.; Özgüroğlu, M.; Bang, Y.J.; Di Bartolomeo, M.; Mandalà, M.; Ryu, M.H.; Fornaro, L.; Olesiński, T.; Caglevic, C.; Chung, H.C.; et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): A randomised, open-label, controlled, phase 3 trial. Lancet 2018, 392, 123–133. [Google Scholar] [CrossRef]
- Kang, Y.K.; Boku, N.; Satoh, T.; Ryu, M.H.; Chao, Y.; Kato, K.; Chung, H.C.; Chen, J.S.; Muro, K.; Kang, W.K.; et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 390, 2461–2471. [Google Scholar] [CrossRef]
| Author | Number of Patients | Type of Cancer | Histology | Disease Stage | Percentage of PD-L1+ | Prognosis (PD-L1+) | Prognostic Value | Reference |
|---|---|---|---|---|---|---|---|---|
| Böger C et al. | 465 | gastric cancer | adenocarcinoma | all stages | tumor cells: 30.1% (primary cancer), 60% (liver metastases) immune cells: 88.4% (primary cancer), 73.3% (liver metastases) | better | improved OS and tumor specific survival | [12] |
| Pereira MA et al. | 287 | gastric cancer | adenocarcinoma | all stages | 8.8% | no impact | no impact | [13] |
| Tamura T et al. | 431 | gastric cancer | adenocarcinoma | all stages | 29.6% | worse | worse OS for stage II/III | [14] |
| Zhang L et al. | 132 | gastric cancer | adenocarcinoma | II/III | 50.8% | worse | worse OS | [15] |
| Eto S et al. | 105 | gastric cancer | adenocarcinoma | II/III | 24.7% | worse | worse OS (statistically non-significant) | [16] |
| Tsutsumi et al. | 90 | esophageal cancer | SCC | localized | 36.6% | worse | worse OS and relapse free survival | [17] |
| Kim R et al. | 200 | esophageal cancer | SCC | localized | 33.5% | worse | worse locoregional relapse rate and distant metastasis rate, no change in OS | [18] |
| Zhang W et al. | 344 | esophageal cancer | SCC | II/III | tumor cells 14.5%, immune cells 24.7% | better | better OS and DFS only in immune cells PD-L1+ | [19] |
| Zhu Y et al. | 133 | esophageal cancer | SCC | pT3pN0M0 | 42.1% | worse | worse DFS and OS | [20] |
| Jiang Y et al. | 428 | esophageal cancer | SCC | localized and metastatic | 79.7% | worse | worse DFS and OS in radically treated patients | [21] |
| Jesinghaus M et al. | 125 | esophageal cancer | SCC | all stages | 71% tumor cells, immune cells 87% | better | better OS, DSS and DFS (PD-L1+ tumor cells) | [22] |
| Chen MF et al. | 162 | esophageal cancer | SCC | not specified | 45.7% | worse | worse treatment response and OS | [23] |
| Kawazoe A et al. | 487 | gastric cancer | adenocarcinoma | localized | tumor cells 22.8%, immune cells 61.4 | no impact | no impact | [24] |
| Thompson ED et al. | 34 | gastric cancer | adenocarcinoma | localized | tumor cells 12%, immune cells 44% | worse | worse PFS and OS | [25] |
| Yang JH et al. | 72 | gastric cancer | adenocarcinoma | IV | 58.3% | better | better PFS | [26] |
| Seo AN et al. | 116 | gastric cancer | adenocarcinoma | localized | tumor cells 49.1%, stromal cells 56.9% | worse | worse DFS, not OS | [27] |
| Ito S et al. | 90 | esophageal cancer | SCC | localized | 19% | worse | worse OS | [28] |
| Hsieh CC et al. | 150 | esophageal cancer | SCC | localized | 64% | worse | worse DFS | [29] |
| Kollmann D et al. | 168 | esophageal cancer | adenocarcinoma | localized | tumor cells 43.5%, immune cells 69% | better | tumor cells expression - better DFS | [30] |
| Tanaka K et al. | 180 | esophageal cancer | SCC | localized | 29.4% | worse | worse OS for patients after neoadjuvant chemotherapy | [31] |
| Li Z et al. | 137 | gastric cancer | adenocarcinoma | all stages | 40.9% | worse | worse OS | [32] |
| Wang L et al. | 550 | gastric cancer | adenocarcinoma | all stages | 37.3% | no impact | not associated | [33] |
| Saito R et al. | 96 | gastric cancer | adenocarcinoma | not specified | tumor cells 34%, stromal cells 45% | worse | worse OS and DSS | [34] |
| Cho J et al. | 78 | gastric cancer | adenocarcinoma | all stages | 9% tumor cells, 60.3% immune cells | better | better OS (immune cells PD-L1+) | [35] |
| Ma C et al. | 44 | gastric cancer | adenocarcinoma | all stages | 72% | no impact | not associated | [36] |
| Koh J et al. | 392 | gastric cancer | adenocarcinoma | II/III | 25% | no impact | not associated | [37] |
| Author | Number of Patients | Type of Cancer | Histology | Disease Stage | Percentage of Positivity | Prognosis | Prognostic Value | Reference | |
|---|---|---|---|---|---|---|---|---|---|
| PD-1 | Chen K et al. | 349 | esophageal cancer | SCC | localized | 33.5% | no impact | no impact | [51] |
| Wu Y et al. | 340 | gastric cancer | adenocarcinoma | all stages | 22.6% | better | improved OS | [52] | |
| Böger C et al. | 465 | gastric cancer | adenocarcinoma | all stages | primary cancer 53.8%, liver metastases 73.3% | better | improved tumor specific survival | [12] | |
| Eto S et al. | 105 | gastric cancer | adenocarcinoma | II/III | 26.7% | worse | worse DFS | [16] | |
| Kollmann D et al. | 168 | esophageal cancer | adenocarcinoma | localized | tumor cells 77.4%, immune cells 81% | worse | worse OS and DFS | [30] | |
| PD-L2 | Seo AN et al. | 116 | gastric cancer | adenocarcinoma | localized | tumor cells 21.6%, stromal cells 38.8% | no impact | not statistically significant trend towards-improved DFS | [27] |
| Hsieh CC et al. | 150 | esophageal cancer | SCC | localized | 42% | no impact | no impact | [29] | |
| Tanaka K et al. | 180 | esophageal cancer | SCC | localized | 48.3% | worse | worse OS for patients after neoadjuvant chemotherapy | [31] |
| Author | Histology | Stage | Number of MSI-H Patients | MSI-H Frequency | Prognostic Role of MSI-H Phenotype | Prognostic/Predictive Value | Reference |
|---|---|---|---|---|---|---|---|
| Kim H et al. | adenocarcinoma | all stages | 161 | 9% | better | improved prognosis | [61] |
| An JY et al. | adenocarcinoma | all stages | 170 | 8.5% | no impact | no benefit from adjuvant chemotherapy in MSI-H patients | [62] |
| Fang WL et al. | adenocarcinoma | I–III | 25 | 11.7% | better | improved 5-year OS (68% vs. 47.6%, p = 0.030), trend towards better DFS at 3 years | [63] |
| Beghelli S et al. | adenocarcinoma | all stages | 83 | 16% | better | improved survival in stage II patients | [64] |
| Kim SY et al. | adenocarcinoma | II–III | 105 | 8.2% | better | improved prognosis without chemotherapy | [65] |
| Smyth EC et al. | adenocarcinoma | localized | 20 | 8.5% | better | worse prognosis when treated with chemotherapy | [59] |
| Polom K et al. | adenocarcinoma | metastatic | 14 | 8.0% | better | improved OS (15.9 vs. 8 months, p = 0.023) | [56] |
| Giampieri R et al. | adenocarcinoma | metastatic | 15 | 14% | better | improved overall survival | [66] |
| Corso G et al. | adenocarcinoma | all stages | 63 | 25.2% | better | improved long term survival | [57] |
| Oki E et al. | adenocarcinoma | all stages | 22 | 9.4% | No prognostic role | No prognostic role | [67] |
| Falchetti M et al. | adenocarcinoma | localized | 27 | 17% | better | improved survival (p = 0.1) | [68] |
| Marrelli D et al. | adenocarcinoma | all stages | 111 | 23.5% | better | improved 5-year survival (67.6% vs. 35%, p < 0.001) | [69] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vrána, D.; Matzenauer, M.; Neoral, Č.; Aujeský, R.; Vrba, R.; Melichar, B.; Rušarová, N.; Bartoušková, M.; Jankowski, J. From Tumor Immunology to Immunotherapy in Gastric and Esophageal Cancer. Int. J. Mol. Sci. 2019, 20, 13. https://doi.org/10.3390/ijms20010013
Vrána D, Matzenauer M, Neoral Č, Aujeský R, Vrba R, Melichar B, Rušarová N, Bartoušková M, Jankowski J. From Tumor Immunology to Immunotherapy in Gastric and Esophageal Cancer. International Journal of Molecular Sciences. 2019; 20(1):13. https://doi.org/10.3390/ijms20010013
Chicago/Turabian StyleVrána, David, Marcel Matzenauer, Čestmír Neoral, René Aujeský, Radek Vrba, Bohuslav Melichar, Nikol Rušarová, Marie Bartoušková, and Janusz Jankowski. 2019. "From Tumor Immunology to Immunotherapy in Gastric and Esophageal Cancer" International Journal of Molecular Sciences 20, no. 1: 13. https://doi.org/10.3390/ijms20010013
APA StyleVrána, D., Matzenauer, M., Neoral, Č., Aujeský, R., Vrba, R., Melichar, B., Rušarová, N., Bartoušková, M., & Jankowski, J. (2019). From Tumor Immunology to Immunotherapy in Gastric and Esophageal Cancer. International Journal of Molecular Sciences, 20(1), 13. https://doi.org/10.3390/ijms20010013





