Proteomic Analysis of a Rice Mutant sd58 Possessing a Novel d1 Allele of Heterotrimeric G Protein Alpha Subunit (RGA1) in Salt Stress with a Focus on ROS Scavenging
Abstract
:1. Introduction
2. Results
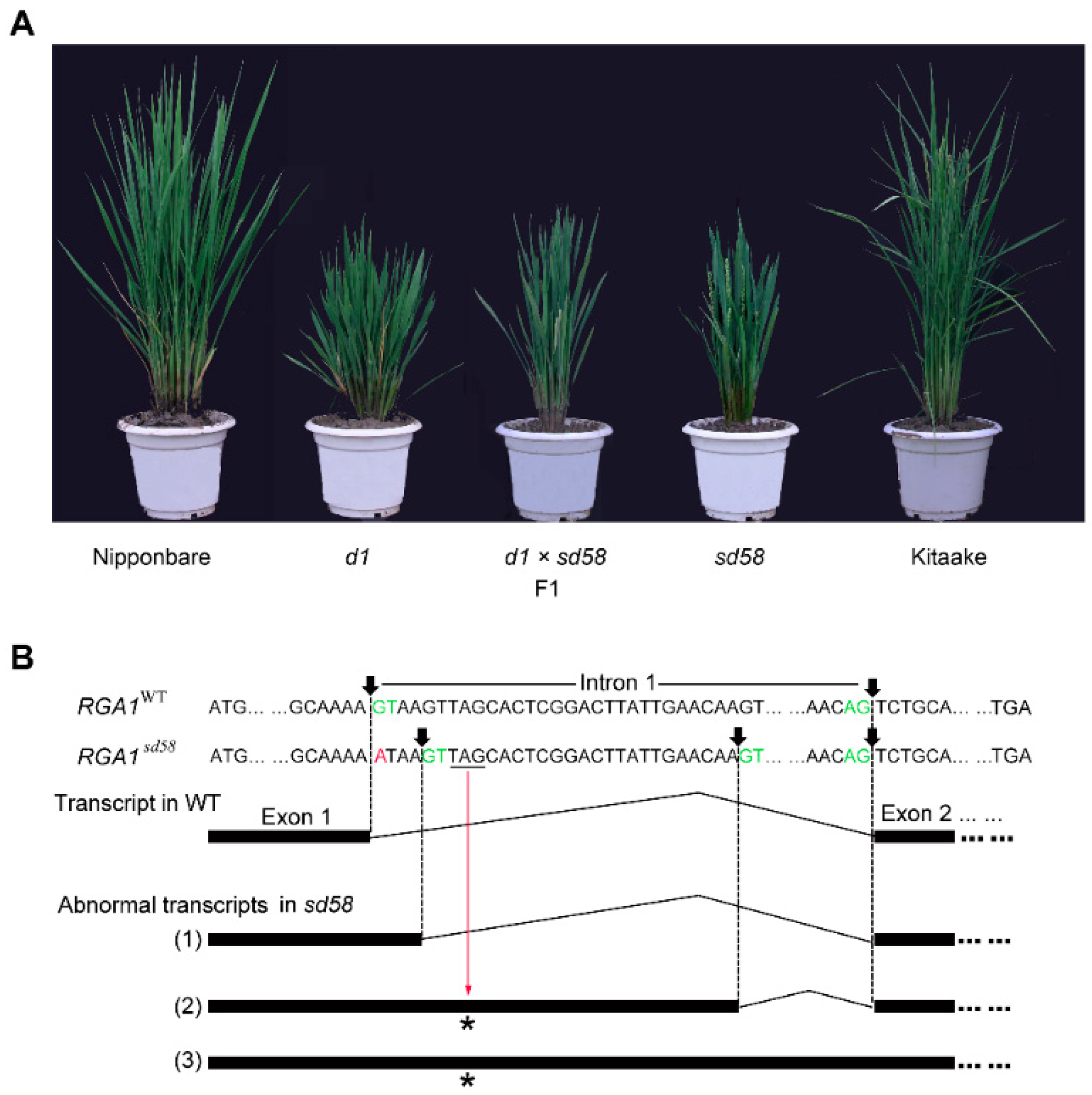
2.1. sd58 Displays Salt Tolerant and Dwarf Phenotypes
2.2. sd58 is an Allele of d1, with Mutation in Heterotrimeric G Protein Alpha Subunit Encoding Gene RGA1
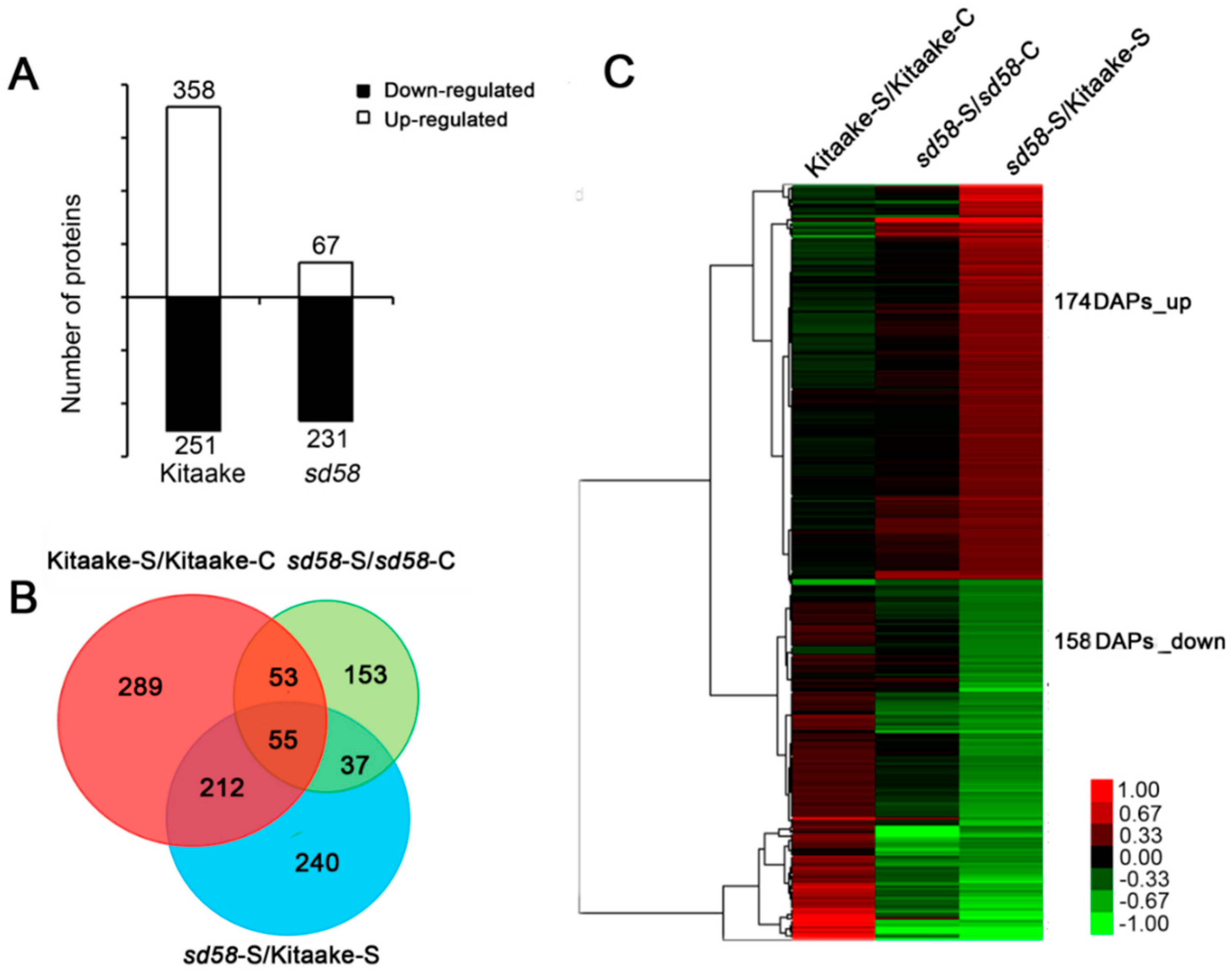
2.3. Loss-of-Function RGA1 Contributes to Significant Change at Proteome Level under Salinity Stress
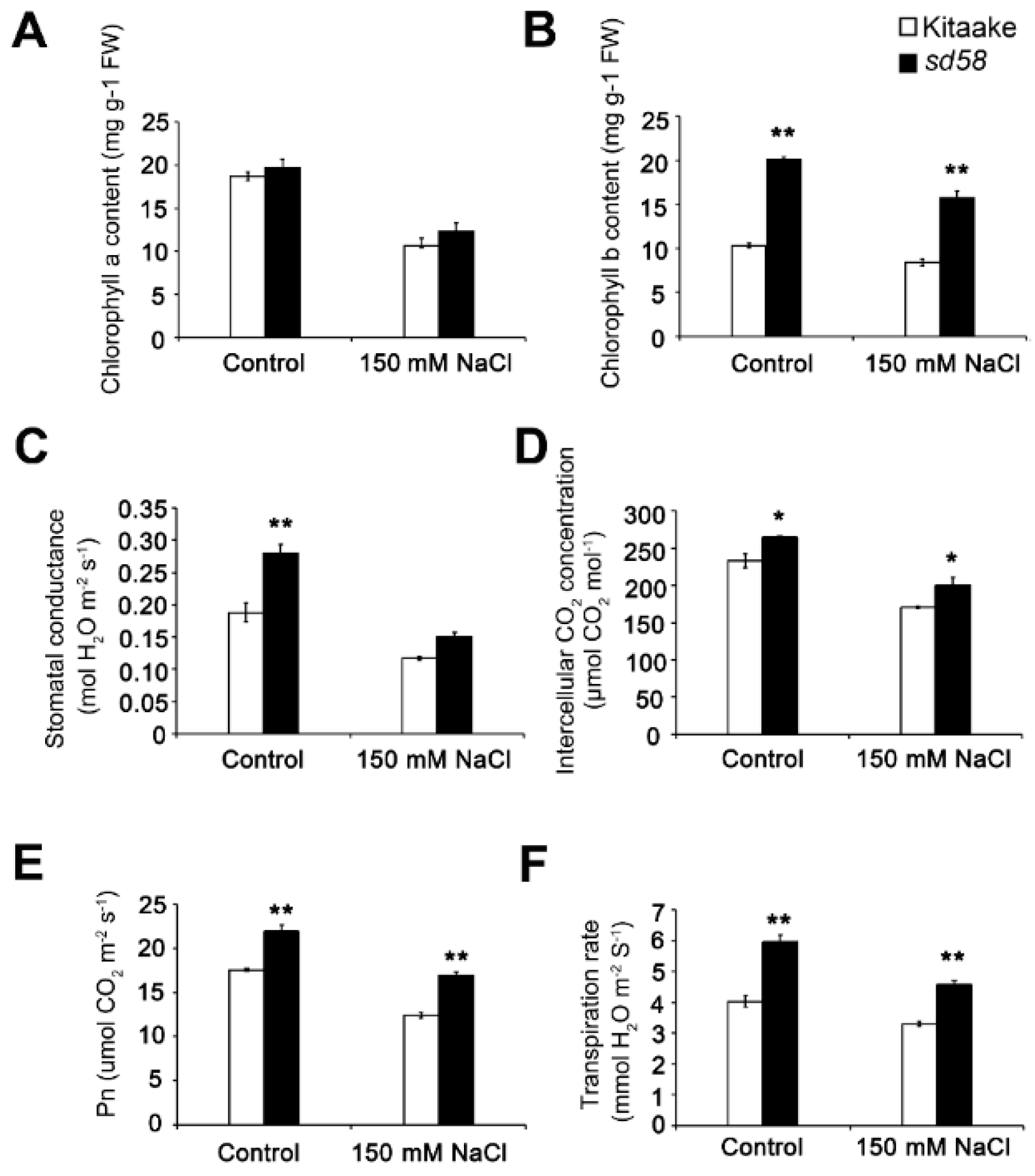
2.4. sd58 Promotes the Chlorophyll Accumulation and Photosynthesis Rate under Salinity Stress
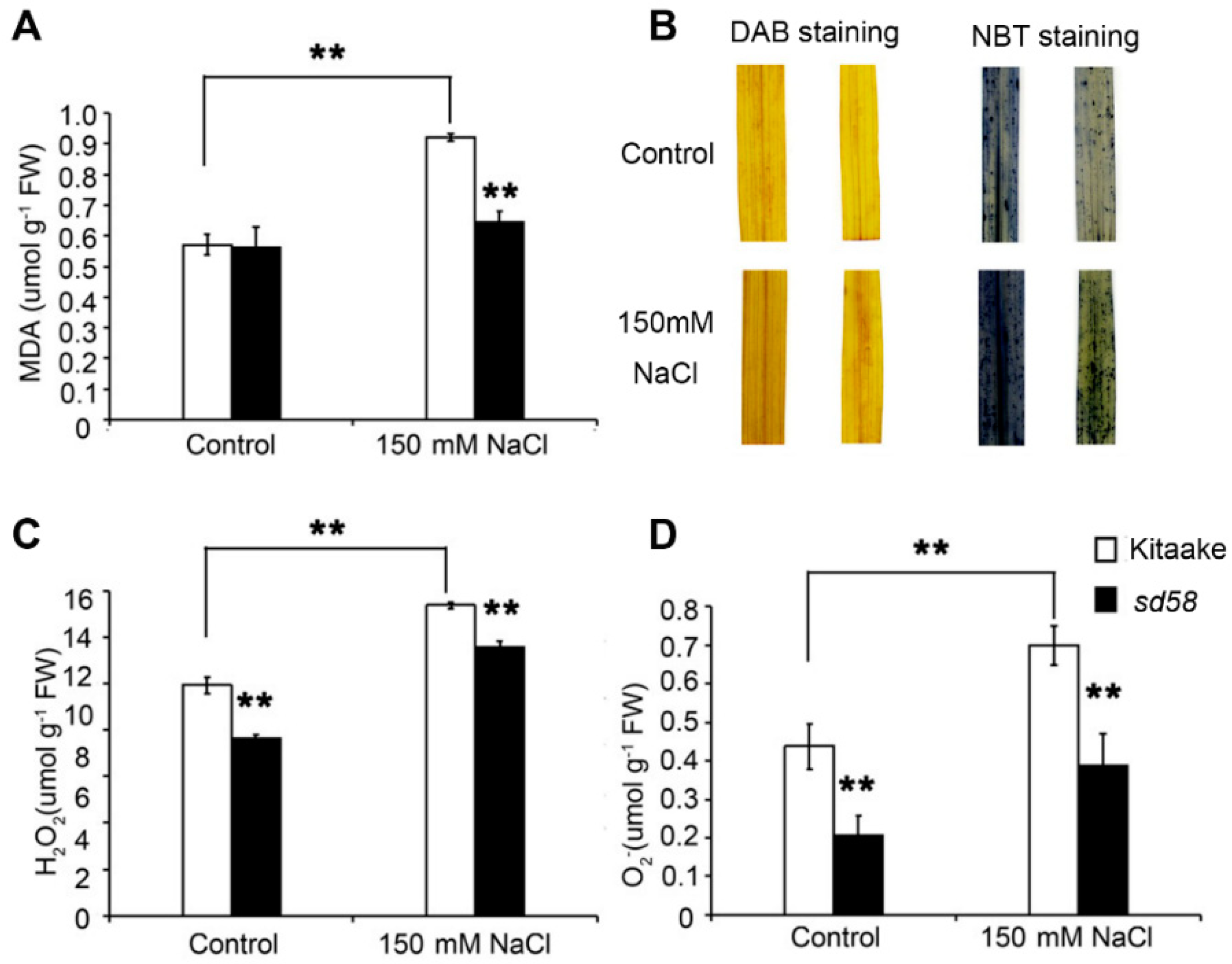
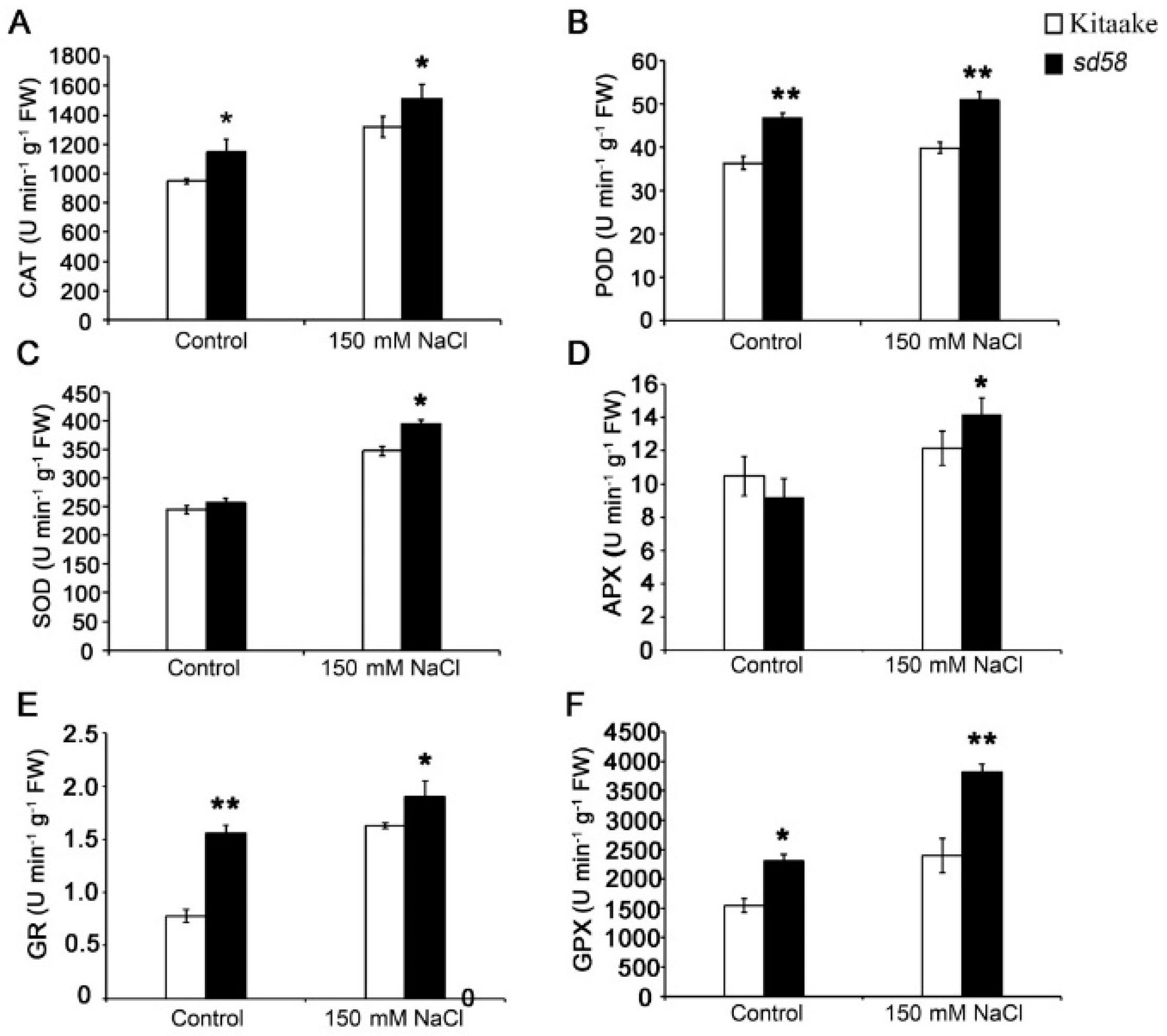
2.5. sd58 Mutant Confers the Tolerance to Salinity Tolerance Partially through Enhancing Antioxidation Capacity
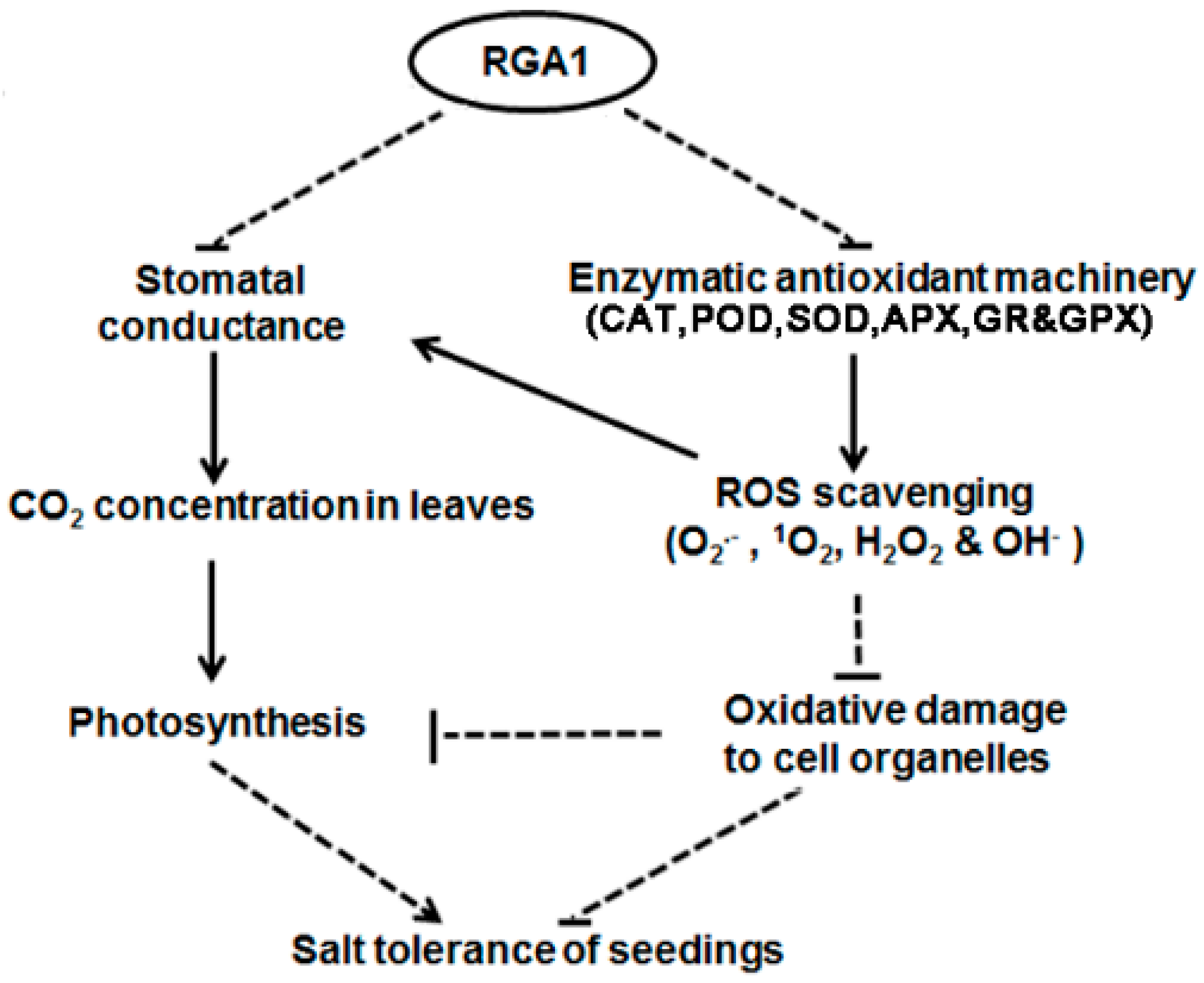
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Screening of Salt-Tolerant Mutants
4.3. Measurement of the Root Growth under Salinity Stress
4.4. Genetic Analysis and Map-Based Cloning
4.5. Protein Extraction, Protein Digestion and iTRAQ Labelling
4.6. Strong Cation Exchange (SCX) Fractionation and LC-ESI-MS/MS Analysis by Q Exactive
4.7. Proteomic Bioinformatic Analysis
4.8. Quantitative Analysis of Chlorophyll Content, Photosynthesis Rate and Chlorophyll Fluorescence
4.9. Visualization of Reactive Oxygen Species
4.10. Determination of Malondialdehyde Equivalents
4.11. Determination of Peroxidase, Superoxide Dismutase and Catalase Activity
4.12. RNA Isolation and Synthesis of First-Strand cDNAs
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.; Schroeder, J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, W.; Ma, X.; Wan, P.; Liu, L. Plant salt-tolerance mechanism: A review. Biochem. Biophys. Res. Commun. 2018, 495, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Hanin, M.; Ebel, C.; Ngom, M.; Laplaze, L.; Masmoudi, K. New Insights on Plant Salt Tolerance Mechanisms and Their Potential Use for Breeding. Front. Plant Sci. 2016, 7, 1787. [Google Scholar] [CrossRef] [PubMed]
- Moradi, F.; Ismail, A.M. Responses of photosynthesis, chlorophyll fluorescence and ROS-scavenging systems to salt stress during seedling and reproductive stages in rice. Ann. Bot. 2007, 99, 1161–1173. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.L.; Che Lah, W.A.; Abd Kadir, N.; Mustaqim, M.; Rahmat, Z.; Ahmad, A.; Lam, S.D.; Ismail, M.R. Susceptibility and tolerance of rice crop to salt threat: Physiological and metabolic inspections. PLoS ONE 2018, 13, e0192732. [Google Scholar] [CrossRef] [PubMed]
- Quan, R.; Wang, J.; Hui, J.; Bai, H.; Lyu, X.; Zhu, Y.; Zhang, H.; Zhang, Z.; Li, S.; Huang, R. Improvement of Salt Tolerance Using Wild Rice Genes. Front. Plant Sci. 2017, 8, 2269. [Google Scholar] [CrossRef] [PubMed]
- Prusty, M.R.; Kim, S.R.; Vinarao, R.; Entila, F.; Egdane, J.; Diaz, M.G.Q.; Jena, K.K. Newly Identified Wild Rice Accessions Conferring High Salt Tolerance Might Use a Tissue Tolerance Mechanism in Leaf. Front. Plant Sci. 2018, 9, 417. [Google Scholar] [CrossRef]
- Cabot, C.; Sibole, J.V.; Barcelo, J.; Poschenrieder, C. Lessons from crop plants struggling with salinity. Plant Sci. 2014, 226, 2–13. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2018, 217, 523–539. [Google Scholar] [CrossRef]
- Jiang, C.; Belfield, E.J.; Mithani, A.; Visscher, A.; Ragoussis, J.; Mott, R.; Smith, J.A.; Harberd, N.P. ROS-mediated vascular homeostatic control of root-to-shoot soil Na delivery in Arabidopsis. EMBO J. 2012, 31, 4359–4370. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Tomar, N.S.; Tittal, M.; Argal, S.; Agarwal, R.M. Plant growth under water/salt stress: ROS production; antioxidants and significance of added potassium under such conditions. Physiol. Mol. Biol. Plants 2017, 23, 731–744. [Google Scholar] [CrossRef]
- Mishra, P.; Bhoomika, K.; Dubey, R.S. Differential responses of antioxidative defense system to prolonged salinity stress in salt-tolerant and salt-sensitive Indica rice (Oryza sativa L.) seedlings. Protoplasma 2013, 250, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Urano, D.; Colaneri, A.; Jones, A.M. Galpha modulates salt-induced cellular senescence and cell division in rice and maize. J. Exp. Bot. 2014, 65, 6553–6561. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Yanofsky, M.F.; Meyerowitz, E.M. Molecular cloning and characterization of GPA1, a G protein alpha subunit gene from Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 1990, 87, 3821–3825. [Google Scholar] [CrossRef] [PubMed]
- Ashikari, M.; Wu, J.; Yano, M.; Sasaki, T.; Yoshimura, A. Rice gibberellin-insensitive dwarf mutant gene Dwarf 1 encodes the alpha-subunit of GTP-binding protein. Proc. Natl. Acad. Sci. USA 1999, 96, 10284–10289. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, Y.; Kato, T.; Ohki, S.; Ishikawa, A.; Kitano, H.; Sasaki, T.; Asahi, T.; Iwasaki, Y. Suppression of the heterotrimeric G protein causes abnormal morphology, including dwarfism, in rice. Proc. Natl. Acad. Sci. USA 1999, 96, 7575–7580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueguchi-Tanaka, M.; Fujisawa, Y.; Kobayashi, M.; Ashikari, M.; Iwasaki, Y.; Kitano, H.; Matsuoka, M. Rice dwarf mutant d1, which is defective in the alpha subunit of the heterotrimeric G protein, affects gibberellin signal transduction. Proc. Natl. Acad. Sci. USA 2000, 97, 11638–11643. [Google Scholar] [CrossRef]
- Ferrero-Serrano, A.; Su, Z.; Assmann, S.M. Illuminating the role of the Galpha heterotrimeric G protein subunit, RGA1, in regulating photoprotection and photoavoidance in rice. Plant Cell Environ. 2018, 41, 451–468. [Google Scholar] [CrossRef]
- Jangam, A.P.; Pathak, R.R.; Raghuram, N. Microarray Analysis of Rice d1 (RGA1) Mutant Reveals the Potential Role of G-Protein Alpha Subunit in Regulating Multiple Abiotic Stresses Such as Drought, Salinity, Heat, and Cold. Front. Plant Sci. 2016, 7, 11. [Google Scholar] [CrossRef]
- Steffens, B.; Sauter, M. G proteins as regulators in ethylene-mediated hypoxia signaling. Plant Signal Behav. 2010, 5, 375–378. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Dai, X.; Xu, Y.; Luo, W.; Zheng, X.; Zeng, D.; Pan, Y.; Lin, X.; Liu, H.; Zhang, D.; et al. COLD1 confers chilling tolerance in rice. Cell 2015, 160, 1209–1221. [Google Scholar] [CrossRef] [PubMed]
- Ferrero-Serrano, A.; Assmann, S.M. The alpha-subunit of the rice heterotrimeric G protein, RGA1, regulates drought tolerance during the vegetative phase in the dwarf rice mutant d1. J. Exp. Bot. 2016, 67, 3433–3443. [Google Scholar] [CrossRef] [PubMed]
- Julkowska, M.M.; Testerink, C. Tuning plant signaling and growth to survive salt. Trends Plant Sci. 2015, 20, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, T.; Morinaka, Y.; Ohnishi, T.; Sunohara, H.; Fujioka, S.; Ueguchi-Tanaka, M.; Mizutani, M.; Sakata, K.; Takatsuto, S.; Yoshida, S.; et al. Erect leaves caused by brassinosteroid deficiency increase biomass production and grain yield in rice. Nat. Biotechnol. 2006, 24, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Hakim, M.A.; Juraimi, A.S.; Hanafi, M.M.; Ali, E.; Ismail, M.R.; Selamat, A.; Karim, S.M. Effect of salt stress on morpho-physiology, vegetative growth and yield of rice. J. Environ. Biol. 2014, 35, 317–326. [Google Scholar] [PubMed]
- Miura, K.; Agetsuma, M.; Kitano, H.; Yoshimura, A.; Matsuoka, M.; Jacobsen, S.E.; Ashikari, M. A metastable DWARF1 epigenetic mutant affecting plant stature in rice. Proc. Natl. Acad. Sci. USA 2009, 106, 11218–11223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.K. Plant salt tolerance. Trends Plant Sci. 2001, 6, 66–71. [Google Scholar] [CrossRef]
- Suo, J.; Zhao, Q.; David, L.; Chen, S.; Dai, S. Salinity Response in Chloroplasts: Insights from Gene Characterization. Int. J. Mol. Sci. 2017, 18, 1011. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Suzuki, N.; Koussevitzky, S.; Mittler, R.; Miller, G. ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ. 2012, 35, 259–270. [Google Scholar] [CrossRef]
- Steffens, B. The role of ethylene and ROS in salinity, heavy metal, and flooding responses in rice. Front. Plant Sci. 2014, 5, 685. [Google Scholar] [CrossRef]
- Chan, Z.; Yokawa, K.; Kim, W.Y.; Song, C.P. Editorial: ROS Regulation during Plant Abiotic Stress Responses. Front. Plant Sci. 2016, 7, 1536. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H. Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ. Exp. Bot. 2018, 154, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Michelmore, R.W.; Paran, I.; Kesseli, R.V. Identification of markers linked to disease-resistance genes by bulked segregant analysis: A rapid method to detect markers in specific genomic regions by using segregating populations. Proc. Natl. Acad. Sci. USA 1991, 88, 9828–9832. [Google Scholar] [CrossRef] [PubMed]
- Porra, R.J. The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth. Res. 2002, 73, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.; Li, X.; Hicks, L.M.; Xiong, L. A Raf-like MAPKKK gene DSM1 mediates drought resistance through reactive oxygen species scavenging in rice. Plant Physiol. 2010, 152, 876–890. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Zhou, J.; Lu, X.; Huang, R.; Zhang, H. Excessive UDPG resulting from the mutation of UAP1 causes programmed cell death by triggering reactive oxygen species accumulation and caspase-like activity in rice. New Phytol. 2018, 217, 332–343. [Google Scholar] [CrossRef]
- Liu, S.; Waqas, M.A.; Wang, S.H.; Xiong, X.Y.; Wan, Y.F. Effects of increased levels of atmospheric CO2 and high temperatures on rice growth and quality. PLoS ONE 2017, 12, e0187724. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.Q.; Zhang, J.H.; Sun, L.M.; Zhu, L.F.; Abliz, B.; Hu, W.J.; Zhong, C.; Bai, Z.G.; Sajid, H.; Cao, X.C.; et al. Hydrogen Sulfide Alleviates Aluminum Toxicity via Decreasing Apoplast and Symplast Al Contents in Rice. Front. Plant Sci. 2018, 9, 294. [Google Scholar] [CrossRef]


| Categories | Accession | Description | Peptide | Unique Peptide | Coverage | PSMs | sd58-C Versus Kitaake-C | Kitaake-S Versus Kitaake-C | sd58-S Versus sd58-C | sd58-S Versus Kitaake-S |
|---|---|---|---|---|---|---|---|---|---|---|
| Photosynthesis | Q0DG05 | Photosystem I reaction center subunit VI | 4 | 4 | 39.44 | 61 | 1.194687663 | 0.884711293 | 1.021167747 | 1.378954377 |
| Q8H4P7 | photosystem II 10 kDa polypeptide | 4 | 4 | 24.09 | 29 | 1.546765379 | 0.90177992 | 0.963631996 | 1.652856287 | |
| Q943W1 | oxygen-evolving enhancer protein 1 | 22 | 22 | 64.56 | 291 | 1.148000937 | 0.908757715 | 1.058357592 | 1.336985081 | |
| P0C2Y7 | ATP synthase subunit a | 2 | 2 | 3.24 | 7 | 1.189606815 | 0.905690444 | 1.040516165 | 1.366697782 | |
| Q40684 | Ferredoxin | 1 | 1 | 6.76 | 2 | 5.256444569 | 1.753843469 | 0.058994804 | 0.176813339 | |
| P0C301 | ATP synthase subunit c | 1 | 1 | 8.64 | 2 | 1.06387461 | 0.977608316 | 1.230407281 | 1.338981108 | |
| Q7XTG4 | Membrane protein | 1 | 1 | 4.93 | 6 | 1.080830998 | 0.945503375 | 1.124059544 | 1.284943482 | |
| P0C2Z6 | ATP synthase subunit alpha | 26 | 2 | 57.59 | 310 | 1.396674248 | 0.838719483 | 1.10045561 | 1.832529283 | |
| Q6ZBV1 | photosystem II 10 kDa polypeptide | 3 | 3 | 23.08 | 48 | 1.079804792 | 0.931293627 | 1.12603067 | 1.305596084 | |
| Q6ZFJ3 | Ferredoxin--NADP reductase | 23 | 19 | 58.74 | 149 | 1.150440812 | 0.955853923 | 1.077095101 | 1.296363527 | |
| P41344 | Ferredoxin--NADP reductase, | 23 | 19 | 58.29 | 155 | 1.113047516 | 0.928829949 | 1.074319895 | 1.287392909 | |
| Q6Z3V7 | Photosystem I reaction center subunit VI | 3 | 3 | 42.28 | 44 | 1.21137611 | 0.948516534 | 1.17008755 | 1.494350445 | |
| Porphyrin and chlorophyll metabolism | Q7XKF3 | Protochlorophyllide reductase A | 18 | 18 | 60.21 | 46 | 1.244582113 | 0.977177448 | 0.996806607 | 1.269582792 |
| Photosynthesis- antenna proteins | Q10HD0 | Chlorophyll a-b binding protein | 7 | 4 | 31.94 | 186 | 1.161584754 | 0.96898888 | 1.071110401 | 1.284003911 |
| Q5ZA98 | Chlorophyll a-b binding protein | 6 | 6 | 17.01 | 45 | 1.210805009 | 0.993780608 | 1.053959837 | 1.284126335 | |
| Q6ZL95 | Chlorophyll a-b binding protein | 3 | 3 | 21.67 | 19 | 1.381846156 | 0.894720871 | 0.994724709 | 1.536296471 | |
| Q6YWJ7 | Chlorophyll a-b binding protein | 6 | 6 | 35.66 | 39 | 1.235309035 | 0.951992885 | 1.070666181 | 1.389299887 | |
| Carbon metabolism | Q7X8A1 | Glyceraldehyde-3-phosphate dehydrogenase | 19 | 15 | 53.23 | 269 | 1.185175004 | 0.893344399 | 1.068761987 | 1.417896607 |
| Q9SE42 | Ribulose-phosphate 3-epimerase | 4 | 4 | 25.88 | 12 | 1.125852891 | 0.92458396 | 1.103994095 | 1.344318091 | |
| Q7XZW5 | Malate dehydrogenase | 15 | 13 | 52.26 | 77 | 1.236084716 | 0.958486603 | 1.042411695 | 1.344316299 | |
| Q40677 | Fructose-bisphosphate aldolase | 19 | 18 | 53.35 | 220 | 1.164792195 | 0.983320615 | 1.086516982 | 1.287033427 | |
| Peroxisome | Q9FW35 | pex14 protein | 2 | 2 | 5.39 | 5 | 1.313339411 | 0.994865066 | 1.019323065 | 1.345626859 |
| B7EBN1 | Os02g0225000 protein | 2 | 1 | 6.61 | 7 | 1.19598591 | 0.930099311 | 0.991836807 | 1.275372245 | |
| Glutathione metabolism | Q6ZJJ1 | Probable L-ascorbate peroxidase 4 | 14 | 12 | 44.67 | 69 | 1.204637136 | 0.986577028 | 1.057649443 | 1.291418471 |
| B7FAE9 | Glutathione peroxidase | 8 | 6 | 45.3 | 25 | 1.189347746 | 1.034130023 | 1.128526366 | 1.297912506 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, P.; Gao, Y.; Li, Z.; Yu, Y.; Qin, H.; Guo, Y.; Huang, R.; Wang, J. Proteomic Analysis of a Rice Mutant sd58 Possessing a Novel d1 Allele of Heterotrimeric G Protein Alpha Subunit (RGA1) in Salt Stress with a Focus on ROS Scavenging. Int. J. Mol. Sci. 2019, 20, 167. https://doi.org/10.3390/ijms20010167
Peng P, Gao Y, Li Z, Yu Y, Qin H, Guo Y, Huang R, Wang J. Proteomic Analysis of a Rice Mutant sd58 Possessing a Novel d1 Allele of Heterotrimeric G Protein Alpha Subunit (RGA1) in Salt Stress with a Focus on ROS Scavenging. International Journal of Molecular Sciences. 2019; 20(1):167. https://doi.org/10.3390/ijms20010167
Chicago/Turabian StylePeng, Peng, Yadi Gao, Zhe Li, Yanwen Yu, Hua Qin, Yan Guo, Rongfeng Huang, and Juan Wang. 2019. "Proteomic Analysis of a Rice Mutant sd58 Possessing a Novel d1 Allele of Heterotrimeric G Protein Alpha Subunit (RGA1) in Salt Stress with a Focus on ROS Scavenging" International Journal of Molecular Sciences 20, no. 1: 167. https://doi.org/10.3390/ijms20010167




