Visualization of Annular Gap Junction Vesicle Processing: The Interplay Between Annular Gap Junctions and Mitochondria
Abstract
:1. Introduction
2. Results
2.1. Characterization of Cx43-Containing Gap Junctions’ Structure Behavior
Live Cell Imaging of Gap Junction Plaque Endoexocytosis in Cells Expressing Cx43-GFP
2.2. Annular Gap Junction Fusion with Other Organelles
2.2.1. Analysis of Lysosomes/Annular Gap Junction Associations
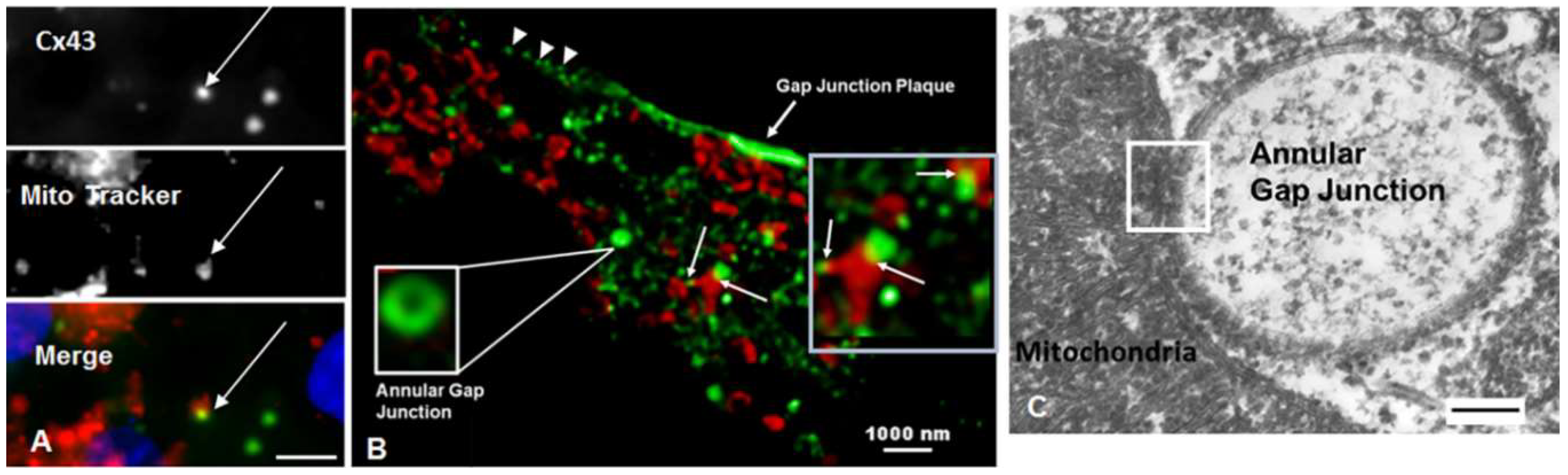
2.2.2. Mitochondria
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Gap Junction Antibodies and Probes
4.3. Immunocytochemistry
4.4. Percent Colocalization
4.5. Transfection with cDNA
4.6. Imaging of Cx43-GFP in Living Cells
4.7. Super-Resolution Live Cell Microscopy
4.8. Transmission Electron Microscopy
4.9. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| TEM | Transmission Electron Microscopy |
| QDs | Quantum Dots |
| AGJ | Annular Gap Junction Vesicle |
References
- Goodenough, D.A.; Goliger, J.A.; Paul, D.L. Connexins, Connexons, and intercellular communication (Review). Ann. Rev. Biochem. 1996, 65, 475–502. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.M. Molecular biology of the interactions between connexins. Novartis Found. Symp. 1999, 219, 6–16; discussion 16–21, 38–43. [Google Scholar] [PubMed]
- Kumar, N.M.; Gilula, N.B. Cloning and characterization of human and rat liver cDNAs coding for a gap junction protein. J. Cell Biol. 1986, 103, 767–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilula, N.B. Topology of gap junction protein and channel function. CIBA Found. Symp. 1987, 125, 128–139. [Google Scholar] [PubMed]
- Sohl, G.; Willecke, K. Gap junctions and the connexin protein family. Cardiovasc. Res. 2004, 62, 228–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, S.; Diez, J.A.; George, C.H.; Evans, W.H. Synthesis and assembly of connexins in vitro into homomeric and heteromeric functional gap junction hemichannels. Biochem. J. 1999, 339, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Perkins, G.A.; Goodenough, D.A.; Sosinsky, G.E. Formation of the gap junction intercellular channel requires a 30 degree rotation for interdigitating two apposing connexons. J. Mol. Biol. 1998, 277, 171–177. [Google Scholar] [CrossRef]
- Murray, S.A.; Larsen, W.J.; Trout, J.; Donta, S.T. Gap junction assembly and endocytosis correlated with patterns of growth in a cultured adrenocortical tumor cell (SW-13). Cancer Res. 1981, 41, 4063–4074. [Google Scholar]
- Falk, M.M.; Bell, C.L.; Kells Andrews, R.M.; Murray, S.A. Molecular Mechanisms Regulating the Formation, Trafficking and Processing of Annular Gap Junctions. BMC Cell Biol. 2016, 17, 22. [Google Scholar] [CrossRef]
- Nickel, B.M.; DeFranco, B.H.; Gay, V.L.; Murray, S.A. Clathrin and Cx43 gap junction plaque endoexocytosis. Biochem. Biophys. Res. Commun. 2008, 374, 679–682. [Google Scholar] [CrossRef]
- Huang, X.D.; Horackova, M.; Pressler, M.L. Changes in the expression and distribution of connexin 43 in isolated cultured adult guinea pig cardiomyocytes. Exp. Cell Res. 1996, 228, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Gumpert, A.M.; Varco, J.S.; Baker, S.M.; Piehl, M.; Falk, M.M. Double-membrane gap junction internalization requires the clathrin-mediated endocytic machinery. FEBS Lett. 2008, 582, 2887–2892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piehl, M.; Lehmann, C.; Gumpert, A.; Denizot, J.P.; Segretain, D.; Falk, M.M. Internalization of Large Double-Membrane Intercellular Vesicles by a Clathrin-dependent Endocytic Process. Mol. Biol. Cell 2007, 18, 337–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsen, W.J.; Tung, H.N.; Murray, S.A.; Swenson, C.A. Evidence for the participation of actin microfilaments and bristle coats in the internalization of gap junction membrane. J. Cell Biol. 1979, 83, 576–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsen, W.J.; Hai, N. Origin and fate of cytoplasmic gap junctional vesicles in rabbit granulosa cells. Tissue Cell 1978, 10, 585–598. [Google Scholar] [CrossRef]
- Jordan, K.; Chodock, R.; Hand, A.R.; Laird, D.W. The origin of annular junctions: A mechanism of gap junction internalization. J. Cell Sci. 2001, 114, 763–773. [Google Scholar] [PubMed]
- Naus, C.C.; Hearn, S.; Zhu, D.; Nicholson, B.J.; Shivers, R.R. Ultrastructural analysis of gap junctions in C6 glioma cells transfected with connexin43 cDNA. Exp. Cell Res. 1993, 206, 72–84. [Google Scholar] [CrossRef]
- Risley, M.S.; Tan, I.P.; Roy, C.; Saez, J.C. Cell-, age- and stage-dependent distribution of connexin43 gap junctions in testes. J. Cell Sci. 1992, 103, 81–96. [Google Scholar]
- Jordan, K.; Solan, J.L.; Dominguez, M.; Sia, M.; Hand, A.; Lampe, P.D.; Laird, D.W. Trafficking, assembly, and function of a connexin43-green fluorescent protein chimera in live mammalian cells. Mol. Biol. Cell 1999, 10, 2033–2050. [Google Scholar] [CrossRef]
- Segretain, D.; Falk, M.M. Regulation of connexin biosynthesis, assembly, gap junction formation, and removal. Biochim. Biophys. Acta 2004, 1662, 3–21. [Google Scholar] [CrossRef]
- Lauf, U.; Giepmans, B.N.; Lopez, P.; Braconnot, S.; Chen, S.C.; Falk, M.M. Dynamic trafficking and delivery of connexons to the plasma membrane and accretion to gap junctions in living cells. Proc. Natl. Acad. Sci. USA 2002, 99, 10446–10451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sosinsky, G.E.; Gaietta, G.M.; Hand, G.; Deerinck, T.J.; Han, A.; Mackey, M.; Adams, S.R.; Bouwer, J.; Tsien, R.Y.; Ellisman, M.H. Tetracysteine genetic tags complexed with biarsenical ligands as a tool for investigating gap junction structure and dynamics. Cell Commun. Adhes. 2003, 10, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Nickel, B.; Boller, M.; Schneider, K.; Shakespeare, T.; Gay, V.; Murray, S.A. Visualizing the effect of dynamin inhibition on annular gap vesicle formation and fission. J. Cell Sci. 2013, 126 Pt 12, 2607–2616. [Google Scholar] [CrossRef] [Green Version]
- Iyyathurai, J.; Decuypere, J.P.; Leybaert, L.; D’Hondt, C.; Bultynck, G. Connexins: Substrates and regulators of autophagy. BMC Cell Biol. 2016, 17 (Suppl. 1), 20. [Google Scholar] [CrossRef]
- Bejarano, E.; Girao, H.; Yuste, A.; Patel, B.; Marques, C.; Spray, D.C.; Pereira, P.; Cuervo, A.M. Autophagy modulates dynamics of connexins at the plasma membrane in a ubiquitin-dependent manner. Mol. Biol. Cell 2012, 23, 2156–2169. [Google Scholar] [CrossRef] [Green Version]
- Girao, H.; Catarino, S.; Pereira, P. Eps15 interacts with ubiquitinated Cx43 and mediates its internalization. Exp. Cell Res. 2009, 315, 3587–3597. [Google Scholar] [CrossRef] [PubMed]
- Leithe, E.; Rivedal, E. Ubiquitination and down-regulation of gap junction protein connexin-43 in response to 12-O-tetradecanoylphorbol 13-acetate treatment. J. Biol. Chem. 2004, 279, 50089–50096. [Google Scholar] [CrossRef]
- Martins-Marques, T.; Catarino, S.; Marques, C.; Matafome, P.; Ribeiro-Rodrigues, T.; Baptista, R.; Pereira, P.; Girao, H. Heart ischemia results in connexin43 ubiquitination localized at the intercalated discs. Biochimie 2015, 112, 196–201. [Google Scholar] [CrossRef]
- Martins-Marques, T.; Catarino, S.; Zuzarte, M.; Marques, C.; Matafome, P.; Pereira, P.; Girao, H. Ischaemia-induced autophagy leads to degradation of gap junction protein connexin43 in cardiomyocytes. Biochem. J. 2015, 467, 231–245. [Google Scholar] [CrossRef]
- Qin, H.; Shao, Q.; Igdoura, S.A.; Alaoui-Jamali, M.A.; Laird, D.W. Lysosomal and proteasomal degradation play distinct roles in the life cycle of Cx43 in gap junctional intercellular communication-deficient and -competent breast tumor cells. J. Biol. Chem. 2003, 278, 30005–30014. [Google Scholar] [CrossRef]
- Boassa, D.; Solan, J.L.; Papas, A.; Thornton, P.; Lampe, P.D.; Sosinsky, G.E. Trafficking and recycling of the connexin43 gap junction protein during mitosis. Traffic 2010, 11, 1471–1486. [Google Scholar] [CrossRef] [PubMed]
- Vanderpuye, O.A.; Bell, C.L.; Murray, S.A. Dynamic Resdistribution of Connexin 43 During Cell Division. Cell Biol. Int. 2016, 40, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.W.; Wessels, A. Cx43 gap junctions in cardiac development. Trends Cardiovasc. Med. 1998, 8, 264–269. [Google Scholar] [CrossRef]
- Loewenstein, W.R.; Rose, B. The cell-cell channel in the control of growth. Semin. Cell Biol. 1992, 3, 59–79. [Google Scholar] [CrossRef]
- Defranco, B.H.; Nickel, B.M.; Baty, C.J.; Martinez, J.S.; Gay, V.L.; Sandulache, V.C.; Hackam, D.J.; Murray, S.A. Migrating cells retain gap junction plaque structure and function. Cell Commun. Adhes. 2008, 15, 273–288. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, P.; Qiu, C.; Frank, S.; Tamber, K.; Becker, D. Dynamic changes in connexin expression correlate with key events in the wound healing process. Cell Biol. Int. 2003, 27, 525–541. [Google Scholar] [CrossRef]
- Schulz, R.; Gres, P.; Skyschally, A.; Duschin, A.; Belosjorow, S.; Konietzka, I.; Heusch, G. Ischemic preconditioning preserves connexin 43 phosphorylation during sustained ischemia in pig hearts in vivo. FASEB J. 2003, 17, 1355–1357. [Google Scholar] [CrossRef] [PubMed]
- Boengler, K.; Schulz, R. Connexin 43 and Mitochondria in Cardiovascular Health and Disease. Adv. Exp. Med. Biol. 2017, 982, 227–246. [Google Scholar]
- Michela, P.; Velia, V.; Aldo, P.; Ada, P. Role of connexin 43 in cardiovascular diseases. Eur. J. Pharmacol. 2015, 768, 71–76. [Google Scholar] [CrossRef]
- Viczenczova, C.; Kura, B.; Chaudagar, K.K.; Szeiffova Bacova, B.; Egan Benova, T.; Barancik, M.; Knezl, V.; Ravingerova, T.; Tribulova, N.; Slezak, J. Myocardial connexin-43 is upregulated in response to acute cardiac injury in rats. Can. J. Physiol. Pharmacol. 2017, 95, 911–919. [Google Scholar] [CrossRef]
- Radosinska, J.; Bacova, B.; Knezl, V.; Benova, T.; Zurmanova, J.; Soukup, T.; Arnostova, P.; Slezak, J.; Goncalvesova, E.; Tribulova, N. Dietary omega-3 fatty acids attenuate myocardial arrhythmogenic factors and propensity of the heart to lethal arrhythmias in a rodent model of human essential hypertension. J. Hypertens. 2013, 31, 1876–1885. [Google Scholar] [CrossRef]
- Saffitz, J.E.; Laing, J.G.; Yamada, K.A. Connexin expression and turnover: Implications for cardiac excitability. Circ. Res. 2000, 86, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Forbes, M.S.; Sperelakis, N. Association between mitochondria and gap junctions in mammalian myocardial cells. Tissue Cell 1982, 14, 25–37. [Google Scholar] [CrossRef]
- Wong, Y.C.; Ysselstein, D.; Krainc, D. Mitochondria-lysosome contacts regulate mitochondrial fission via RAB7 GTP hydrolysis. Nature 2018, 554, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Van der Bliek, A.M.; Shen, Q.; Kawajiri, S. Mechanisms of mitochondrial fission and fusion. Cold Spring Harb. Perspect. Biol. 2013, 5, a011072. [Google Scholar] [CrossRef]
- Lin, H.; Mitasikova, M.; Dlugosova, K.; Okruhlicova, L.; Imanaga, I.; Ogawa, K.; Weismann, P.; Tribulova, N. Thyroid hormones suppress epsilon-PKC signalling, down-regulate connexin-43 and increase lethal arrhythmia susceptibility in non-diabetic and diabetic rat hearts. J. Physiol. Pharmacol. 2008, 59, 271–285. [Google Scholar]
- Tribulova, N.; Knezl, V.; Szeiffova Bacova, B.; Egan Benova, T.; Viczenczova, C.; Goncalvesova, E.; Slezak, J. Disordered myocardial Ca(2+) homeostasis results in substructural alterations that may promote occurrence of malignant arrhythmias. Physiol. Res. 2016, 65 (Suppl. 1), S139–S148. [Google Scholar] [PubMed]
- Emdad, L.; Uzzaman, M.; Takagishi, Y.; Honjo, H.; Uchida, T.; Severs, N.J.; Kodama, I.; Murata, Y. Gap junction remodeling in hypertrophied left ventricles of aortic-banded rats: Prevention by angiotensin II type 1 receptor blockade. J. Mol. Cell. Cardiol. 2001, 33, 219–231. [Google Scholar] [CrossRef]
- Boengler, K.; Dodoni, G.; Rodriguez-Sinovas, A.; Cabestrero, A.; Ruiz-Meana, M.; Gres, P.; Konietzka, I.; Lopez-Iglesias, C.; Garcia-Dorado, D.; Di Lisa, F.; et al. Connexin 43 in cardiomyocyte mitochondria and its increase by ischemic preconditioning. Cardiovasc. Res. 2005, 67, 234–244. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Sinovas, A.; Boengler, K.; Cabestrero, A.; Gres, P.; Morente, M.; Ruiz-Meana, M.; Konietzka, I.; Miro, E.; Totzeck, A.; Heusch, G.; et al. Translocation of connexin 43 to the inner mitochondrial membrane of cardiomyocytes through the heat shock protein 90-dependent TOM pathway and its importance for cardioprotection. Circ. Res. 2006, 99, 93–101. [Google Scholar] [CrossRef]
- Miro-Casas, E.; Ruiz-Meana, M.; Agullo, E.; Stahlhofen, S.; Rodriguez-Sinovas, A.; Cabestrero, A.; Jorge, I.; Torre, I.; Vazquez, J.; Boengler, K.; et al. Connexin43 in cardiomyocyte mitochondria contributes to mitochondrial potassium uptake. Cardiovasc. Res. 2009, 83, 747–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boengler, K.; Konietzka, I.; Buechert, A.; Heinen, Y.; Garcia-Dorado, D.; Heusch, G.; Schulz, R. Loss of ischemic preconditioning’s cardioprotection in aged mouse hearts is associated with reduced gap junctional and mitochondrial levels of connexin 43. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H1764–H1769. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhang, S.S.; Xiao, S.; Basheer, W.A.; Baum, R.; Epifantseva, I.; Hong, T.; Shaw, R.M. Cx43 Isoform GJA1-20k Promotes Microtubule Dependent Mitochondrial Transport. Front. Physiol. 2017, 8, 905. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.N.; Kwon, H.J.; Im, S.W.; Son, Y.H.; Akindehin, S.; Jung, Y.S.; Lee, S.J.; Rhyu, I.J.; Kim, I.Y.; Seong, J.K.; et al. Connexin 43 is required for the maintenance of mitochondrial integrity in brown adipose tissue. Sci. Rep. 2017, 7, 7159. [Google Scholar] [CrossRef] [PubMed]
- Boengler, K.; Ruiz-Meana, M.; Gent, S.; Ungefug, E.; Soetkamp, D.; Miro-Casas, E.; Cabestrero, A.; Fernandez-Sanz, C.; Semenzato, M.; Di Lisa, F.; et al. Mitochondrial connexin 43 impacts on respiratory complex I activity and mitochondrial oxygen consumption. J. Cell. Mol. Med. 2012, 16, 1649–1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halestrap, A.P. Mitochondria and preconditioning: A connexin connection? Circ. Res. 2006, 99, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Goubaeva, F.; Mikami, M.; Giardina, S.; Ding, B.; Abe, J.; Yang, J. Cardiac mitochondrial connexin 43 regulates apoptosis. Biochem. Biophys. Res. Commun. 2007, 352, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Meana, M.; Rodriguez-Sinovas, A.; Cabestrero, A.; Boengler, K.; Heusch, G.; Garcia-Dorado, D. Mitochondrial connexin43 as a new player in the pathophysiology of myocardial ischaemia-reperfusion injury. Cardiovasc. Res. 2008, 77, 325–333. [Google Scholar] [CrossRef]
- Rivedal, E.; Leithe, E. Connexin43 synthesis, phosphorylation, and degradation in regulation of transient inhibition of gap junction intercellular communication by the phorbol ester TPA in rat liver epithelial cells. Exp. Cell Res. 2005, 302, 143–152. [Google Scholar] [CrossRef]
- Rivedal, E.; Opsahl, H. Role of PKC and MAP kinase in EGF- and TPA-induced connexin43 phosphorylation and inhibition of gap junction intercellular communication in rat liver epithelial cells. Carcinogenesis 2001, 22, 1543–1550. [Google Scholar] [CrossRef] [Green Version]
- Wynn, J.; Shah, U.; Murray, S.A. Redistribution of connexin 43 by cAMP: A mechanism for growth control in adrenal cells. Endocr. Res. 2002, 28, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Sirnes, S.; Kjenseth, A.; Leithe, E.; Rivedal, E. Interplay between PKC and the MAP kinase pathway in Connexin43 phosphorylation and inhibition of gap junction intercellular communication. Biochem. Biophys. Res. Commun. 2009, 382, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Rhett, J.M.; Jourdan, J.; Gourdie, R.G. Connexin 43 connexon to gap junction transition is regulated by zonula occludens-1. Mol. Biol. Cell 2011, 22, 1516–1528. [Google Scholar] [CrossRef] [PubMed]
- Gilleron, J.; Fiorini, C.; Carette, D.; Avondet, C.; Falk, M.M.; Segretain, D.; Pointis, G. Molecular reorganization of Cx43, Zo-1 and Src complexes during the endocytosis of gap junction plaques in response to a non-genomic carcinogen. J. Cell Sci. 2008, 121, 4069–4078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barker, R.J.; Price, R.L.; Gourdie, R.G. Increased association of ZO-1 with connexin43 during remodeling of cardiac gap junctions. Circ Res. 2002, 90, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Barker, R.J.; Price, R.L.; Gourdie, R.G. Increased co-localization of connexin43 and ZO-1 in dissociated adult myocytes. Cell Commun. Adhes. 2001, 8, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Dunn, C.A.; Lampe, P.D. Injury-triggered Akt phosphorylation of Cx43: A ZO-1-driven molecular switch that regulates gap junction size. J. Cell Sci. 2014, 127, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Hunter, A.W.; Jourdan, J.; Gourdie, R.G. Fusion of GFP to the carboxyl terminus of connexin43 increases gap junction size in HeLa cells. Cell Commun. Adhes. 2003, 10, 211–214. [Google Scholar] [CrossRef]
- Kostin, S. Zonula occludens-1 and connexin 43 expression in the failing human heart. J. Cell. Mol. Med. 2007, 11, 892–895. [Google Scholar] [CrossRef] [Green Version]
- Larsen, W.J. Structural diversity of gap junctions. A review. Tissue Cell 1977, 9, 373–394. [Google Scholar] [CrossRef]
- Kostin, S.; Hein, S.; Bauer, E.P.; Schaper, J. Spatiotemporal development and distribution of intercellular junctions in adult rat cardiomyocytes in culture. Circ. Res. 1999, 85, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Carette, D.; Gilleron, J.; Denizot, J.P.; Grant, K.; Pointis, G.; Segretain, D. New cellular mechanisms of gap junction degradation and recycling. Biol. Cell 2015, 107, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.M.; Gilula, N.B. Molecular biology and genetics of gap junction channels. Semin. Cell Biol. 1992, 3, 3–16. [Google Scholar] [CrossRef]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bell, C.L.; Shakespeare, T.I.; Smith, A.R.; Murray, S.A. Visualization of Annular Gap Junction Vesicle Processing: The Interplay Between Annular Gap Junctions and Mitochondria. Int. J. Mol. Sci. 2019, 20, 44. https://doi.org/10.3390/ijms20010044
Bell CL, Shakespeare TI, Smith AR, Murray SA. Visualization of Annular Gap Junction Vesicle Processing: The Interplay Between Annular Gap Junctions and Mitochondria. International Journal of Molecular Sciences. 2019; 20(1):44. https://doi.org/10.3390/ijms20010044
Chicago/Turabian StyleBell, Cheryl L., Teresa I. Shakespeare, Amber R. Smith, and Sandra A. Murray. 2019. "Visualization of Annular Gap Junction Vesicle Processing: The Interplay Between Annular Gap Junctions and Mitochondria" International Journal of Molecular Sciences 20, no. 1: 44. https://doi.org/10.3390/ijms20010044




