Biosynthesis of Natural Rubber: Current State and Perspectives
Abstract
1. Introduction
2. Biosynthesis of Natural Rubber and the Involved Genes/Proteins
2.1. Biosynthesis and Physiological Roles of Natural Rubber
2.2. Structure, Components and Biogenesis of Rubber Particles
2.3. Genes/Proteins Involved in Natural Rubber Biosynthesis
2.3.1. cis-Prenyltransferase
2.3.2. Rubber Elongation Factor and Small Rubber Particle Protein
2.3.3. CPT-Like/CPT-Binding Protein
2.3.4. Other Involved Genes/Proteins/Pathways
3. Omics Analyses Provide New Insights into Natural Rubber Biosynthesis
3.1. Genome Analysis
3.2. Transcriptome Analysis
3.3. Proteome Analysis
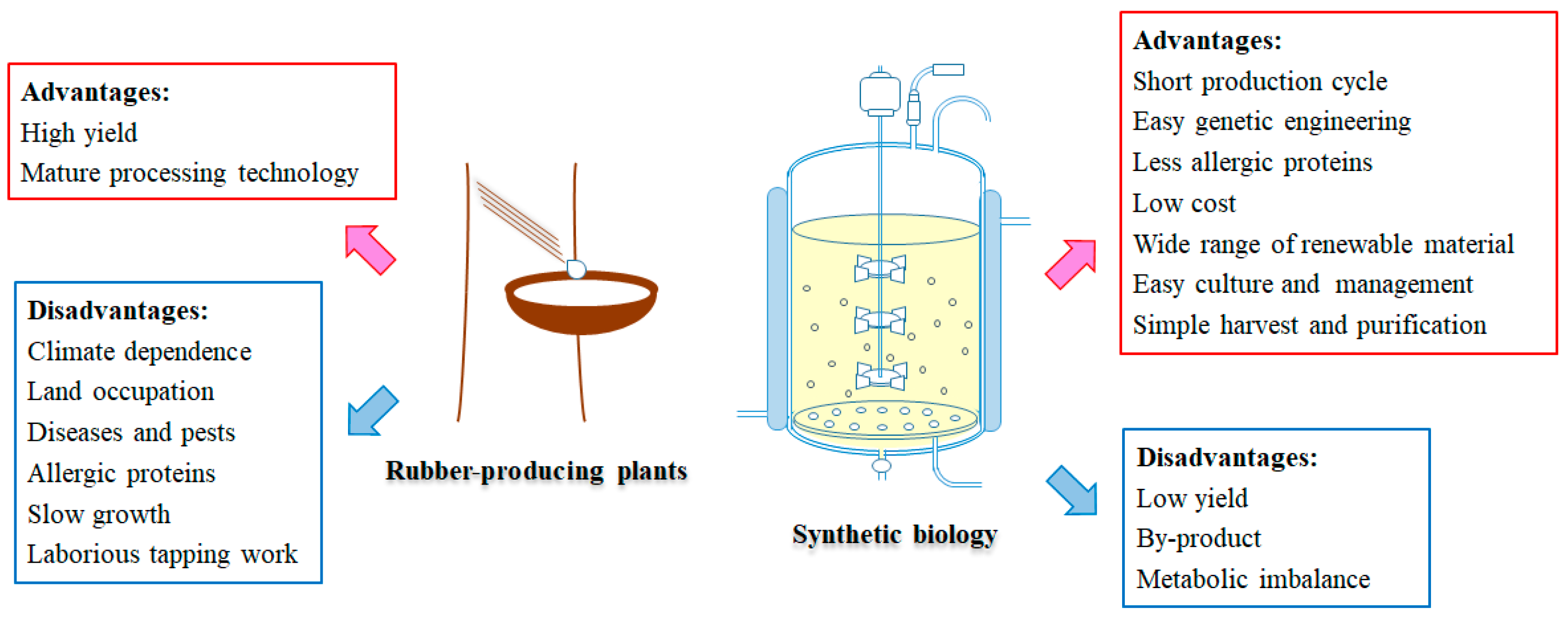
4. Possibility of Natural Rubber Biosynthesis In Vitro or in Genetically Engineered Microorganisms
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Cornish, K. Similarities and differences in rubber biochemistry among plant species. Phytochemistry 2001, 57, 1123–1134. [Google Scholar] [CrossRef]
- Van Beilen, J.B.; Poirier, Y. Establishment of new crops for the production of natural rubber. Trends Biotechnol. 2007, 25. [Google Scholar] [CrossRef] [PubMed]
- Whalen, M.; McMahan, C.; Shintani, D. Development of crops to produce industrially useful natural rubber. In Isoprenoid Synthesis in Plants and Microorganisms: New Concepts and Experimental Approaches; Bach, T.J., Rohmer, M., Eds.; Springer: New York, NY, USA, 2013; pp. 329–345. [Google Scholar] [CrossRef]
- Mooibroek, H.; Cornish, K. Alternative sources of natural rubber. Appl. Microbiol. Biotechnol. 2000, 53, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Wuyun, T.; Wang, L.; Liu, H.; Wang, X.; Zhang, L.; Bennetzen, J.L.; Li, T.; Yang, L.; Liu, P.; Du, L.; et al. The hardy rubber tree genome provides insights into the evolution of polyisoprene biosynthesis. Mol. Plant. 2018, 11, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Uefuji, H.; Nishikawa, T.; Mukai, Y.; Yamashita, A.; Hattori, M.; Ogasawara, N.; Bamba, T.; Fukusaki, E.-I.; Kobayashi, A.; et al. Construction and analysis of EST libraries of the trans-polyisoprene producing plant, Eucommia ulmoides Oliver. Planta 2012, 236, 1405–1417. [Google Scholar] [CrossRef] [PubMed]
- Iaffaldano, B.; Zhang, Y.; Cornish, K. CRISPR/Cas9 genome editing of rubber producing dandelion Taraxacum kok-saghyz using Agrobacterium rhizogenes without selection. Ind. Crops Prod. 2016, 89, 356–362. [Google Scholar] [CrossRef]
- Tang, C.; Xiao, X.; Li, H.; Fan, Y.; Yang, J.; Qi, J.; Li, H. Comparative analysis of latex transcriptome reveals putative molecular mechanisms underlying super productivity of Hevea brasiliensis. PLoS ONE 2013, 8, e75307. [Google Scholar] [CrossRef] [PubMed]
- Blanc, G.; Baptiste, C.; Oliver, G.; Martin, F.; Montoro, P. Efficient Agrobacterium tumefaciens-mediated transformation of embryogenic calli and regeneration of Hevea brasiliensis Müll Arg. plants. Plant. Cell. Rep. 2006, 24, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Stolze, A.; Wanke, A.; van Deenen, N.; Geyer, R.; Prüfer, D.; Schulze Gronover, C. Development of rubber-enriched dandelion varieties by metabolic engineering of the inulin pathway. Plant. Biotechnol. J. 2017, 15, 740–753. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Xu, X.; Ruan, J.; Liu, S.; Wu, S.; Shao, X.; Wang, X.; Gan, L.; Qin, B.; Yang, Y.; et al. Genome analysis of Taraxacum kok-saghyz Rodin provides new insights into rubber biosynthesis. Natl. Sci. Rev. 2018, 5, 78–87. [Google Scholar] [CrossRef]
- Chiang, C.K.; Xie, W.; McMahan, C.; Puskas, J.E. Unraveling the mystery of natural rubber biosynthesis. part I: Investigation of the composition and growth of in vitro natural rubber using high resolution size exclusion choromatography. Rubber Chem. Technol. 2011, 84, 166–177. [Google Scholar] [CrossRef]
- da Costa, B.M.T.; Keasling, J.D.; Cornish, K. Regulation of rubber biosynthetic rate and molecular weight in Hevea brasiliensis by metal cofactor. Biomacromolecules 2005, 6, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Espy, S.C.; Keasling, J.D.; Castillón, J.; Cornish, K. Initiator-independent and initiator-dependent rubber biosynthesis in Ficus elastica. Arch. Biochem. Biophys. 2006, 448, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; McMahan, C.M.; DeGraw, A.J.; Distefano, M.D.; Cornish, K.; Whalen, M.C.; Shintani, D.K. Initiation of rubber biosynthesis: In vitro comparisons of benzophenone-modified diphosphate analogues in three rubber-producing species. Phytochemistry 2008, 69, 2539–2545. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, B.M.T.; Keasling, J.D.; McMahan, C.M.; Cornish, K. Magnesium ion regulation of in vitro rubber biosynthesis by Parthenium argentatum Gray. Phytochemistry 2006, 67, 1621–1628. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.J.; da Costa, B.M.T.; Espy, S.C.; Keasling, J.D.; Cornish, K. Activation and inhibition of rubber transferases by metal cofactors and pyrophosphate substrates. Phytochemistry 2003, 64, 123–134. [Google Scholar] [CrossRef]
- Ouardad, S.; Bakleh, M.-E.; Kostjuk, S.V.; Ganachaud, F.; Puskas, J.E.; Deffieux, A.; Peruch, F. Bio-inspired cationic polymerization of isoprene and analogues: State-of-the-art. Polym. Int. 2012, 61, 149–156. [Google Scholar] [CrossRef]
- Puskas, J.E.; Gautriaud, E.; Deffieux, A.; Kennedy, J.P. Natural rubber biosynthesis—A living carbocationic polymerization? Prog. Polym. Sci. 2006, 31, 533–548. [Google Scholar] [CrossRef]
- Cornish, K.; Castillón, J.; Scott, D.J. Rubber molecular weight regulation, in vitro, in plant species that produce high and low molecular weights in vivo. Biomacromolecules 2000, 1, 632–641. [Google Scholar] [CrossRef]
- Wang, D.; Sun, Y.; Chang, L.; Tong, Z.; Xie, Q.; Jin, X.; Zhu, L.; He, P.; Li, H.; Wang, X. Subcellular proteome profiles of different latex fractions revealed washed solutions from rubber particles contain crucial enzymes for natural rubber biosynthesis. J. Proteom. 2018, 182, 53–64. [Google Scholar] [CrossRef]
- Lau, N.S.; Makita, Y.; Kawashima, M.; Taylor, T.D.; Kondo, S.; Othman, A.S.; Shu-Chien, A.C.; Matsui, M. The rubber tree genome shows expansion of gene family associated with rubber biosynthesis. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Cornish, K.; Blakeslee, J.J. Rubber biosynthesis in plants. Plant Lipid Biochem. 2011. Available online: http://lipidlibrary.aocs.org/Biochemistry/content.cfm?ItemNumber=40312 (accessed on 20 December 2018).
- Ko, J.-H.; Chow, K.-S.; Han, K.-H. Transcriptome analysis reveals novel features of the molecular events occurring in the laticifers of Hevea brasiliensis (para rubber tree). Plant. Mol. Biol. 2003, 53, 479–492. [Google Scholar] [CrossRef] [PubMed]
- Konno, K.; Hirayama, C.; Nakamura, M.; Tateishi, K.; Tamura, Y.; Hattori, M.; Kohno, K. Papain protects papaya trees from herbivorous insects: Role of cysteine proteases in latex. Plant. J. 2004, 37, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Konno, K. Plant latex and other exudates as plant defense systems: Roles of various defense chemicals and proteins contained therein. Phytochemistry 2011, 72, 1510–1530. [Google Scholar] [CrossRef] [PubMed]
- Salvucci, M.E.; Barta, C.; Byers, J.A.; Canarini, A. Photosynthesis and assimilate partitioning between carbohydrates and isoprenoid products in vegetatively active and dormant guayule: Physiological and environmental constraints on rubber accumulation in a semiarid shrub. Physiol. Plant. 2010, 140, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Rio, M.; Leclercq, J.; Bonnot, F.; Oliver, G.; Montoro, P. Gene expression pattern in response to wounding, methyl jasmonate and ethylene in the bark of Hevea brasiliensis. Tree Physiol. 2010, 30, 1349–1359. [Google Scholar] [CrossRef]
- Kajiura, H.; Suzuki, N.; Mouri, H.; Watanabe, N.; Nakazawa, Y. Elucidation of rubber biosynthesis and accumulation in the rubber producing shrub, guayule (Parthenium argentatum Gray). Planta 2018, 247, 513–526. [Google Scholar] [CrossRef]
- Nakazawa, Y.; Takeda, T.; Suzuki, N.; Hayashi, T.; Harada, Y.; Bamba, T.; Kobayashi, A. Histochemical study of trans-polyisoprene accumulation by spectral confocal laser scanning microscopy and a specific dye showing fluorescence solvatochromism in the rubber-producing plant, Eucommia ulmoides Oliver. Planta 2013, 238, 549–560. [Google Scholar] [CrossRef]
- Berthelot, K.; Lecomte, S.; Estevez, Y.; Peruch, F. Hevea brasiliensis REF (Hev b 1) and SRPP (Hev b 3): An overview on rubber particle proteins. Biochimie 2014, 106, 1–9. [Google Scholar] [CrossRef]
- Tang, C.; Qi, J.; Li, H.; Zhang, C.; Wang, Y. A convenient and efficient protocol for isolating high-quality RNA from latex of Hevea brasiliensis (para rubber tree). J. Biochem. Bioph. Methods 2007, 70, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.; Lenders, M.; Hillebrand, A.; Deenen, N.V.; Munt, O.; Reichelt, R.; Eisenreich, W.; Fischer, R.; Prüfer, D.; Gronover, C.S. Characterization of rubber particles and rubber chain elongation in Taraxacum koksaghyz. BMC Biochem. 2010, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y. Structural characterization of natural polyisoprenes: Solve the mystery of natural rubber based on structural study. Rubber Chem. Technol. 2001, 74, 355–375. [Google Scholar] [CrossRef]
- Singh, A.P.; Wi, S.G.; Chung, G.C.; Kim, Y.S.; Kang, H. The micromorphology and protein characterization of rubber particles in Ficus carica, Ficus benghalensis and Hevea brasiliensis. J. Exp. Bot. 2003, 54, 985–992. [Google Scholar] [CrossRef]
- Spanò, D.; Pintus, F.; Esposito, F.; Loche, D.; Floris, G.; Medda, R. Euphorbia characias latex: Micromorphology of rubber particles and rubber transferase activity. Plant. Physiol. Biochem. 2015, 87, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Xia, K.; Dai, L.; Kang, G.; Li, Y.; Nie, Z.; Duan, C.; Zeng, R. Proteome analysis of the large and the small rubber particles of Hevea brasiliensis using 2D-DIGE. Plant. Physiol. Biochem. 2012, 60, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Yamaguchi, H.; Waki, T.; Aoki, Y.; Mizuno, M.; Yanbe, F.; Ishii, T.; Funaki, A.; Tozawa, Y.; Miyagi-Inoue, Y.; et al. Identification and reconstitution of the rubber biosynthetic machinery on rubber particles from Hevea brasiliensis. eLife 2016, 5, e19022. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Kang, G.; Li, Y.; Nie, Z.; Duan, C.; Zeng, R. In-depth proteome analysis of the rubber particle of Hevea brasiliensis (para rubber tree). Plant. Mol. Biol. 2013, 82, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Wahler, D.; Colby, T.; Kowalski Natalie, A.; Harzen, A.; Wotzka Sandra, Y.; Hillebrand, A.; Fischer, R.; Helsper, J.; Schmidt, J.; Schulze Gronover, C.; et al. Proteomic analysis of latex from the rubber-producing plant Taraxacum brevicorniculatum. Proteomics 2012, 12, 901–905. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, A.; Bachi, A.; Fasoli, E.; Boschetti, E.; Peltre, G.; Sénéchal, H.; Sutra, J.P.; Citterio, A.; Righetti, P.G. In-depth exploration of Hevea brasiliensis latex proteome and “hidden allergens” via combinatorial peptide ligand libraries. J. Proteom. 2010, 73, 1368–1380. [Google Scholar] [CrossRef] [PubMed]
- Chrispeels, M.J.; Herman, E.M. Endoplasmic reticulum-derived compartments function in storage and as mediators of vacuolar remodeling via a new type of organelle, precursor protease vesicles. Plant. Physiol. 2000, 123, 1227–1233. [Google Scholar] [CrossRef] [PubMed]
- Epping, J.; Deenen, N.V.; Niephaus, E.; Stolze, A.; Fricke, J.; Huber, C.; Eisenreich, W.; Twyman, R.M.; Prüfer, D.; Gronover, C.S. A rubber transferase activator is necessary for natural rubber biosynthesis in dandelion. Nat. Plants 2015, 1, 15048. [Google Scholar] [CrossRef]
- Brown, D.; Feeney, M.; Ahmadi, M.; Lonoce, C.; Sajari, R.; Di Cola, A.; Frigerio, L. Subcellular localization and interactions among rubber particle proteins from Hevea brasiliensis. J. Exp. Bot. 2017, 68, 5045–5055. [Google Scholar] [CrossRef] [PubMed]
- Herman, E.; Schmidt, M. Endoplasmic reticulum to vacuole trafficking of endoplasmic reticulum bodies provides an alternate pathway for protein transfer to the vacuole. Plant. Physiol. 2004, 136, 3440–3446. [Google Scholar] [CrossRef] [PubMed]
- Herman, E.M. Endoplasmic reticulum bodies: Solving the insoluble. Curr. Opin. Plant. Biol. 2008, 11, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Hillebrand, A.; Post, J.J.; Wurbs, D.; Wahler, D.; Lenders, M.; Krzyzanek, V.; Prüfer, D.; Gronover, C.S. Down-regulation of small rubber particle protein expression affects integrity of rubber particles and rubber content in Taraxacum brevicorniculatum. PLoS ONE 2012, 7, e41874. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Chakrabarty, R.; Tran, H.T.; Kwon, E.-J.G.; Kwon, M.; Nguyen, T.-D.; Ro, D.-K. A lettuce (Lactuca sativa) homolog of human Nogo-B receptor interacts with cis-prenyltransferase and is necessary for natural rubber biosynthesis. J. Biol. Chem. 2015, 290, 1898–1914. [Google Scholar] [CrossRef]
- Ohya, N.; Tanaka, Y.; Wititsuwannakul, R.; Koyama, T. Activity of rubber transferase and rubber particle size in Hevea latex. J. Rubber Res. 2000, 3, 214–221. [Google Scholar]
- Yamashita, S.; Mizuno, M.; Hayashi, H.; Yamaguchi, H.; Miyagi-Inoue, Y.; Fushihara, K.; Koyama, T.; Nakayama, T.; Takahashi, S. Purification and characterization of small and large rubber particles from Hevea brasiliensis. Biosci. Biotechnol. Biochem. 2018, 82, 1011–1020. [Google Scholar] [CrossRef]
- Qu, W.; Zhu, Y.; Huang, G.; Huang, C.; Luo, M.-C.; Zheng, J. Study of molecular weight and chain branching architectures of natural rubber. J. Appl. Polym. Sci. 2016, 133, 43975. [Google Scholar] [CrossRef]
- Archer, B.L.; Cockbain, E.G. Rubber transferase from Hevea brasiliensis latex. In Methods in Enzymology; Clayton, R.B., Ed.; Elsevier Academic Press: San Diego, CA, USA, 1969; Volume 15, pp. 476–480. [Google Scholar] [CrossRef]
- Oh, S.K.; Han, K.H.; Ryu, S.B.; Kang, H. Molecular cloning, expression, and functional analysis of a cis-prenyltransferase from Arabidopsis thaliana. J. Biol. Chem. 2000, 275, 18482–18488. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Fujisaki, S.; Sato, K.; Nishimura, Y.; Nakano, A. Yeast Saccharomyces cerevisiae has two cis-prenyltransferases with different properties and localizations. Implication for their distinct physiological roles in dolichol synthesis. Genes Cells 2001, 6, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Grabińska, K.; Park, E.J.; Sessa, W.C. cis-Prenyltransferase: New insights into protein glycosylation, rubber synthesis and human diseases. J. Biol. Chem. 2016, 291, 18582–18590. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.; Kwon, E.J.G.; Ro, D.K. cis-Prenyltransferase and polymer analysis from a natural rubber perspective. In Methods in Enzymology; O’Connor, S.E., Ed.; Elsevier Academic Press: San Diego, CA, USA, 2016; Volume 576, pp. 121–145. [Google Scholar] [CrossRef]
- Fujihashi, M.; Zhang, Y.-W.; Higuchi, Y.; Li, X.-Y.; Koyama, T.; Miki, K. Crystal structure of cis-prenyl chain elongating enzyme, undecaprenyl diphosphate synthase. Proc. Natl. Acad. Sci. USA 2001, 98, 4337–4342. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Dong, C.; McNeil, M.; Kaur, D.; Mahapatra, S.; Crick, D.C.; Naismith, J.H. The structural basis of chain length control in Rv1086. J. Mol. Biol. 2008, 381, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Asawatreratanakul, K.; Zhang, Y.-W.; Wititsuwannakul, D.; Wititsuwannakul, R.; Takahashi, S.; Rattanapittayaporn, A.; Koyama, T. Molecular cloning, expression and characterization of cDNA encoding cis-prenyltransferases from Hevea brasiliensis: A key factor participating in natural rubber biosynthesis. FEBS J. 2003, 270, 4671–4680. [Google Scholar] [CrossRef]
- Kharel, Y.; Koyama, T. Molecular analysis of cis-prenyl chain elongating enzymes. Nat. Prod. Rep. 2003, 20, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Lee, H.-J.; Yamashita, S.; Koyama, T. Characterization of cis-prenyltransferases from the rubber producing plant Hevea brasiliensis heterologously expressed in yeast and plant cells. Plant. Biotechnol. 2012, 29, 411–417. [Google Scholar] [CrossRef]
- Schmidt, T.; Hillebrand, A.; Wurbs, D.; Wahler, D.; Lenders, M.; Gronover, C.S.; Prüfer, D. Molecular cloning and characterization of rubber biosynthetic genes from Taraxacum koksaghyz. Plant. Mol. Biol. Rep. 2010, 28, 277–284. [Google Scholar] [CrossRef]
- Post, J.J.; van Deenen, N.; Fricke, J.; Kowalski, N.; Wurbs, D.; Schaller, H.; Eisenreich, W.; Huber, C.; Twyman, R.M.; Prüfer, D.; et al. Laticifer-specific cis-prenyltransferase silencing affects the rubber, triterpene, and inulin content of Taraxacum brevicorniculatum. Plant. Physiol. 2012, 158, 1406–1417. [Google Scholar] [CrossRef] [PubMed]
- Ponciano, G.; McMahan, C.M.; Xie, W.; Lazo, G.R.; Coffelt, T.A.; Collins-Silva, J.; Nural-Taban, A.; Gollery, M.; Shintani, D.K.; Whalen, M.C. Transcriptome and gene expression analysis in cold-acclimated guayule (Parthenium argentatum) rubber-producing tissue. Phytochemistry 2012, 79, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Goyvaerts, E.; Dennis, M.; Light, D.; Chua, N.-H. Cloning and sequencing of the cDNA encoding the rubber elongation factor of Hevea brasiliensis. Plant. Physiol. 1991, 97, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Attanyaka, D.P.S.T.G.; Kekwick, R.G.O.; Franklin, F.C.H. Molecular cloning and nucleotide sequencing of the rubber elongation factor gene from Hevea brasiliensis. Plant. Mol. Biol. 1991, 16, 1079–1081. [Google Scholar] [CrossRef] [PubMed]
- Dennis, M.S.; Light, D.R. Rubber Elongation Factor from Hevea brasiliensis: Identification, characterization, and role in rubber biosynthesis. J. Biol. Chem. 1989, 264, 18608–18617. [Google Scholar] [PubMed]
- Dennis, M.S.; Henzel, W.J.; Bell, J.; Kohr, W.; Light, D.R. Amino acid sequence of rubber elongation factor protein associated with rubber particles in Hevea Latex. J. Biol. Chem. 1989, 264, 18618–18626. [Google Scholar] [PubMed]
- Oh, S.K.; Kang, H.; Shin, D.H.; Yang, J.; Chow, K.-S.; Yeang, H.Y.; Wagner, B.; Breiteneder, H.; Han, K.-H. Isolation, characterization, and functional analysis of a novel cDNA clone encoding a small rubber particle protein from Hevea brasiliensis. J. Biol. Chem. 1999, 274, 17132–17138. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Wang, D.; Sun, Y.; Yang, Q.; Meng, X.; Wang, L.; Feng, W.; Li, L.; Wurtele, S.E.; Wang, X. Comparative proteomics of rubber latex revealed multiple protein species of REF/SRPP family respond diversely to ethylene stimulation among different rubber tree clones. Int. J. Mol. Sci. 2017, 18, 958. [Google Scholar] [CrossRef] [PubMed]
- Berthelot, K.; Lecomte, S.; Estevez, Y.; Coulary-Salin, B.d.; Bentaleb, A.; Cullin, C.; Deffieux, A.; Peruch, F. Rubber elongation factor (REF), a major allergen component in Hevea brasiliensis latex has amyloid properties. PLoS ONE 2012, 7, e48065. [Google Scholar] [CrossRef]
- Dai, L.; Nie, Z.; Kang, G.; Li, Y.; Zeng, R. Identification and subcellular localization analysis of two rubber elongation factor isoforms on Hevea brasiliensis rubber particles. Plant. Physiol. Biochem. 2017, 111, 97–106. [Google Scholar] [CrossRef]
- Berthelot, K.; Lecomte, S.; Estevez, Y.; Coulary-Salin, B.; Peruch, F. Homologous Hevea brasiliensis REF (Hevb1) and SRPP (Hevb3) present different auto-assembling. BBA Proteins Proteom. 2014, 1844, 473–485. [Google Scholar] [CrossRef]
- Kim, I.J.; Ryu, S.B.; Kwak, Y.S.; Kang, H. A novel cDNA from Parthenium argentatum Gray enhances the rubber biosynthetic activity in vitro. J. Exp. Bot. 2004, 55, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Laibach, N.; Hillebrand, A.; Twyman Richard, M.; Prüfer, D.; Schulze Gronover, C. Identification of a Taraxacum brevicorniculatum rubber elongation factor protein that is localized on rubber particles and promotes rubber biosynthesis. Plant. J. 2015, 82, 609–620. [Google Scholar] [CrossRef]
- Priya, P.; Venkatachalam, P.; Thulaseedharan, A. Differential expression pattern of rubber elongation factor (REF) mRNA transcripts from high and low yielding clones of rubber tree (Hevea brasiliensis Muell. Arg.). Plant. Cell. Rep. 2007, 26, 1833–1838. [Google Scholar] [CrossRef] [PubMed]
- Collins-Silva, J.; Nural, A.T.; Skaggs, A.; Scott, D.; Hathwaik, U.; Woolsey, R.; Schegg, K.; McMahan, C.; Whalen, M.; Cornish, K.; et al. Altered levels of the Taraxacum kok-saghyz (Russian dandelion) small rubber particle protein, TkSRPP3, result in qualitative and quantitative changes in rubber metabolism. Phytochemistry 2012, 79, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, R.; Qu, Y.; Ro, D.-K. Silencing the lettuce homologs of small rubber particle protein does not influence natural rubber biosynthesis in lettuce (Lactuca sativa). Phytochemistry 2015, 113, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Miao, R.Q.; Gao, Y.; Harrison, K.D.; Prendergast, J.; Acevedo, L.M.; Yu, J.; Hu, F.; Strittmatter, S.M.; Sessa, W.C. Identification of a receptor necessary for Nogo-B stimulated chemotaxis and morphogenesis of endothelial cells. Proc. Natl. Acad. Sci. USA 2006, 103, 10997–11002. [Google Scholar] [CrossRef]
- Harrison, K.D.; Park, E.J.; Gao, N.; Kuo, A.; Rush, J.S.; Waechter, C.J.; Lehrman, M.A.; Sessa, W.C. Nogo-B receptor is necessary for cellular dolichol biosynthesis and protein N-glycosylation. EMBO J. 2011, 30, 2490–2500. [Google Scholar] [CrossRef]
- Brasher Megan, I.; Surmacz, L.; Leong, B.; Pitcher, J.; Swiezewska, E.; Pichersky, E.; Akhtar Tariq, A. A two-component enzyme complex is required for dolichol biosynthesis in tomato. Plant. J. 2015, 82, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Grabińska, K.A.; Edani, B.H.; Park, E.J.; Kraehling, J.R.; Sessa, W.C. A conserved carboxy-terminal RxG motif in the NgBR subunit of cis-prenyltransferase is critical for prenyltransferase activity. J. Biol. Chem. 2017, 292, 17351–17361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ohyama, K.; Boudet, J.; Chen, Z.; Yang, J.; Zhang, M.; Muranaka, T.; Maurel, C.; Zhu, J.-K.; Gong, Z. Dolichol biosynthesis and its effects on the unfolded protein response and abiotic stress resistance in Arabidopsis. Plant. Cell. 2008, 20, 1879–1898. [Google Scholar] [CrossRef] [PubMed]
- Sando, T.; Takeno, S.; Watanabe, N.; Okumoto, H.; Kuzuyama, T.; Yamashita, A.; Hattori, M.; Ogasawara, N.; Fukusaki, E.; Kobayashi, A. Cloning and characterization of the 2-C-Methyl-D-erythritol 4-phosphate (MEP) pathway genes of a natural-rubber producing plant, Hevea brasiliensis. Biosci. Biotechnol. Biochem. 2008, 72, 2903–2917. [Google Scholar] [CrossRef] [PubMed]
- Sando, T.; Takaoka, C.; Mukai, Y.; Yamashita, A.; Hattori, M.; Ogasawara, N.; Fukusaki, E.; Kobayashi, A. Cloning and characterization of mevalonate pathway genes in a natural rubber producing plant, Hevea brasiliensis. Biosci. Biotechnol. Biochem. 2008, 72, 2049–2060. [Google Scholar] [CrossRef] [PubMed]
- Seetang-Nun, Y.; Sharkey, T.D.; Suvachittanont, W. Molecular cloning and characterization of two cDNAs encoding 1-deoxy-d-xylulose 5-phosphate reductoisomerase from Hevea brasiliensis. J. Plant. Physiol. 2008, 165, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Sun, L.; Li, Y.; Zeng, R. Molecular characterization and expression analysis of two farnesyl pyrophosphate synthase genes involved in rubber biosynthesis in Hevea brasiliensis. Ind. Crops Prod. 2017, 108, 398–409. [Google Scholar] [CrossRef]
- Chow, K.-S.; Mat-Isa, M.N.; Bahari, A.; Ghazali, A.-K.; Alias, H.; Mohd.-Zainuddin, Z.; Hoh, C.-C.; Wan, K.-L. Metabolic routes affecting rubber biosynthesis in Hevea brasiliensis latex. J. Exp. Bot. 2012, 63, 1863–1871. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jing, T.; Li, T.; Du, H.; Wuyun, T.-N. Identification and expression analysis of the Eucommia ulmoides farnesyl diphosphate synthase gene family to reveal the key gene involved in rubber biosynthesis. Acta Physiol. Plant. 2017, 40, 11. [Google Scholar] [CrossRef]
- Tokumoto, Y.; Uefuji, H.; Yamamoto, N.; Kajiura, H.; Takeno, S.; Suzuki, N.; Nakazawa, Y. Gene coexpression network for trans-1,4-polyisoprene biosynthesis involving mevalonate and methylerythritol phosphate pathways in Eucommia ulmoides Oliver. Plant. Biotechnol. 2017, 34, 165–172. [Google Scholar] [CrossRef]
- Rahman, A.Y.A.; Usharraj, A.O.; Misra, B.B.; Thottathil, G.P.; Jayasekaran, K.; Feng, Y.; Hou, S.; Ong, S.Y.; Ng, F.L.; Lee, L.S.; et al. Draft genome sequence of the rubber tree Hevea brasiliensis. BMC Genom. 2013, 14, 75. [Google Scholar] [CrossRef]
- Tang, C.; Yang, M.; Fang, Y.; Luo, Y.; Gao, S.; Xiao, X.; An, Z.; Zhou, B.; Zhang, B.; Tan, X. The rubber tree genome reveals new insights into rubber production and species adaptation. Nat. Plants 2016, 2, 16073. [Google Scholar] [CrossRef]
- Makita, Y.; Kawashima, M.; Lau, N.S.; Othman, A.S.; Matsui, M. Construction of Pará rubber tree genome and multi-transcriptome database accelerates rubber researches. BMC Genom. 2018, 19, 922. [Google Scholar] [CrossRef]
- Feng, S.P.; Li, W.G.; Huang, H.S.; Wang, J.Y.; Wu, Y.T. Development, characterization and cross-species/genera transferability of EST-SSR markers for rubber tree (Hevea brasiliensis). Mol. Breed. 2009, 23, 85–97. [Google Scholar] [CrossRef]
- Yu, F.; Wang, B.-H.; Feng, S.-P.; Wang, J.-Y.; Li, W.-G.; Wu, Y.-T. Development, characterization, and cross-species/genera transferability of SSR markers for rubber tree (Hevea brasiliensis). Plant. Cell. Rep. 2011, 30, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Mantello, C.C.; Cardoso-Silva, C.B.; da Silva, C.C.; de Souza, L.M.; Scaloppi Junior, E.J.; de Souza Gonçalves, P.; Vicentini, R.; de Souza, A.P. De novo assembly and transcriptome analysis of the rubber tree (Hevea brasiliensis) and SNP markers development for rubber biosynthesis pathways. PLoS ONE 2014, 9, e102665. [Google Scholar] [CrossRef] [PubMed]
- Shearman, J.R.; Sangsrakru, D.; Jomchai, N.; Ruangareerate, P.; Sonthirod, C.; Naktang, C.; Theerawattanasuk, K.; Tragoonrung, S.; Tangphatsornruang, S. SNP identification from RNA sequencing and linkage map construction of rubber tree for anchoring the draft genome. PLoS ONE 2015, 10, e0121961. [Google Scholar] [CrossRef] [PubMed]
- Pootakham, W.; Ruang-Areerate, P.; Jomchai, N.; Sonthirod, C.; Sangsrakru, D.; Yoocha, T.; Theerawattanasuk, K.; Nirapathpongporn, K.; Romruensukharom, P.; Tragoonrung, S.; et al. Construction of a high-density integrated genetic linkage map of rubber tree (Hevea brasiliensis) using genotyping-by-sequencing (GBS). Front. Plant. Sci. 2015, 6, 367. [Google Scholar] [CrossRef]
- De Souza, L.M.; Toledo-Silva, G.; Cardoso-Silva, C.B.; da Silva, C.C.; de Araujo Andreotti, I.A.; Conson, A.R.O.; Mantello, C.C.; Le Guen, V.; de Souza, A.P. Development of single nucleotide polymorphism markers in the large and complex rubber tree genome using next-generation sequence data. Mol. Breed. 2016, 36, 115. [Google Scholar] [CrossRef]
- Triwitayakorn, K.; Chatkulkawin, P.; Kanjanawattanawong, S.; Sraphet, S.; Yoocha, T.; Sangsrakru, D.; Chanprasert, J.; Ngamphiw, C.; Jomchai, N.; Therawattanasuk, K.; et al. Transcriptome sequencing of Hevea brasiliensis for development of microsatellite markers and construction of a genetic linkage map. DNA Res. 2011, 18, 471–482. [Google Scholar] [CrossRef]
- Li, D.; Deng, Z.; Qin, B.; Liu, X.; Men, Z. De novo assembly and characterization of bark transcriptome using Illumina sequencing and development of EST-SSR markers in rubber tree (Hevea brasiliensis Muell. Arg.). BMC Genom. 2012, 13, 192. [Google Scholar] [CrossRef]
- Salgado, L.R.; Koop, D.M.; Pinheiro, D.G.; Rivallan, R.; Le Guen, V.; Nicolás, M.F.; de Almeida, L.G.; Rocha, V.R.; Magalhães, M.; Gerber, A.L. De novo transcriptome analysis of Hevea brasiliensis tissues by RNA-seq and screening for molecular markers. BMC Genom. 2014, 15, 236. [Google Scholar] [CrossRef]
- Sanchez, P.L.; Costich, D.E.; Friebe, B.; Coffelt, T.A.; Jenks, M.A.; Gore, M.A. Genome size variation in guayule and mariola: Fundamentaldescriptors for polyploid plant taxa. Ind. Crops Prod. 2014, 54, 1–5. [Google Scholar] [CrossRef]
- Ilut, D.C.; Sanchez, P.L.; Costich, D.E.; Friebe, B.; Coffelt, T.A.; Dyer, J.M.; Jenks, M.A.; Gore, M.A. Genomic diversity and phylogenetic relationships in the genus Parthenium (Asteraceae). Ind. Crops Prod. 2015, 76, 920–929. [Google Scholar] [CrossRef]
- Chow, K.S.; Wan, K.L.; Isa, M.N.; Bahari, A.; Tan, S.H.; Harikrishna, K.; Yeang, H.Y. Insights into rubber biosynthesis from transcriptome analysis of Hevea brasiliensis latex. J. Exp. Bot. 2007, 58, 2429–2440. [Google Scholar] [CrossRef] [PubMed]
- Makita, Y.; Ng, K.K.; Veera Singham, G.; Kawashima, M.; Hirakawa, H.; Sato, S.; Othman, A.S.; Matsui, M. Large-scale collection of full-length cDNA and transcriptome analysis in Hevea brasiliensis. DNA Res. 2017, 24, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Hao, L.; Liu, H.; Zhao, M.; Deng, Z.; Li, Y.; Zeng, R.; Tian, W. Next-generation sequencing, assembly, and comparative analyses of the latex transcriptomes from two elite Hevea brasiliensis varieties. Tree Genet. Genomes 2015, 11, 98. [Google Scholar] [CrossRef]
- Aoki, Y.; Takahashi, S.; Takayama, D.; Ogata, Y.; Sakurai, N.; Suzuki, H.; Asawatreratanakul, K.; Wititsuwannakul, D.; Wititsuwannakul, R.; Shibata, D.; et al. Identification of laticifer-specific genes and their promoter regions from a natural rubber producing plant Hevea brasiliensis. Plant. Sci. 2014, 225, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhan, D.-F.; Li, H.-L.; Guo, D.; Zhu, J.-H.; Peng, S.-Q. Transcriptome-wide identification and characterization of MYB transcription factor genes in the laticifer cells of Hevea brasiliensis. Front. Plant. Sci. 2017, 8, 1974. [Google Scholar] [CrossRef] [PubMed]
- Chao, J.; Chen, Y.; Wu, S.; Tian, W.-M. Comparative transcriptome analysis of latex from rubber tree clone CATAS8-79 and PR107 reveals new cues for the regulation of latex regeneration and duration of latex flow. BMC Plant. Biol. 2015, 15, 104. [Google Scholar] [CrossRef]
- Chao, J.; Chen, Y.; Wu, S.; Tian, W.-M. Comparative transcriptome analysis of latex from rubber tree clone CATAS8-79 and PR107. Genom. Data 2015, 5, 120–121. [Google Scholar] [CrossRef]
- Liu, J.P.; Zhuang, Y.F.; Guo, X.L.; Li, Y.J. Molecular mechanism of ethylene stimulation of latex yield in rubber tree (Hevea brasiliensis) revealed by de novo sequencing and transcriptome analysis. BMC Genom. 2016, 17, 257. [Google Scholar] [CrossRef]
- Luo, Z.; Iaffaldano, B.J.; Zhuang, X.; Fresnedo-Ramírez, J.; Cornish, K. Analysis of the first Taraxacum kok-saghyz transcriptome reveals potential rubber yield related SNPs. Sci. Rep. 2017, 7, 9939. [Google Scholar] [CrossRef]
- Cao, X.; Yan, J.; Lei, J.; Li, J.; Zhu, J.; Zhang, H. De novo transcriptome sequencing of MeJA-induced Taraxacum koksaghyz Rodin to identify genes related to rubber formation. Sci. Rep. 2017, 7, 15697. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.K.; Chen, X.-Y.; Uddin, N.M.; Rim, Y.; Moon, J.; Jung, J.-H.; Shi, C.; Chu, H.; Kim, S.; Kim, S.-W.; et al. Comprehensive proteome analysis of lettuce latex using multidimensional protein-identification technology. Phytochemistry 2009, 70, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shi, M.; Lu, X.; Ma, R.; Wu, C.; Guo, A.; Peng, M.; Tian, W. A method for protein extraction from different subcellular fractions of laticifer latex in Hevea brasiliensis compatible with 2-DE and MS. Proteome Sci. 2010, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, D.; Sun, Y.; Yang, Q.; Chang, L.; Wang, L.; Meng, X.; Huang, Q.; Jin, X.; Tong, Z. Comprehensive proteomics analysis of laticifer latex reveals new insights into ethylene stimulation of natural rubber production. Sci. Rep. 2015, 5, 13778. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Kang, G.; Nie, Z.; Li, Y.; Zeng, R. Comparative proteomic analysis of latex from Hevea brasiliensis treated with Ethrel and methyl jasmonate using iTRAQ-coupled two-dimensional LC–MS/MS. J. Proteom. 2016, 132, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Harada, Y.; Bamba, T.; Nakazawa, Y.; Gyokusen, K. Overexpression of an isopentenyl diphosphate isomerase gene to enhance trans-polyisoprene production in Eucommia ulmoides Oliver. BMC Biotechnol. 2012, 12, 78. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.C.M.; Chyan, C.-L.; Lee, T.T.T.; Huang, S.-H.; Tzen, J.T.C. Constitution of stable artificial oil bodies with triacylglycerol, phospholipid, and caleosin. J. Agric. Food Chem. 2004, 52, 3982–3987. [Google Scholar] [CrossRef]
- Bhatla, S.C.; Kaushik, V.; Yadav, M.K. Use of oil bodies and oleosins in recombinant protein production and other biotechnological applications. Biotechnol. Adv. 2010, 28, 293–300. [Google Scholar] [CrossRef]
- Yang, J.; Xian, M.; Su, S.; Zhao, G.; Nie, Q.; Jiang, X.; Zheng, Y.; Liu, W. Enhancing production of bio-isoprene using hybrid MVA pathway and isoprene synthase in E. coli. PLoS ONE 2012, 7, e33509. [Google Scholar] [CrossRef]
- Liu, C.; Men, X.; Chen, H.; Li, M.; Ding, Z.; Chen, G.; Wang, F.; Liu, H.; Wang, Q.; Zhu, Y.; et al. A systematic optimization of styrene biosynthesis in Escherichia coli BL21(DE3). Biotechnol. Biofuels 2018, 11, 14. [Google Scholar] [CrossRef]
- Steinbuchel, A. Production of rubber-like polymers by microorganisms. Curr. Opin. Microbiol. 2003, 6, 261–270. [Google Scholar] [CrossRef]
- Tanimura, A.; Takashima, M.; Sugita, T.; Endoh, R.; Kikukawa, M.; Yamaguchi, S.; Sakuradani, E.; Ogawa, J.; Ohkuma, M.; Shima, J. Cryptococcus terricola is a promising oleaginous yeast for biodiesel production from starch through consolidated bioprocessing. Sci. Rep. 2014, 4, 4776. [Google Scholar] [CrossRef] [PubMed]




| Species | Chromosome Number | Assembly Length/Estimated Length | Predicted Gene Number | BioProject/Accession No. | Ref. |
|---|---|---|---|---|---|
| Hevea brasiliensis RRIM 600 | 2N = 2X = 36 | 1.1 Gb/2.15 Gb | 68955 8 CPTs, 10 SRPPs, 12 REFs | GenBank: AJJZ01000000 | [91] |
| Hevea brasiliensis Reyan 7-33-97 | 2N = 2X = 36 | 1.37 Gb/1.46 Gb | 43792 11 CPTs, 8 REFs, 10 SRPPs | GenBank: LVXX01000000 | [92] |
| Hevea brasiliensis RRIM 600 | 2N = 2X = 36 | 1.55 Gb/2.15 Gb | 84440 7 CPTs, 1 CPTL, 9 REFs, 8 SRPPs | GenBank: PRJDB4387 | [22] |
| Taraxacum kok-saghyz line 1151 | 2N = 2X = 16 | 1.29 Gb/1.4 Gb | 46731 9 CPTs, 2 CPTLs, 1 REF, 9 SRPPs | Genome Warehouse: PRJCA000437 GWHAAAA00000000 | [11] |
| Eucommia ulmoides a wild E. ulmoides tree in Shennongjia | 2N = 2X = 34 | 1.2 Gb/1.1 Gb | 26723 5 FPSs, 5 REFs, 7 SRPPs | Genome Warehouse: PRJCA000677 GWHAAAL00000000 | [5] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Men, X.; Wang, F.; Chen, G.-Q.; Zhang, H.-B.; Xian, M. Biosynthesis of Natural Rubber: Current State and Perspectives. Int. J. Mol. Sci. 2019, 20, 50. https://doi.org/10.3390/ijms20010050
Men X, Wang F, Chen G-Q, Zhang H-B, Xian M. Biosynthesis of Natural Rubber: Current State and Perspectives. International Journal of Molecular Sciences. 2019; 20(1):50. https://doi.org/10.3390/ijms20010050
Chicago/Turabian StyleMen, Xiao, Fan Wang, Guo-Qiang Chen, Hai-Bo Zhang, and Mo Xian. 2019. "Biosynthesis of Natural Rubber: Current State and Perspectives" International Journal of Molecular Sciences 20, no. 1: 50. https://doi.org/10.3390/ijms20010050
APA StyleMen, X., Wang, F., Chen, G.-Q., Zhang, H.-B., & Xian, M. (2019). Biosynthesis of Natural Rubber: Current State and Perspectives. International Journal of Molecular Sciences, 20(1), 50. https://doi.org/10.3390/ijms20010050




