Electro-Kinetic Instability in a Laminar Boundary Layer Next to an Ion Exchange Membrane
Abstract
:1. Introduction
2. Theory
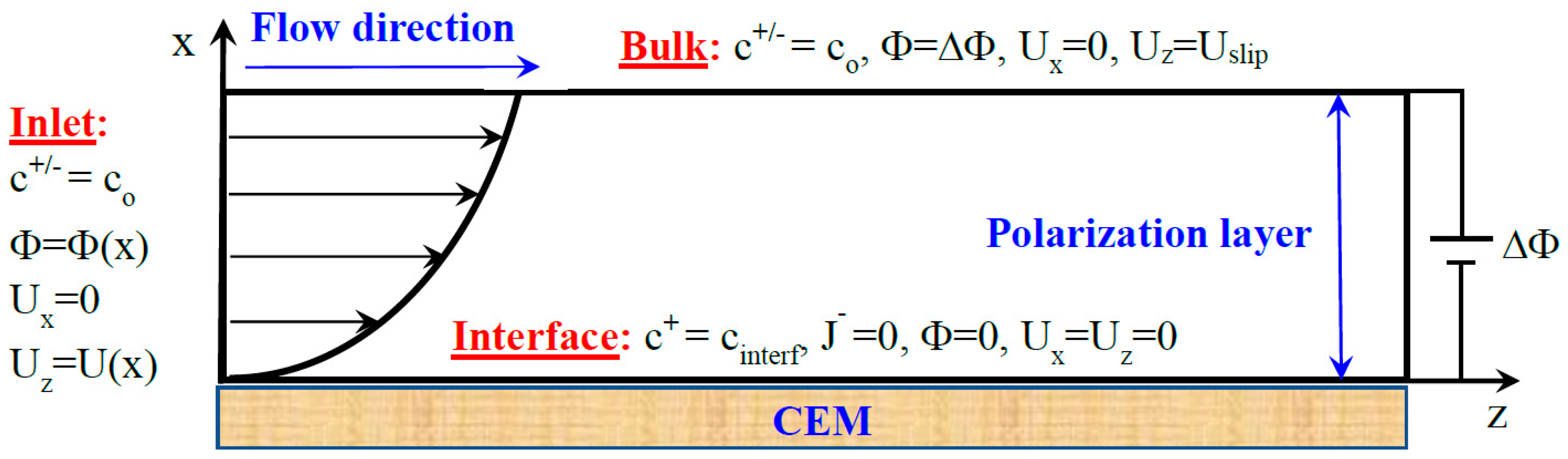
2.1. Model and Governing Equations
2.2. Numerical Method
2.2.1. Stokes and Poisson–Nernst–Planck Equations
2.2.2. Streamlines
3. Numerical Results
3.1. Comparison with Published Results
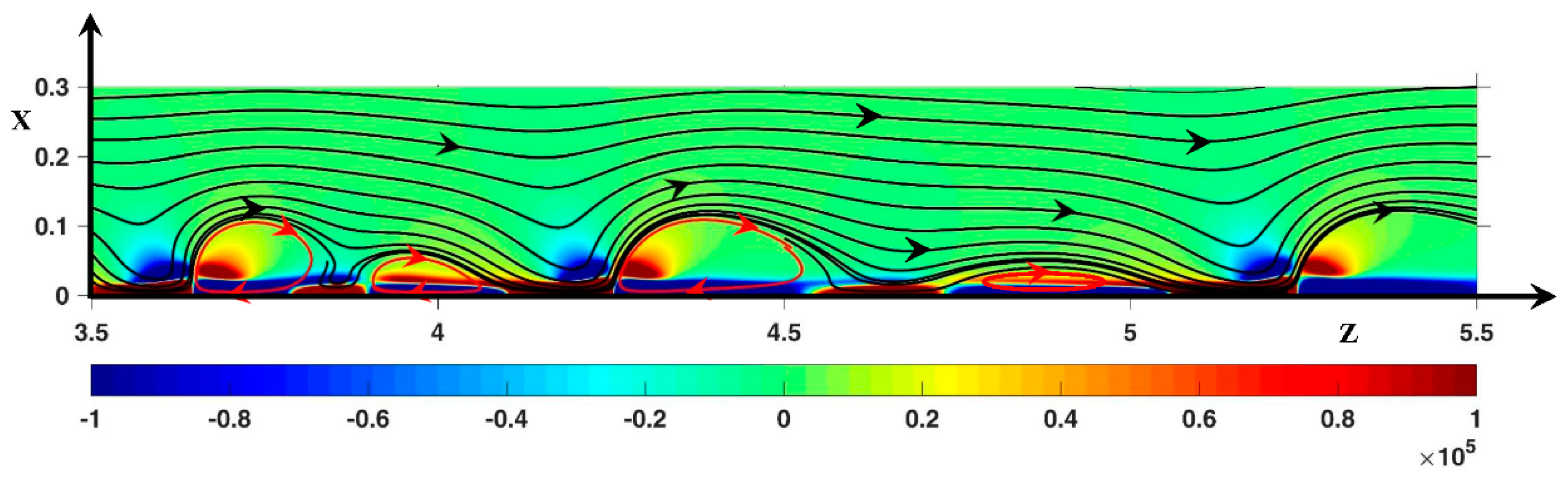
3.2. Hydrodynamic Instability
3.2.1. Periodic Mode Flow ()
3.2.2. Non-Periodic Mode Flow (∆Φ = 27)
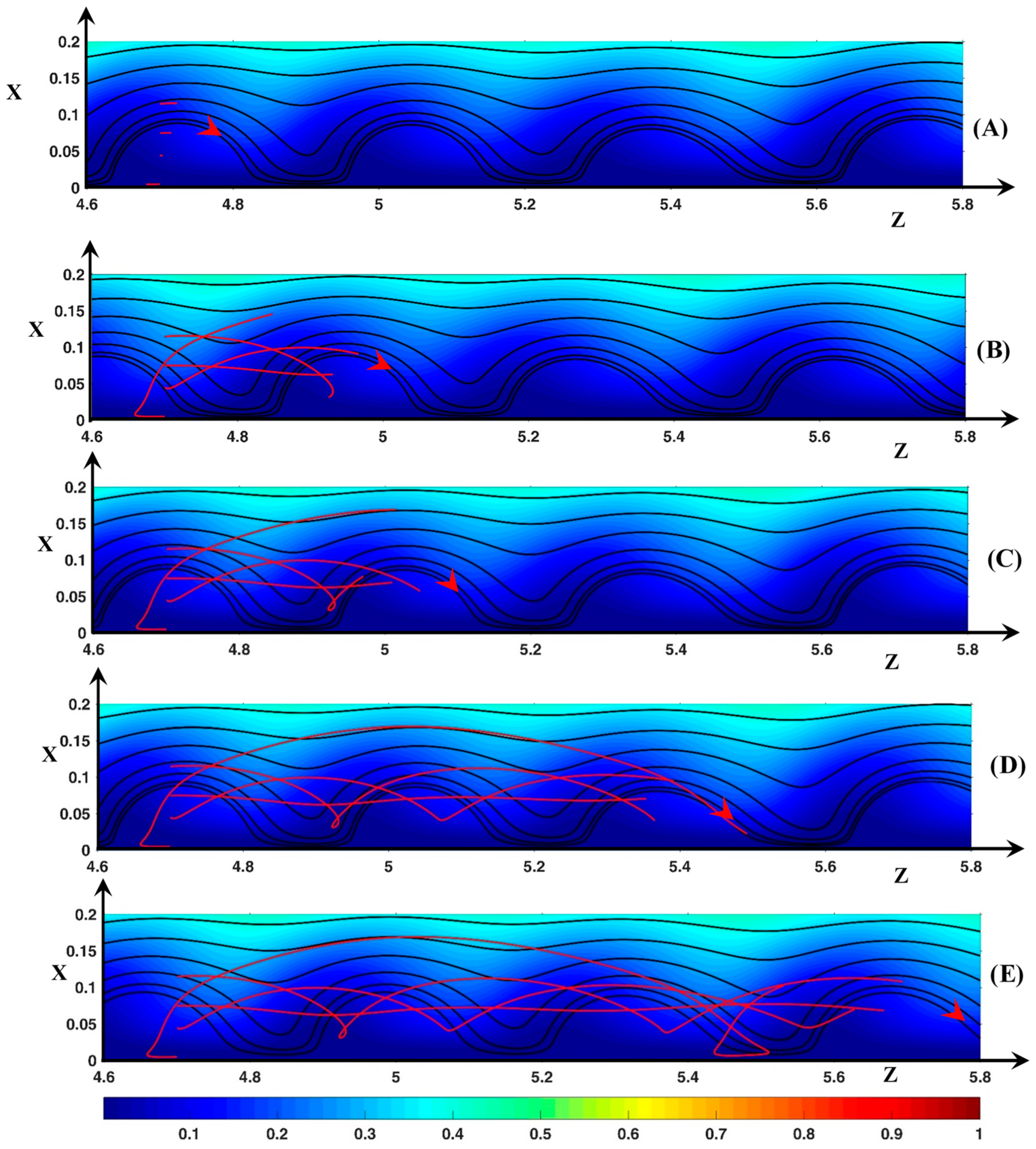
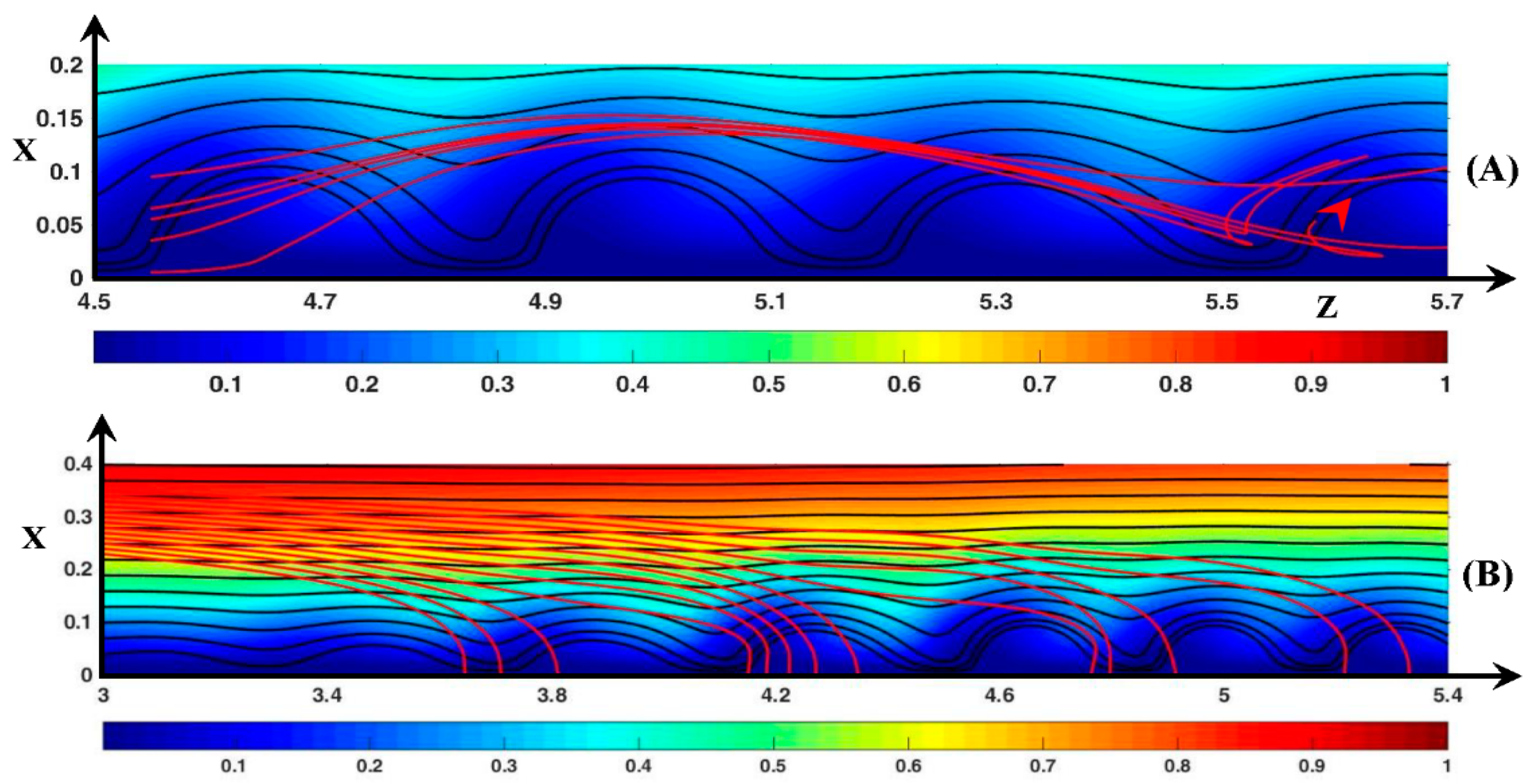
3.3. Dynamic Streamlines of Fluid Flow and Ionic Transfer
3.3.1. Fluid DSL in Periodic Regime ()
3.3.2. Fluid DSL in Aperiodic Regime (∆Φ = 27)
3.3.3. Ionic DSL in the Periodic Regime ()
4. Conclusions and Summary
Funding
Conflicts of Interest
Abbreviations
| PNP | Poisson–Nernst–Planck |
| EDL | Electric double layer |
| ESC | Extended space charge |
| SSL | Static streamline |
| DSL | Dynamic streamline |
| Symbols | |
| A | Surface of the domain |
| , , | Cation and anion concentration, bulk salt concentration, cation concentration at the membrane surface |
| e | Elementary charge |
| Kinetic energy | |
| , | Cation and anion diffusion coefficient, reference diffusion coefficient |
| , , | Electric force, Pressure force, total force |
| Boltzmann constant | |
| I, Im, Ilim | Mean current density, current density at the membrane surface, limiting current |
| , , , | Cation and anion flux, convective, electro-migration and Fickian flux contribution |
| , , | Longitudinal and transverse domain length, inter-membrane distance |
| P, Po | Pressure, osmotic pressure |
| Pe | Peclet number |
| Q, | Charge density, maximum charge density in the ESC |
| t, tdiff, | Time, diffusional time, time step |
| T | Temperature |
| , , Uslip, , | Fluid velocity, cation and anion diffusional velocity, imposed velocity at the upper edge of the domain, longitudinal vortex velocity, longitudinal velocity of the kinetic energy spot |
| Cation and anion charge | |
| Greek Symbols | |
| Vortex height | |
| , | Vacuum and relative permittivity |
| Debye length | |
| Dynamic viscosity | |
| Debye length to the longitudinal domain length ratio | |
| Fluid density | |
| Local angle between the membrane surface and the vortex front | |
| ,, | Electric potential, potential in the vortex layer, thermodynamic potential |
| Domain | |
Appendix A. Influence of the Length Ratio on the Instability Modes

References
- Ran, J.; Wu, L.; He, Y.; Yang, Z.; Wang, Y.; Jiang, C.; Ge, L.; Bakangura, E.; Xu, T. Ion exchange membranes: New developments and applications. J. Membr. Sci. 2017, 522, 267–291. [Google Scholar] [CrossRef]
- Hong, J.G.; Zhang, B.; Glabman, S.; Uzal, N.; Dou, X.; Zhang, H.; Wei, X.; Chen, Y. Potential ion exchange membranes and system performance in reverse electrodialysis for power generation: A review. J. Membr. Sci. 2015, 486, 71–88. [Google Scholar] [CrossRef]
- Weber, A.Z.; Mench, M.M.; Meyers, J.P.; Ross, P.N.; Gostick, J.T.; Liu, Q. Redox flow batteries: A review. J. Appl. Electrochem. 2011, 41, 1137. [Google Scholar] [CrossRef]
- De Jong, J.; Lammertink, R.G.H.; Wessling, M. Membranes and microfluidics: A review. Lab Chip 2006, 6, 1125–1139. [Google Scholar] [CrossRef]
- Kim, S.J.; Song, Y.; Han, J. Nanofluidic concentration devices for biomolecules utilizing ion concentration polarization: Theory, fabrication and applications. Chem. Soc. Rev. 2010, 39, 912–922. [Google Scholar] [CrossRef]
- Suss, M.E.; Mani, A.; Zangle, T.A.; Santiago, J.G. Electroosmotic jump performance is affected by concentration polarizations on both electrodes and pump. Sens. Actuators A 2011, 165, 310. [Google Scholar] [CrossRef]
- Ulbricht, M. Advanced functional polymer membranes. Polymer 2006, 47, 2217–2262. [Google Scholar] [CrossRef] [Green Version]
- Moore, C.M.; Minteer, S.D.; Martin, R.S. Microchip-based ethanol/oxygen biofuel cell. Lab Chip 2005, 5, 218–225. [Google Scholar] [CrossRef]
- Suzuki, H.; Tabata, K.V.; Noji, H.; Takeushi, S. Highly reproducible method of planar lipid bilayer reconstruction in polymethyl methacrylate microfluidic chip. Langmuir 2006, 22, 1937–1942. [Google Scholar] [CrossRef]
- Sandison, M.E.; Morgan, H. Rapid fabrication of polymer microfluidic systems for the production of artificial lipid bilayers. J. Micromech. Microeng. 2005, 15, S139–S144. [Google Scholar] [CrossRef]
- Montané, X.; Bogdanowicz, K.A.; Prats-Reig, J.; Colace, G.; Reina, J.A.; Giamberini, M. Advances in the design of self-supported ion-conducting membranes-New family of columnar liquid crystalline polyamines. Part 2: Ion transport characterization and comparison to hybrid membranes. Polymer 2016, 105, 234–242. [Google Scholar] [CrossRef]
- Rubinstein, I.; Zaltzman, B. Electro-osmotically induced convection at a permselective membrane. Phys. Rev. E 2000, 62, 2238–2251. [Google Scholar] [CrossRef]
- Rubinstein, I.; Zaltzman, B. Electro-osmotic slip of the second kind and instability in concentration polarization at electrodialysis membranes. Math. Models Methods Appl. Sci. 2001, 11, 263–300. [Google Scholar] [CrossRef]
- Zaltzman, B.; Rubinstein, I. Electro-osmotic slip and electro-convective instability. J. Fluid Mech. 2007, 579, 173–226. [Google Scholar] [CrossRef]
- Rubinstein, I.; Zaltzman, B.; Lerman, I. Electroconvective instability in concentration polarization and non-equilibrium electro-osmotic slip. Phys. Rev. E 2005, 72, 011505. [Google Scholar] [CrossRef] [PubMed]
- Abu-Rjal, R.; Rubinstein, I.; Zaltzman, B. Driving factors of electro-convective instability in concentration polarization. Phys. Rev. Fluids 2016, 1, 023601. [Google Scholar] [CrossRef]
- Kim, S.J.; Wang, Y.C.; Lee, J.H.; Jang, H.; Han, J. Concentration polarization and nonlinear electrokinetic flow near a nanofluidic channel. Phys. Rev. Lett. 2007, 99, 044501. [Google Scholar] [CrossRef]
- Kwak, R.; Pham, V.S.; Lim, K.M.; Han, J. Shear flow of an electrically charged fluid by ion concentration polarization: Scaling laws for electroconvective vortices. Phys. Rev. Lett. 2013, 110, 11450. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, S.M.; Manukyan, G.; Staicu, A.; Rubinstein, I.; Zaltzman, B.; Lammertink, R.G.H.; Mugele, F.; Wessling, M. Direct observation of a nonequilibrium electro-osmotic instability. Phys. Rev. Lett. 2008, 101, 236101. [Google Scholar] [CrossRef]
- Chang, H.C.; Yossifon, G. Understanding electrokinetics at the nanoscale: A perspective. Biomicrofluidics 2009, 3, 012001. [Google Scholar] [CrossRef] [Green Version]
- Kwak, R.; Guan, G.; Peng, W.K.; Han, J. Microscale electrodialysis: Concentration profiling and vortex visualization. Desalination 2013, 308, 138–146. [Google Scholar] [CrossRef]
- Valenca, J.C.; Wagterveld, R.M.; Lammertink, R.G.H.; Tsai, P.A. Dynamics of microvortices induced by ion concentration polarization. Phys. Rev. E 2015, 92, 031003R. [Google Scholar] [CrossRef]
- Davidson, S.M.; Wessling, M.; Mani, A. On the Dynamical Regimes of Pattern-Accelerated Electroconvection. Sci. Rep. 2016, 6, 22505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manzanares, J.A.; Murphy, W.D.; Mafe, S.; Reiss, H. Numerical simulation of the nonequilibrium diffuse double layer in ion-exchange membranes. J. Phys. Chem. 1993, 97, 8524–8530. [Google Scholar] [CrossRef]
- Zabolotskii, V.I.; Manzanares, J.A.; Mafe, S.; Nikonenko, V.V.; Lebedev, K.A. Steady-state ion transport through a three-layered membrane system: A mathematical model allowing for violation of the electroneutrality condition. Russ. J. Electrochem. 2002, 38, 819–827. [Google Scholar] [CrossRef]
- Magnico, P. Influence of the ion-solvent interactions on ionic transport through ion-exchange-membranes. J. Membr. Sci. 2013, 442, 272–285. [Google Scholar] [CrossRef]
- Nikonenko, V.V.; Kovalenko, A.V.; Urtenov, M.K.; Pismenskaya, N.D.; Han, J.; Sistat, P.; Pourcelly, G. Desalination at overlimiting currents: State-of-the-art and perspectives. Desalination 2014, 342, 85–106. [Google Scholar] [CrossRef]
- Nikonenko, V.V.; Vasil’eva, V.I.; Akberova, E.M.; Uzdenova, A.M.; Urtenov, M.K.; Kovalenko, A.V.; Pismenskaya, N.P.; Mareev, S.A.; Pourcelly, G. Competition between diffusion and electroconvection at an ion-selective surface in intensive current regimes. Adv. Colloid Interface Sci. 2016, 235, 233–246. [Google Scholar] [CrossRef]
- Długołecki, P.; Anet, B.; Metz, S.J.; Nijmeijer, K.; Wessling, M. Transport limitations in ion exchange membranes at low salt concentrations. J. Membr. Sci. 2010, 346, 163–171. [Google Scholar] [CrossRef]
- Kwak, R.; Pham, V.S.; Kim, B.; Chen, L.; Han, J. Enhanced salt removal by unipolar ion conduction in ion concentration polarization desalination. Sci. Rep. 2016, 6, 25349. [Google Scholar] [CrossRef] [PubMed]
- Druzgalski, C.L.; Andersen, M.B.; Mani, A. Direct numerical simulation of electroconvective instability and hydrodynamic chaos near an ion-selective surface. Phys. Fluids 2013, 25, 110804. [Google Scholar] [CrossRef]
- Druzgalski, C.; Mani, A. Statistical analysis of electroconvection near an ion-selective membrane in the highly chaotic regime. Phys. Rev. Fluids 2016, 1, 073601. [Google Scholar] [CrossRef]
- Demekhin, E.A.; Nikitin, N.V.; Shelistov, V.S. Direct numerical simulation of electrokinetic instability and transition to chaos motion. Phys. Fluids 2013, 25, 122001. [Google Scholar] [CrossRef]
- Demekhin, E.A.; Nikitin, N.V.; Shelistov, V.S. Three-dimensional coherent structures of electrokinetic instability. Phys. Rev. E 2014, 90, 013031. [Google Scholar] [CrossRef]
- Ganchenko, G.S.; Kalaydin, E.N.; Schiffbauer, J.; Demekhin, E.A. Modes of electrokinetic instability for imperfect membranes. Phys. Rev. E 2016, 94, 063106. [Google Scholar] [CrossRef]
- Pham, V.S.; Li, Z.; Lim, K.M.; White, J.K.; Han, J. Direct numerical simulation of electroconvective instability and hysteretic current-voltage response of a permselective membrane. Phys. Rev. E 2012, 86, 046310. [Google Scholar] [CrossRef]
- Magnico, P. Spatial distribution of mechanical forces and ionic flux in electro-kinetic instability near a permselective membrane. Phys. Fluids 2018, 30, 014101. [Google Scholar] [CrossRef] [Green Version]
- Roghmans, F.; Evdochenko, E.; Stockmeier, F.; Schneider, S.; Smailji, A.; Tiwari, R.; Mikosch, A.; Karatay, E.; Kühne, A.; Walther, A.; et al. 2D Patterned Ion-Exchange Membranes Induce Electroconvection. Adv. Mater. Interfaces 2018, 342, 1801309. [Google Scholar] [CrossRef]
- Valença, J.; Jogi, M.; Wagterveld, R.M.; Karatay, E.; Wood, J.A.; Lammertink, R.G.H. Confined electroconvective vortices at structured ion exchange membranes. Langmuir 2018, 34, 2455–2463. [Google Scholar] [CrossRef]
- Pham, S.V.; Kwon, H.; Kim, B.; White, J.K.; Lim, G.; Han, J. Helical vortex formation in three-dimensional electrochemical systems with ion-selective membranes. Phys. Rev. E 2016, 93, 033114. [Google Scholar] [CrossRef]
- Urtenov, M.K.; Uzdenova, A.M.; Kovalenko, A.V.; Nikonenko, V.V.; Pimenskaya, N.D.; Vasil’eva, V.I.; Sistat, P.; Pourcelly, G. Basic mathematic model of overlimiting transfer enhanced by electroconvection in flow-through electrodialysis membrane cells. J. Membr. Sci. 2013, 447, 190–202. [Google Scholar] [CrossRef]
- Tsai, P.; Peters, A.M.; Pirat, C.; Wessling, M.; Lammertink, R.G.H.; Lohse, D. Quantifying effective slip length over micropatterned hydrophobic surfaces. Phys. Fluids 2009, 21, 112002. [Google Scholar] [CrossRef] [Green Version]
- Maali, A.; Bhushan, B. Measurement of slip length on superhydrophobic surfaces. Philos. Trans. R. Soc. A 2012, 370, 2304–2320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korzhova, E.; Pismenskaya, N.; Lopatin, D.; Baranov, O.; Dammak, L.; Nikonenko, V.V. Effect of surface hydrophobization on chronopotentiometric behavior of an AMX anion-exchange membrane at overlimiting currents. J. Membr. Sci. 2016, 500, 161–170. [Google Scholar] [CrossRef]
- Andreeva, M.A.; Gil, V.V.; Pismenskaya, N.D.; Nikonenko, V.V.; Dammak, L.; Larchet, C.; Grande, D.; Kononenko, N.A. Effect of homogenization and hydrophobization of a cation-exchange membrane surface on its scaling in the presence of calcium and magnesium chlorides during electrodialysis. J. Membr. Sci. 2017, 540, 183–191. [Google Scholar] [CrossRef]
- Shelistov, V.S.; Demekhin, E.A.; Ganchenko, G.S. Electrokinetic instability near charge-selective hydrophobic surfaces. Phys. Rev. E 2014, 90, 013001. [Google Scholar] [CrossRef]
- Newmann, J.; Thomas-Alyea, K.E. Electrochemical Systems, 3rd ed.; Electrochemical Society Series; Wiley-Interscience: Hoboken, NJ, USA, 2004. [Google Scholar]











| 100 µm [41] | 140 µm [41] | 90 µm [28] | |
| 100 µm | 150 µm | 75 µm |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magnico, P. Electro-Kinetic Instability in a Laminar Boundary Layer Next to an Ion Exchange Membrane. Int. J. Mol. Sci. 2019, 20, 2393. https://doi.org/10.3390/ijms20102393
Magnico P. Electro-Kinetic Instability in a Laminar Boundary Layer Next to an Ion Exchange Membrane. International Journal of Molecular Sciences. 2019; 20(10):2393. https://doi.org/10.3390/ijms20102393
Chicago/Turabian StyleMagnico, Pierre. 2019. "Electro-Kinetic Instability in a Laminar Boundary Layer Next to an Ion Exchange Membrane" International Journal of Molecular Sciences 20, no. 10: 2393. https://doi.org/10.3390/ijms20102393
APA StyleMagnico, P. (2019). Electro-Kinetic Instability in a Laminar Boundary Layer Next to an Ion Exchange Membrane. International Journal of Molecular Sciences, 20(10), 2393. https://doi.org/10.3390/ijms20102393





