Topical Spilanthol Inhibits MAPK Signaling and Ameliorates Allergic Inflammation in DNCB-Induced Atopic Dermatitis in Mice
Abstract
1. Introduction
2. Results
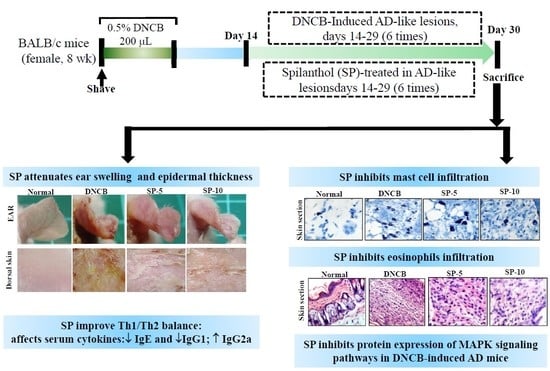
2.1. Spilanthol Attenuates Ear Swelling in BALB/c Mice with DNCB-Induced AD
2.2. Spilanthol Attenuates Collagen Deposition and Reduces Epidermal and Dermal Thickness in BALB/c Mice with DNCB-Induced AD
2.3. Spilanthol Inhibits Mast Cell Infiltration and Affects Serum Cytokines in BALB/c Mice with DNCB-Induced AD
2.4. Spilanthol Suppresses Eosinophil Infiltration and Inhibits Protein Expression of MAPK Signaling Pathways in BALB/c Mice with DNCB-Induced AD
2.5. Spilanthol Inhibits the Expression of Pro-Inflammatory Factors COX-2 and iNOS in BALB/c Mice with DNCB-Induced AD
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. DNCB Induction of AD-Like Skin Lesions and Spilanthol Treatment
4.3. Measurement of Ear and Epidermal Thickness
4.4. Histopathological Analysis
4.5. Measurement of Serum IgE and Cytokines
4.6. Western Blot Analysis
4.7. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AD | Atopic dermatitis |
| SP | Spilanthol |
| IgE | Immunoglobulin (Ig) E |
| IgG2a | Immunoglobulin (Ig) G2a |
| IgG1 | Immunoglobulin (Ig) G1 |
| COX-2 | Cyclooxygenase-2 |
| iNOS | Inducible NO synthase |
| DNCB | 2,4-dinitrochlorobenzene |
| MAPK | Mitogen-activated protein kinase |
| ERK | Extracellular signal-regulated kinase |
| JNK | c-jun N-terminal kinase |
References
- Boguniewicz, M.; Leung, D.Y. Atopic dermatitis: A disease of altered skin barrier and immune dysregulation. Immunol. Rev. 2011, 242, 233–246. [Google Scholar] [CrossRef]
- Kim, Y.J.; Choi, M.J.; Bak, D.H. Topical administration of EGF suppresses immune response and protects skin barrier in DNCB-induced atopic dermatitis in NC/Nga mice. Sci. Rep. 2018, 8, 11895. [Google Scholar] [CrossRef]
- Lan, C.C.; Fang, A.H.; Wu, P.H.; Wu, C.S. Tacrolimus abrogates TGF-β1-induced type I collagen production in normal human fibroblasts through suppressing p38MAPK signalling pathway: Implications on treatment of chronic atopic dermatitis lesions. J. Eur. Acad. Dermatol. 2014, 28, 204–215. [Google Scholar] [CrossRef]
- Brandt, E.B.; Sivaprasad, U. Th2 cytokines and atopic dermatitis. J. Clin. Cell. Immuno. 2011, 2, 110. [Google Scholar] [CrossRef]
- Turner, M.J.; Travers, J.B.; Kaplan, M.H. T helper cell subsets in the development of atopic dermatitis. J. Drugs. Dermatol. 2012, 11, 1174–1178. [Google Scholar]
- Lefeber, D.J.; Benaissa-Trouw, B.; Vliegenthart, J.F. Th1-Directing Adjuvants Increase the Immunogenicity of Oligosaccharide-Protein Conjugate Vaccines Related to Streptococcus pneumoniae Type 3. Infect. Immun. 2003, 12, 6915–6920. [Google Scholar] [CrossRef] [PubMed]
- Yosipovitch, G.; Papoiu, A.D.P. What causes itch in atopic dermatitis? Curr. Allergy. Asthm. R. 2008, 8, 306–311. [Google Scholar] [CrossRef]
- Holgate, S.T. The role of mast cells and basophils in inflammation. Clin. Exp. Allergy. 2000, 30, 28–32. [Google Scholar] [CrossRef]
- Rostamian, M.; Sohrabi, S.; Kavosifard, H. Lower levels of IgG1 in comparison with IgG2a are associated with protective immunity against Leishmania tropica infection in BALB/c mice. J. Microbiol. Immunol. Infect. 2017, 50, 160e166. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, S.; Yamada, Y.; Abe, T. Association of epithelial damage and signs of neutrophil mobilization in the airways during acute exacerbations of paediatric asthma. Clin. Exp. Immunol. 2006, 144, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Hommes, D.W.; Peppelenbosc, M.P.; van Deventer, S.J. Mitogen activated protein (MAP) kinase signal transduction pathways and novel anti-inflammatory targets. Gut 2003, 52, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Arthur, J.S.; Ley, S.C. Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 2013, 13, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Pathophysiology of allergic inflammation. Immunol. Rev. 2011, 242, 31–50. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.C.; Fan, N.C.; Lin, M.H. Anti-inflammatory effect of spilanthol from Spilanthes acmella on murine macrophage by down-regulating LPS-induced inflammatory mediators. J. Agr. Food. Chem. 2008, 9, 2341–2349. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Devi, B.R.; Sanjebam, R. Preliminary studies on local anesthetic and antipyretic activities of Spilanthes acmella Murr. in experimental animal models. Indian. J. Pharmacol. 2010, 42, 277–279. [Google Scholar] [CrossRef]
- Gerbino, A.; Schena, G.; Milano, S. Spilanthol from Acmella Oleracea Lowers the Intracellular Levels of cAMP Impairing NKCC2 Phosphorylation and Water Channel AQP2 Membrane Expression in Mouse Kidney. PLoS ONE 2016, 11, e0156021. [Google Scholar] [CrossRef]
- Cheng, Y.-B.; Liu, R.H.; Ho, M.-C.; Wu, T.-Y.; Chen, C.-Y.; Lo, I.-W.; Hou, M.-F.; Yuan, S.-S.; Wu, Y.-C.; Chang, F.-R. Alkylamides of Acmella oleracea. Molecules 2015, 20, 6970–6977. [Google Scholar] [CrossRef]
- Singh, M.; Pradhan, S. In vitro production of spilanthol from Spilanthes acmella Murr.: State of the art and future prospect. Int. J. Adv. Res. 2015, 3, 1559–1567. [Google Scholar]
- Joseph, B.; George, J.; Jeevitha, M.V. Tohle of Acmella Olerecea in Medicine—A review. World J. Pharm. Res. 2017, 2, 2781–2792. [Google Scholar]
- Barbosaa, A.F.; de Carvalhoa, M.G.; Smithb, R.E. Spilanthol: Occurrence, extraction, chemistry and biological activities. Rev. Bras. Farmacogn. 2016, 26, 128–133. [Google Scholar] [CrossRef]
- Prachayasittukal, V.; Prachayasittukal, S.; Ruchiwarat, S.; Prachayasittukal, V. High therapeutic potential of Spilanthes acmella: A review. Excli. J. 2013, 12, 291–312. [Google Scholar]
- Dubey, S.; Maity, S.; Singh, M. Phytochemistry, pharmacol-ogy and toxicology of Spilanthes acmella: A review. Adv. Pharmacol. Sci. 2013, 2013, 423750. [Google Scholar] [PubMed]
- Prachayasittikul, S.; Suphapong, S.; Worachartcheewan, A. Bioactive metabolites from Spilanthes acmella Murr. Molecules 2009, 14, 850–886. [Google Scholar] [CrossRef]
- Huang, C.H.; Chang, L.C.; Hu, S. Spilanthol inhibits TNF α induced ICAM 1 expression and pro inflammatory responses by inducing heme oxygenase 1 expression and suppressing pJNK in HaCaT keratinocytes. Mol. Med. Rep. 2018, 18, 2987–2994. [Google Scholar] [CrossRef]
- Leung, D.Y.; Soter, N.A. Cellular and immunologic mechanisms in atopic dermatitis. J. Am. Acad.Dermatol. 2001, 44, S1–S12. [Google Scholar] [CrossRef]
- Galli, S.J.; Tsai, M. IgE and mast cells in allergic disease. Nat. Med. 2015, 18, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.W.; Cheng, C.C.; Hwang, T.S. Danggui Buxue Tang Inhibits 2,4-Dinitrochlorobenzene: Induced Atopic Dermatitis in Mice. Evid.-Based Complementary Altern. Med. 2015, 2015, 672891. [Google Scholar]
- Ahn, J.Y.; Choi, S.E.; Jeong, M.S.; Park, K.H.; Moon, N.J.; Joo, S.S.; Lee, C.S.; Choi, Y.W.; Li, K.; Lee, M.K.; et al. Effect of taxifolin glycoside on atopic dermatitis-like skin lesions in NC/Nga mice. Phytother. Res. 2010, 24, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Ku, J.M.; Hong, S.H.; Kim, S.R. The prevention of 2,4-dinitrochlorobenzene-induced inflammation in atopic dermatitis-like skin lesions in BALB/c mice by Jawoongo. BMC. Complem. Altern. Med. 2018, 18, 215. [Google Scholar] [CrossRef]
- Jegal, J.; Park, N.J.; Bong, S.K. Dioscorea quinqueloba Ameliorates Oxazolone- and 2,4-Dinitrochlorobenzene-induced Atopic Dermatitis Symptoms in Murine Models. Nutrients 2017, 9, 1324. [Google Scholar] [CrossRef]
- Darlenski, R.; Kazandjieva, J.; Hristakieva, E.; Fluhr, J.W. Atopic dermatitis as a systemic disease. Clin. Dermatol. 2014, 32, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S. A novel model for human atopic dermatitis: Application of repeated DNCB patch in BALB/c mice, in comparison with NC/Nga mice. Lab. Anim. Res. 2010, 26, 95–102. [Google Scholar] [CrossRef]
- Danso, M.O. TNF-α and Th2 cytokines induce atopic dermatitis–like features on epidermal differentiation proteins and stratum corneum lipids in human skin equivalents. J. Invest. Dermatol. 2014, 134, 1941–1950. [Google Scholar] [CrossRef]
- Deo, S.S.; Mistry, K.J.; Kakade, A.M. Role played by Th2 type cytokines in IgE mediated allergy and asthma. Lung. India. 2010, 27, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Baumann, U.; Chouchakova, N.; Gewecke, B. Distinct Tissue Site-Specific Requirements of Mast Cells and Complement Components C3/C5a Receptor in IgG Immune Complex-Induced Injury of Skin and Lung. J. Immunol. 2001, 167, 1022–1027. [Google Scholar] [CrossRef] [PubMed]
- Govindaraj, D.; Sharma, S.; Singh, N.; Arora, N. T cell epitopes of Per a 10 modulate local-systemic immune responses and airway inflammation by augmenting Th1 and T regulatory cell functions in murine model. Immunobiology 2019, 18. [Google Scholar] [CrossRef]
- Piaoa, C.H.; Kim, T.G.; Buic, T.T. Ethanol extract of Dryopteris crassirhizoma alleviates allergic inflammation via inhibition of Th2 response and mast cell activation in a murine model of allergic rhinitis. J. Ethnopharmacol. 2019, 232, 21–29. [Google Scholar] [CrossRef]
- Johansen, C.; Kragballe, K.; Westergaard, M. The mitogen-activated protein kinases p38 and ERK1/2 are increased in lesional psoriatic skin. Brit. J. Dermatol. 2005, 152, 37–42. [Google Scholar] [CrossRef]
- Senthil, K.J.; Hsieh, H.W.; Wang, S.Y. Anti-inflammatory effect of lucidone in mice via inhibition of NF-κB/MAP kinase pathway. Int. Immunopharmacol. 2010, 10, 385–392. [Google Scholar] [CrossRef]
- Giuliano, F.; Warner, T.D. Origins of prostaglandin E2: Involvements of cyclooxygenase (COX)-1 and COX-2 in human and rat systems. J. Pharmacol. Exp. Ther. 2002, 303, 1001. [Google Scholar] [CrossRef]
- Shah, G.; Zhang, G.; Chen, F. iNOS expression and NO production contribute to the direct effects of BCG on urothelial carcinoma cell biology. Urol. Oncol. 2014, 32, 45e1–45e9. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chan, C.C.; Liou, C.J.; Xu, P.Y. Effect of dehydroepiandrosterone on atopic dermatitis-like skin lesions induced by 1-chloro-2,4-dinitrobenzene in mouse. J. Dermatol. Sci. 2013, 72, 149–157. [Google Scholar] [CrossRef] [PubMed]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.-C.; Huang, C.-H.; Hu, S.; Peng, H.-L.; Wu, S.-J. Topical Spilanthol Inhibits MAPK Signaling and Ameliorates Allergic Inflammation in DNCB-Induced Atopic Dermatitis in Mice. Int. J. Mol. Sci. 2019, 20, 2490. https://doi.org/10.3390/ijms20102490
Huang W-C, Huang C-H, Hu S, Peng H-L, Wu S-J. Topical Spilanthol Inhibits MAPK Signaling and Ameliorates Allergic Inflammation in DNCB-Induced Atopic Dermatitis in Mice. International Journal of Molecular Sciences. 2019; 20(10):2490. https://doi.org/10.3390/ijms20102490
Chicago/Turabian StyleHuang, Wen-Chung, Chun-Hsun Huang, Sindy Hu, Hui-Ling Peng, and Shu-Ju Wu. 2019. "Topical Spilanthol Inhibits MAPK Signaling and Ameliorates Allergic Inflammation in DNCB-Induced Atopic Dermatitis in Mice" International Journal of Molecular Sciences 20, no. 10: 2490. https://doi.org/10.3390/ijms20102490
APA StyleHuang, W.-C., Huang, C.-H., Hu, S., Peng, H.-L., & Wu, S.-J. (2019). Topical Spilanthol Inhibits MAPK Signaling and Ameliorates Allergic Inflammation in DNCB-Induced Atopic Dermatitis in Mice. International Journal of Molecular Sciences, 20(10), 2490. https://doi.org/10.3390/ijms20102490