Melatonin Reduces Excitability in Dorsal Root Ganglia Neurons with Inflection on the Repolarization Phase of the Action Potential
Abstract
:1. Introduction
2. Results
2.1. Neuronal Sample Characterization
2.2. Melatonin Effect on the Excitability of Ninf DRG Neurons
2.3. Melatonin Effect on Passive Properties of Ninf DRG Neurons
2.4. Melatonin Effect on Active Properties of Ninf Cells
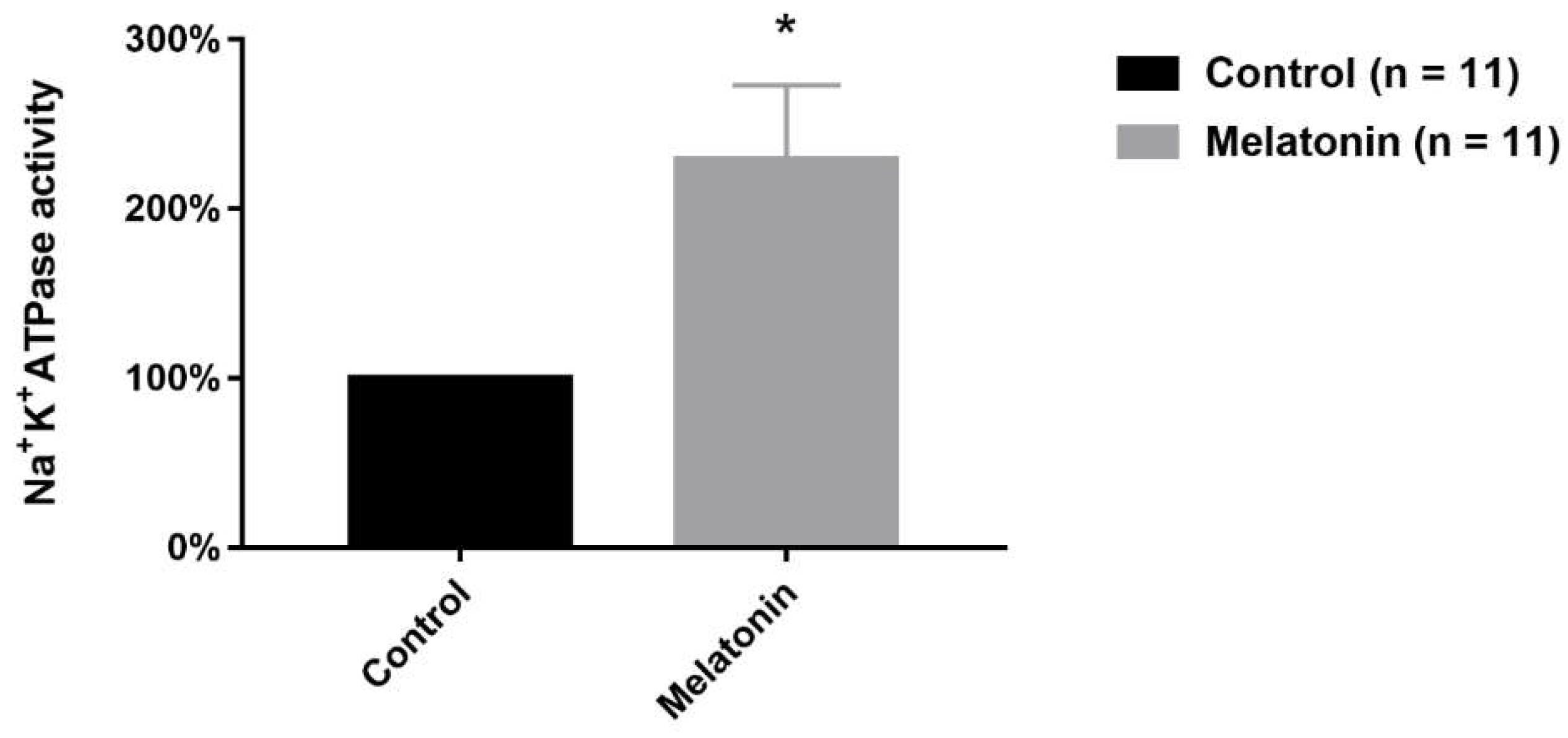
2.5. Melatonin Effect on Na+/K+-ATPase Activity
3. Discussion
4. Materials and Methods
4.1. Animals and Tissue Dissection
4.2. Solutions and Drugs
4.3. Electrophysiological Measurements and Analysis
4.4. Determination of Na+/K+-ATPase Activity
4.5. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AP | Action potential |
| DRG | Dorsal root ganglia |
| dV/dtasc | Maximum ascendant inclination |
| dV/dtdesc | Maximum descendant inclination |
| EMNS | Excitability melatonin not sensitive |
| EMS | Excitability melatonin sensitive |
| Rin | Input resistance |
| RMP | Resting membrane potential |
| SCN | Suprachiasmatic nucleus |
References
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. Isolation of melatonin, the pineal gland factor that lightens melanocytes. J. Am. Chem. Soc. 1958, 80, 2587. [Google Scholar] [CrossRef]
- Cipolla-Neto, J.; do Amaral, F.G. Melatonin As a Hormone: New Physiological and Clinical Insights. Endocr. Rev. 2018, 39, 990–1028. [Google Scholar] [CrossRef]
- Jiang, Z.G.; Nelson, C.S.; Allen, C.N. Melatonin activates an outward current and inhibits Ih in rat suprachiasmatic nucleus neurons. Brain Res. 1995, 687, 125–132. [Google Scholar] [CrossRef]
- Huang, F.; Guan, X.; Yan, Y.; Fan, W.; You, Y.; He, H.; Cheng, B. Electrophysiological effects of melatonin on rat trigeminal ganglion neurons that participate in nociception in vitro. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 3234–3239. [Google Scholar] [PubMed]
- Meng, X.; Li, Y.; Li, S.; Zhou, Y.; Gan, R.Y.; Xu, D.P.; Li, H. Bin Dietary sources and bioactivities of melatonin. Nutrients 2017, 9, 367. [Google Scholar] [CrossRef]
- Xie, Z.; Chen, F.; Li, W.A.; Geng, X.; Li, C. A review of sleep disorders and melatonin. Neurol. Res. 2017, 39, 559–565. [Google Scholar] [CrossRef]
- Zephy, D.; Ahmad, J. Type 2 diabetes mellitus: Role of melatonin and oxidative stress. Diabetes Metab. Syndr. Clin. Res. Rev. 2015, 9, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Mack, J.M.; Schamne, M.G.; Sampaio, T.B.; Pértile, R.A.N.; Fernandes, P.A.C.M.; Markus, R.P.; Prediger, R.D. Melatoninergic System in Parkinson’s Disease: From Neuroprotection to the Management of Motor and Nonmotor Symptoms. Oxid. Med. Cell. Longev. 2016, 2016, 3472032. [Google Scholar] [CrossRef]
- Alghamdi, B.S. The neuroprotective role of melatonin in neurological disorders. J. Neurosci. Res. 2018, 96, 1136–1149. [Google Scholar] [CrossRef]
- Shukla, M.; Govitrapong, P.; Boontem, P.; Reiter, R.J.; Satayavivad, J. Mechanisms of Melatonin in Alleviating Alzheimer’s Disease. Curr. Neuropharmacol. 2017, 15, 1010–1031. [Google Scholar] [CrossRef]
- Chen, W.W.; Zhang, X.; Huang, W.J. Pain control by melatonin: Physiological and pharmacological effects. Exp. Ther. Med. 2016, 12, 1963–1968. [Google Scholar] [CrossRef]
- Kurganova, Y.M.; Danilov, A.B. Melatonin in Chronic Pain Syndromes. Neurosci. Behav. Physiol. 2017, 47, 806–812. [Google Scholar] [CrossRef] [Green Version]
- Harper, A.; Lawson, S. Electrical properties of rat dorsal root ganglion neurones with different peripheral nerve conduction velocities. J. Physiol. 1985, 47–63. [Google Scholar] [CrossRef]
- Silva-Alves, K.S.; Ferreira-da-Silva, F.W.; Peixoto-Neves, D.; Viana-Cardoso, K.V.; Moreira-Júnior, L.; Oquendo, M.B.; Oliveira-Abreu, K.; Albuquerque, A.A.; Coelho-de-Souza, A.N.; Leal-Cardoso, J.H. Estragole blocks neuronal excitability by direct inhibition of Na+ channels. Braz. J. Med. Biol. Res. 2013, 46, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Abreu, K.; Ferreira-da-Silva, F.W.; Silva-Alves, K.S.; Silva-dos-Santos, N.M.; Cardoso-Teixeira, A.C.; Gaspar, F.; Cipolla-Neto, J.; Leal-Cardoso, J.H. Melatonin decreases neuronal excitability in a sub-population of dorsal root ganglion neurons. Brain Res. 2018, 1692, 1–8. [Google Scholar] [CrossRef]
- Harper, A.; Lawson, S. Conduction velocity is related to morphological cell type in rat dorsal root ganglion neurones. J. Physiol. 1985, 359, 31–46. [Google Scholar] [CrossRef]
- Villière, V.; McLachlan, E.M. Electrophysiological properties of neurons in intact rat dorsal root ganglia classified by conduction velocity and action potential duration. J. Neurophysiol. 1996, 76, 1924–1941. [Google Scholar] [CrossRef]
- Fang, X.; McMullan, S.; Lawson, S.N.; Djouhri, L. Electrophysiological differences between nociceptive and non-nociceptive dorsal root ganglion neurones in the rat in vivo. J. Physiol. 2005, 565, 927–943. [Google Scholar] [CrossRef]
- Pack, W.; Hill, D.D.; Wong, K.Y. Melatonin modulates M4-type ganglion-cell photoreceptors. Neuroscience 2015, 303, 178–188. [Google Scholar] [CrossRef] [Green Version]
- Van den Top, M.; Buijs, R.M.; Ruijter, J.M.; Delagrange, P.; Spanswick, D.; Hermes, M.L. Melatonin generates an outward potassium current in rat suprachiasmatic nucleus neurones in vitro independent of their circadian rhythm. Neuroscience 2001, 107, 99–108. [Google Scholar] [CrossRef]
- Scott, F.F.; Belle, M.D.C.; Delagrange, P.; Piggins, H.D. Electrophysiological effects of melatonin on mouse Per1 and non-Per1 suprachiasmatic nuclei neurones in vitro. J. Neuroendocrinol. 2010, 22, 1148–1156. [Google Scholar] [CrossRef]
- Squecco, R.; Tani, A.; Zecchi-Orlandini, S.; Formigli, L.; Francini, F. Melatonin affects voltage-dependent calcium and potassium currents in MCF-7 cell line cultured either in growth or differentiation medium. Eur. J. Pharmacol. 2015, 758, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Vanecek, J.; Klein, D.C. Sodium-dependent effects of melatonin on membrane potential of neonatal rat pituitary cells. Endocrinology 1992, 131, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, E.E. Na+, K+-ATPase: Functions in the nervous system and involvement in neurologic disease. Neurology 2011, 76, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Aidley, D.J. The physiology of excitable cells. Cambrige Univ. Press 1998, 61, 35–71. [Google Scholar]
- Ritter, L.; Kleemann, D.; Hickmann, F.H.; Amaral, A.U.; Sitta, Â.; Wajner, M.; Ribeiro, C.A.J. Disturbance of energy and redox homeostasis and reduction of Na+,K+-ATPase activity provoked by in vivo intracerebral administration of ethylmalonic acid to young rats. Biochim. Biophys. Acta 2015, 1852, 1–9. [Google Scholar] [CrossRef]
- González, M.A.; Contini, M.D.C.; Millen, N.; Mahieu, S.T. Role of melatonin in the oxidative damage prevention at different times of hepatic regeneration. Cell Biochem. Funct. 2012, 30, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Ersahin, M.; Toklu, H.Z.; Çetinel, Ş.; Yüksel, M.; Yeğen, B.Ç.; Şener, G. Melatonin reduces experimental subarachnoid hemorrhage-induced oxidative brain damage and neurological symptoms. J. Pineal Res. 2009, 46, 324–332. [Google Scholar] [CrossRef]
- Hodgkin, A.L.; Huxley, A.F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 1952, 117, 500–544. [Google Scholar] [CrossRef]
- Inyushkin, A.N.; Bhumbra, G.S.; Gonzalez, J.A.; Dyball, R.E. Melatonin modulates spike coding in the rat suprachiasmatic nucleus. J. Neuroendocrinol. 2007, 19, 671–681. [Google Scholar] [CrossRef]
- Catterall, W.A. Forty Years of Sodium Channels: Structure, Function, Pharmacology, and Epilepsy. Neurochem. Res. 2017, 42, 2495–2504. [Google Scholar] [CrossRef] [PubMed]
- Kanda, H.; Gu, J.G.; Medicine, P. Effects of cold temperatures on the excitability of rat trigeminal ganglion neurons that are not for cold-sensing. J. Neuroendocrinol. 2017, 141, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, T.C.; Benoit, E.; Partiseti, M.; Servent, D. The NaV1.7 channel subtype as an antinociceptive target for spider toxins in adult dorsal root ganglia neurons. Front. Pharmacol. 2018, 9, 1000. [Google Scholar] [CrossRef]
- Leal-Cardoso, J.H.; da Silva-Alves, K.S.; Ferreira-da-Silva, F.W.; dos Santos-Nascimento, T.; Joca, H.C.; de Macedo, F.H.P.; de Albuquerque-Neto, P.M.; Magalhães, P.J.C.; Lahlou, S.; Cruz, J.S.; et al. Linalool blocks excitability in peripheral nerves and voltage-dependent Na+ current in dissociated dorsal root ganglia neurons. Eur. J. Pharmacol. 2010, 645, 86–93. [Google Scholar] [CrossRef]
- Joca, H.C.; Cruz-Mendes, Y.; Oliveira-Abreu, K.; Maia-Joca, R.P.M.; Barbosa, R.; Lemos, T.L.; Lacerda Beirão, P.S.; Leal-Cardoso, J.H. Carvacrol decreases neuronal excitability by inhibition of voltage-gated sodium channels. J. Nat. Prod. 2012, 75, 1511–1517. [Google Scholar] [CrossRef]
- Axon. The Axon TM Guide. Prod. Man. 2012. [Google Scholar]
- Green, R.J.; King, R.H.M.; Thomas, P.K.; Baron, D.N. Sodium-potassium-ATPase activity in the dorsal root ganglia of rats with streptozotocin-induced diabetes. Diabetologia 1985, 28, 104–107. [Google Scholar] [CrossRef]
- Chakravarty, S.; Rizvi, S.I. Circadian modulation of sodium-potassium ATPase and sodium—Proton exchanger in human erythrocytes: In vitro effect of melatonin. Cell. Mol. Biol. 2011, 57, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Fiske, C.H.; Subbarow, Y. The colorimetric determination of phosphorus. J. Biol. Chem. 1925, 66, 375–400. [Google Scholar]
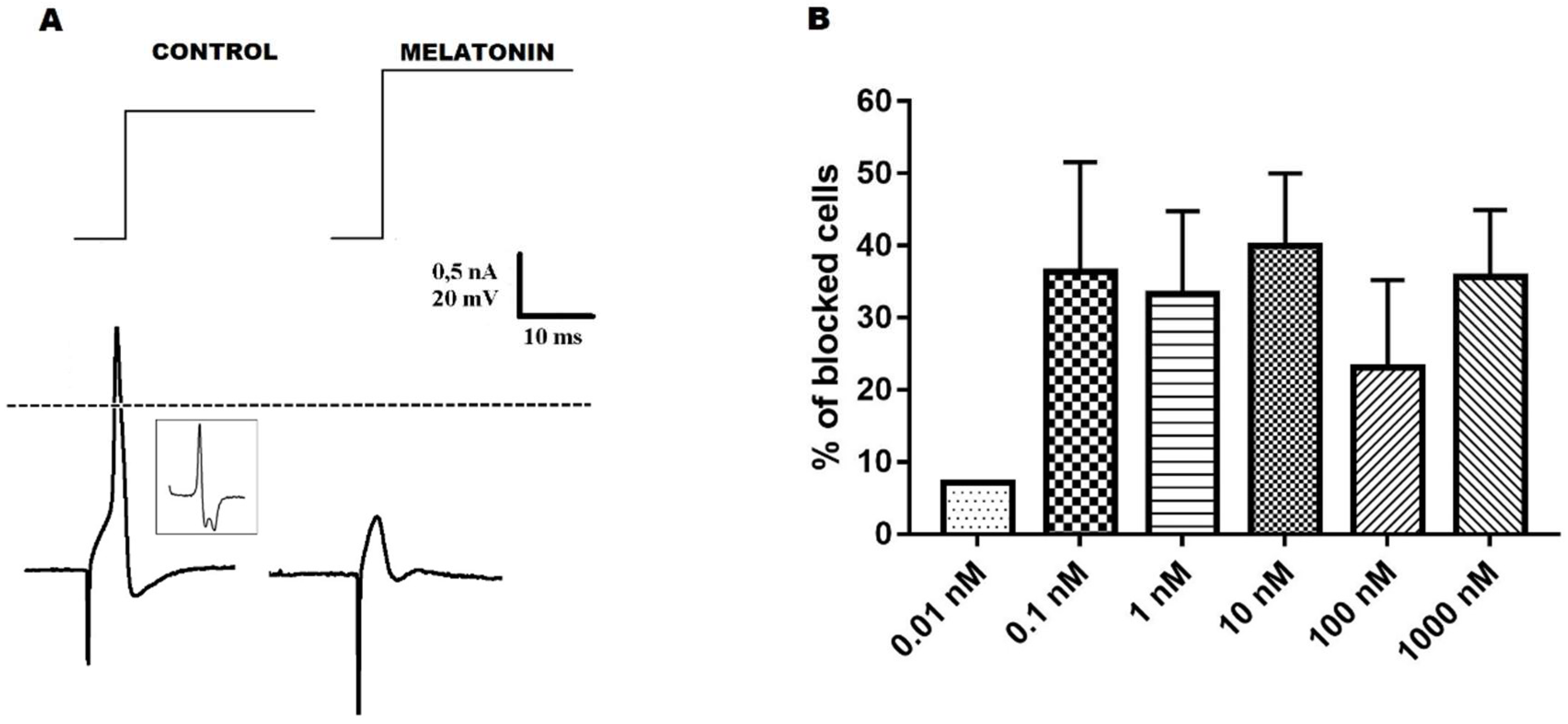
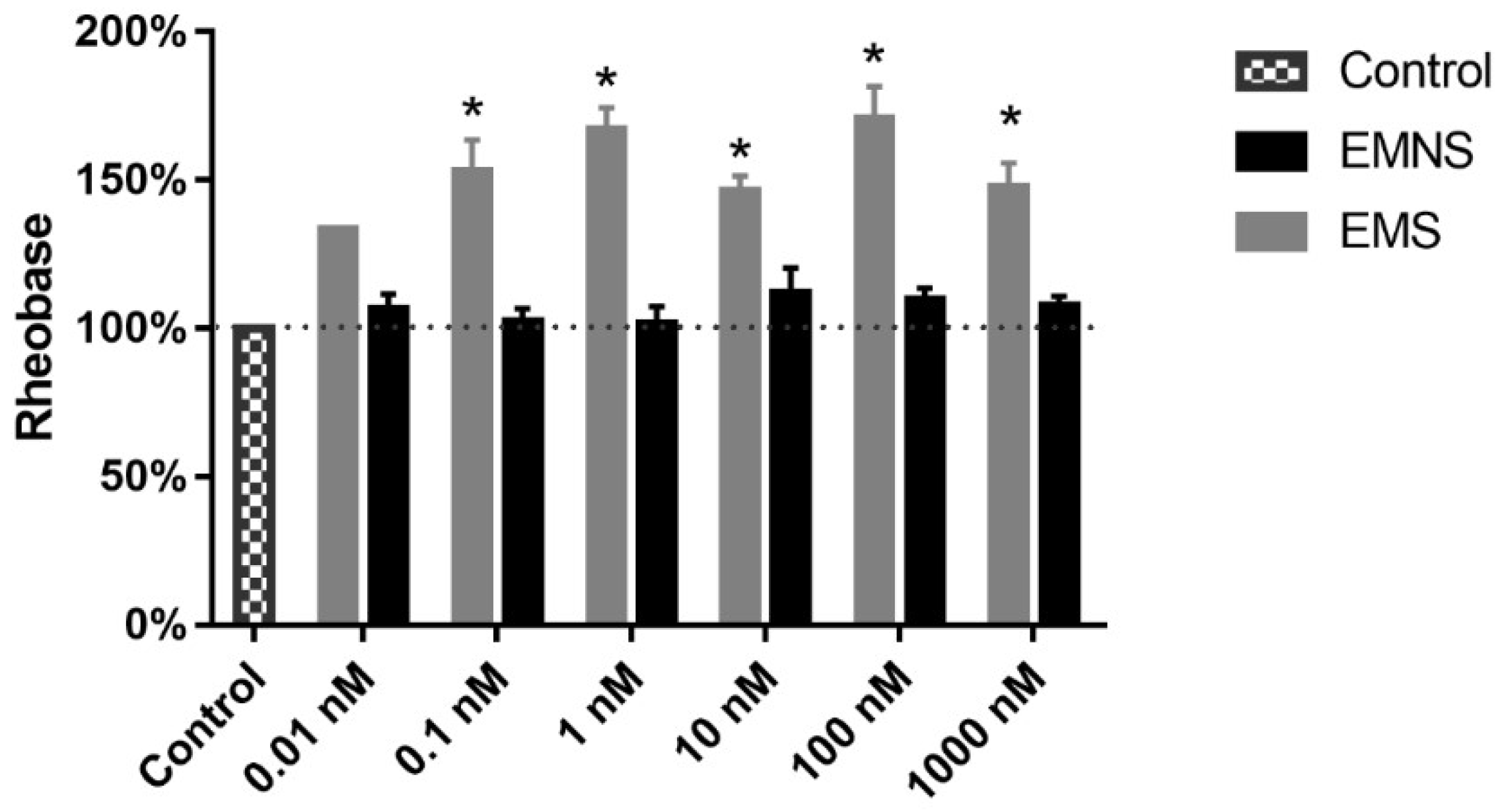

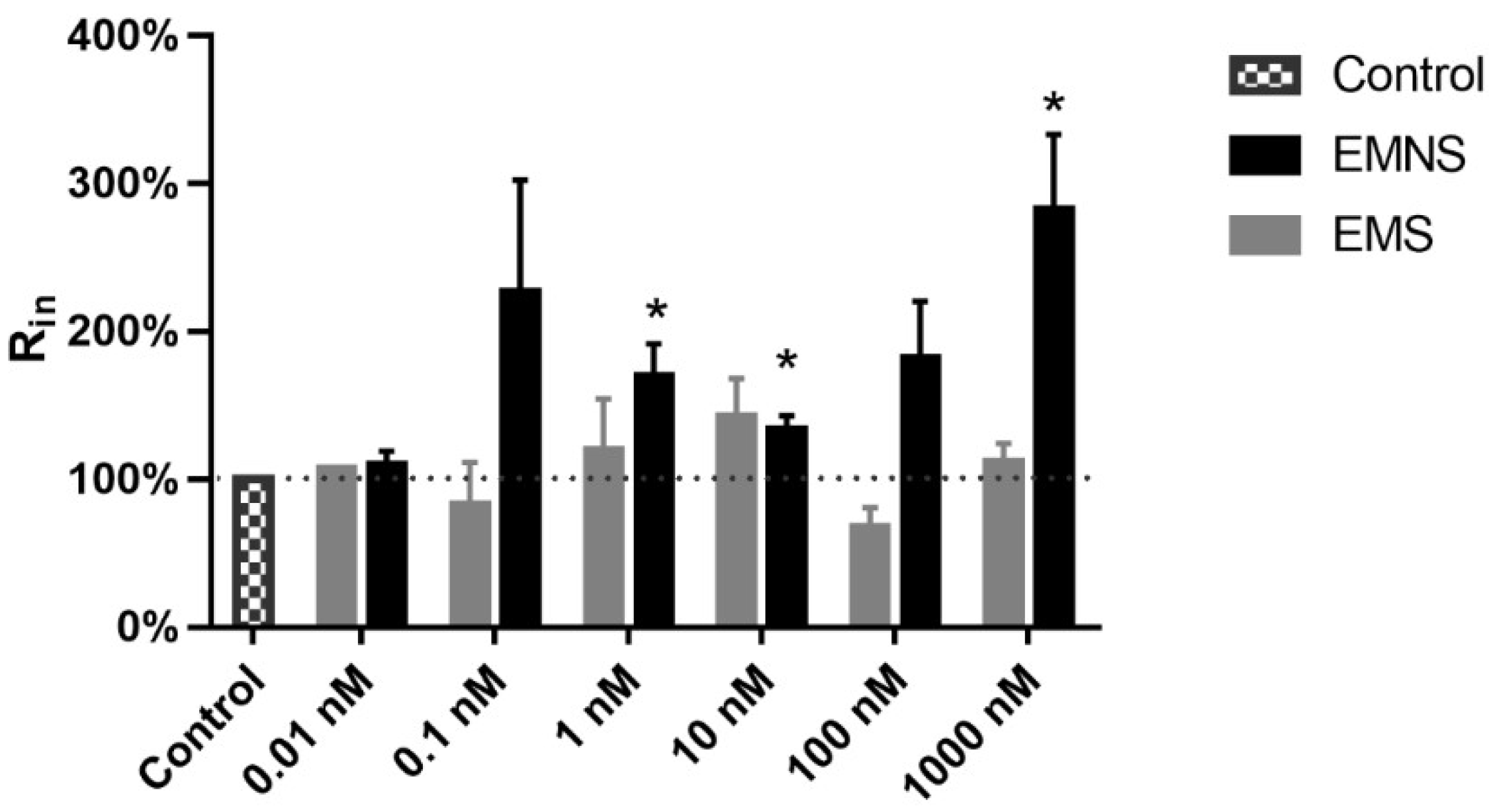
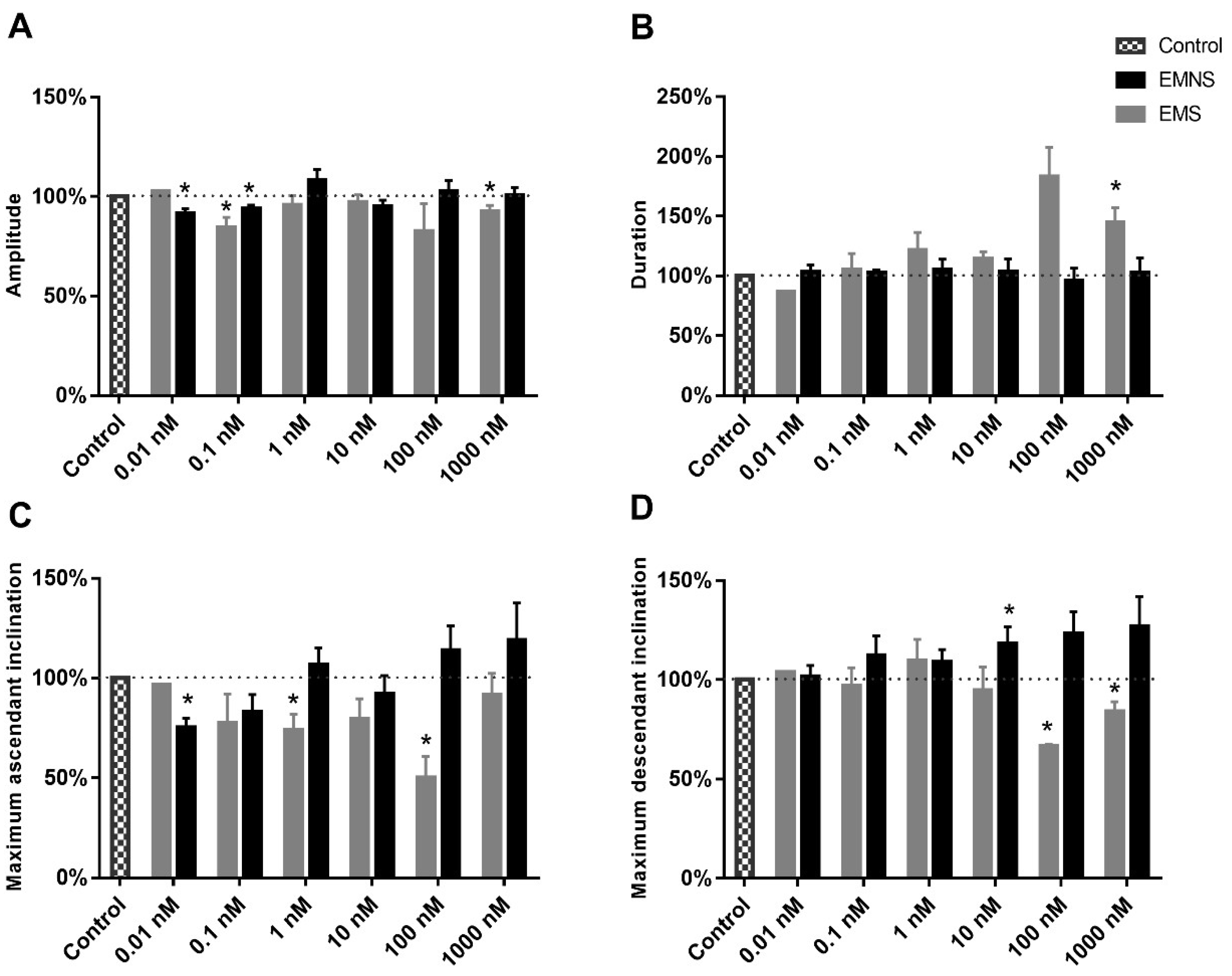
| Parameters 1 | Ninf Parameters (EMS) 5 | Ninf Parameters (EMNS) 6 | N0 Parameters 7 |
|---|---|---|---|
| RMP 2 (mV) | −56.0 ± 0.98 # | −59.3 ± 0.93 | −58.8 ± 0.59 |
| Input resistance (MΩ) | 31.8 ± 9.22 | 63.6 ± 14.69 * | 13.0 ± 0.93 |
| Rheobase (nA) | 1.5 ± 0.14 | 1.6 ± 0.10 | 1.5 ± 0.07 |
| Amplitude (mV) | 80.8 ± 2.13 * | 78.6 ± 1.34 * | 71.3 ± 0.88 |
| Duration (ms) | 1.9 ± 0.16 * | 2.1 ± 0.19 * | 0.8 ± 0.02 |
| dV/dtasc 3 (V/s) | 115.7 ± 5.85 * | 110.6 ± 5.47 * | 167.1 ± 4.81 |
| dV/dtdesc 4 (V/s) | −54.1 ± 2.69 * | −58.4 ± 2.56 * | −108.5 ± 3.14 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira-Abreu, K.; Silva-dos-Santos, N.M.; Coelho-de-Souza, A.N.; Ferreira-da-Silva, F.W.; Silva-Alves, K.S.d.; Cardoso-Teixeira, A.C.; Cipolla-Neto, J.; Leal-Cardoso, J.H. Melatonin Reduces Excitability in Dorsal Root Ganglia Neurons with Inflection on the Repolarization Phase of the Action Potential. Int. J. Mol. Sci. 2019, 20, 2611. https://doi.org/10.3390/ijms20112611
Oliveira-Abreu K, Silva-dos-Santos NM, Coelho-de-Souza AN, Ferreira-da-Silva FW, Silva-Alves KSd, Cardoso-Teixeira AC, Cipolla-Neto J, Leal-Cardoso JH. Melatonin Reduces Excitability in Dorsal Root Ganglia Neurons with Inflection on the Repolarization Phase of the Action Potential. International Journal of Molecular Sciences. 2019; 20(11):2611. https://doi.org/10.3390/ijms20112611
Chicago/Turabian StyleOliveira-Abreu, Klausen, Nathalia Maria Silva-dos-Santos, Andrelina Noronha Coelho-de-Souza, Francisco Walber Ferreira-da-Silva, Kerly Shamyra da Silva-Alves, Ana Carolina Cardoso-Teixeira, José Cipolla-Neto, and José Henrique Leal-Cardoso. 2019. "Melatonin Reduces Excitability in Dorsal Root Ganglia Neurons with Inflection on the Repolarization Phase of the Action Potential" International Journal of Molecular Sciences 20, no. 11: 2611. https://doi.org/10.3390/ijms20112611
APA StyleOliveira-Abreu, K., Silva-dos-Santos, N. M., Coelho-de-Souza, A. N., Ferreira-da-Silva, F. W., Silva-Alves, K. S. d., Cardoso-Teixeira, A. C., Cipolla-Neto, J., & Leal-Cardoso, J. H. (2019). Melatonin Reduces Excitability in Dorsal Root Ganglia Neurons with Inflection on the Repolarization Phase of the Action Potential. International Journal of Molecular Sciences, 20(11), 2611. https://doi.org/10.3390/ijms20112611





