Stemarane Diterpenes and Diterpenoids †
Abstract
:1. Introduction
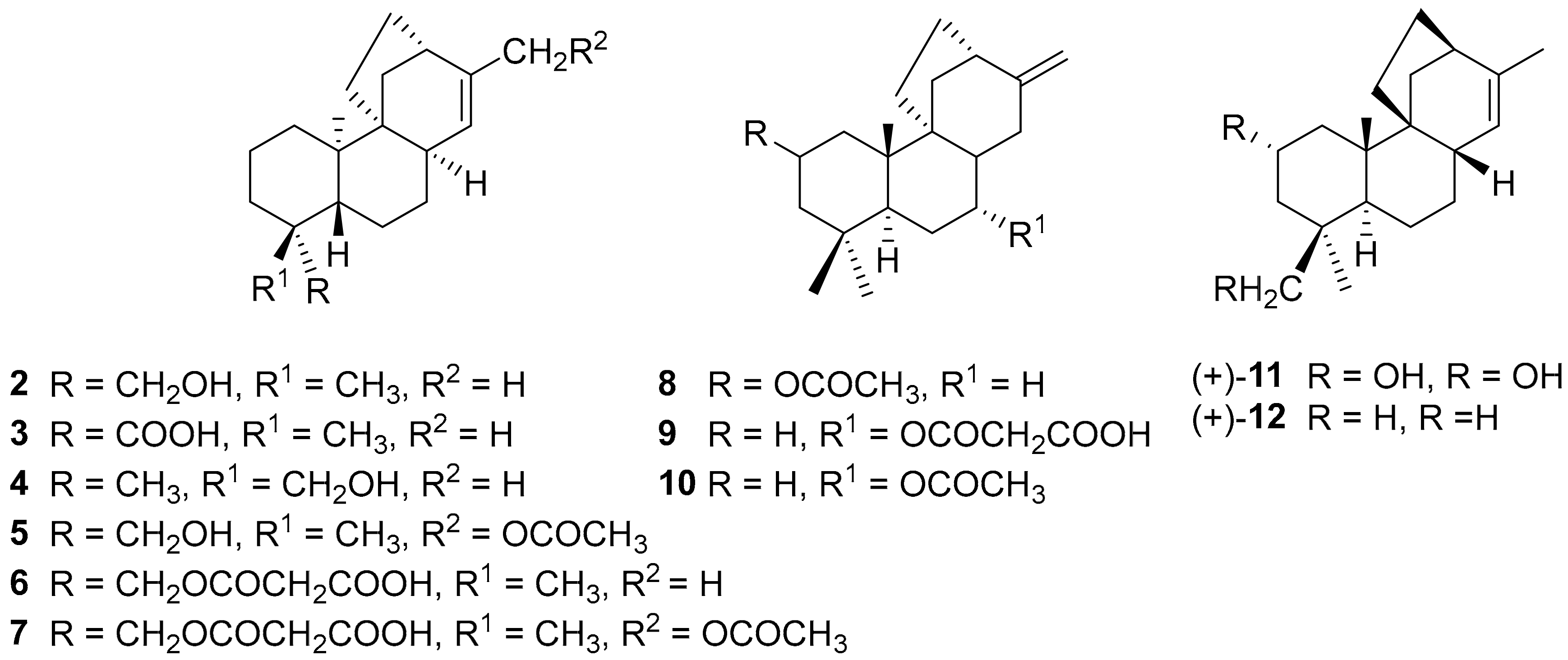
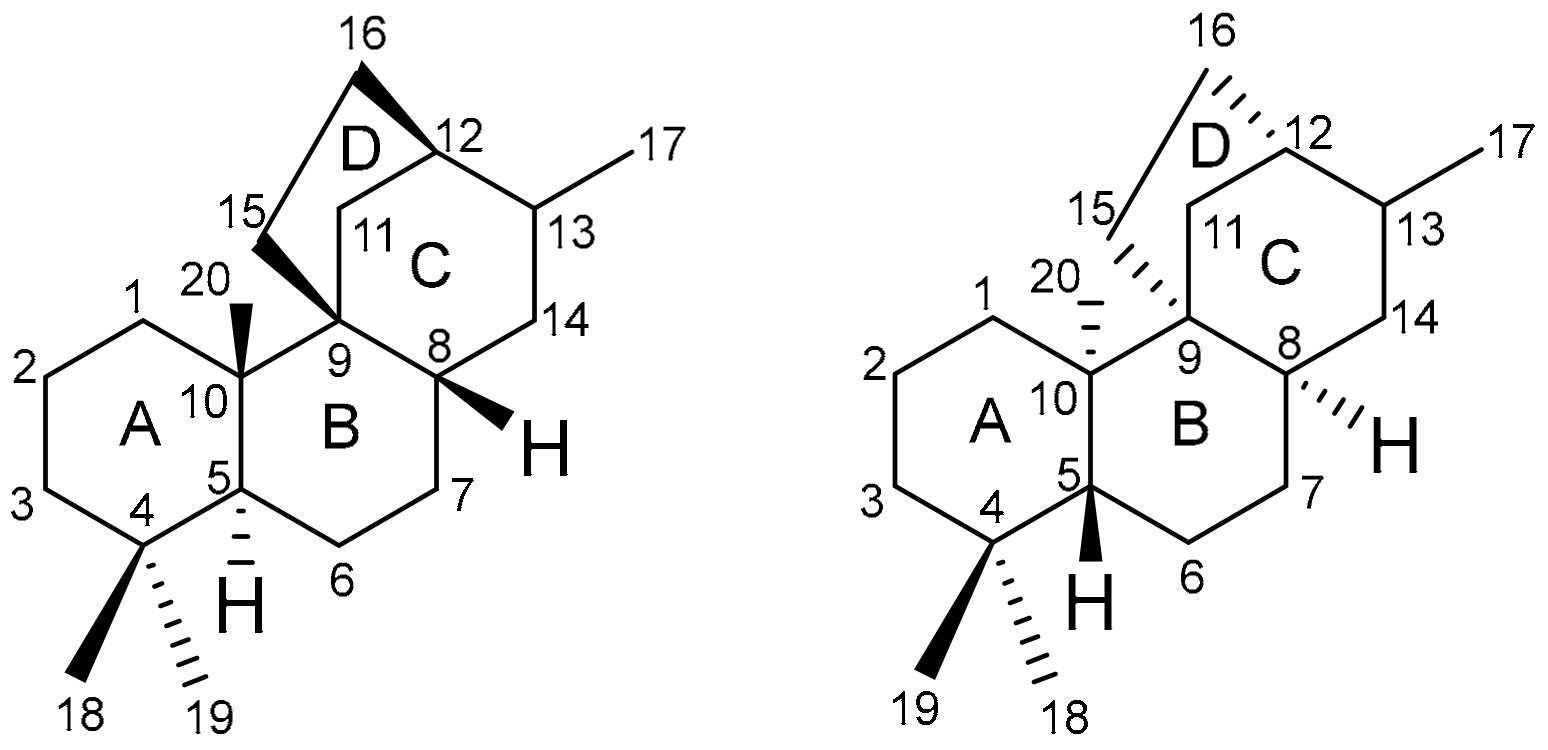
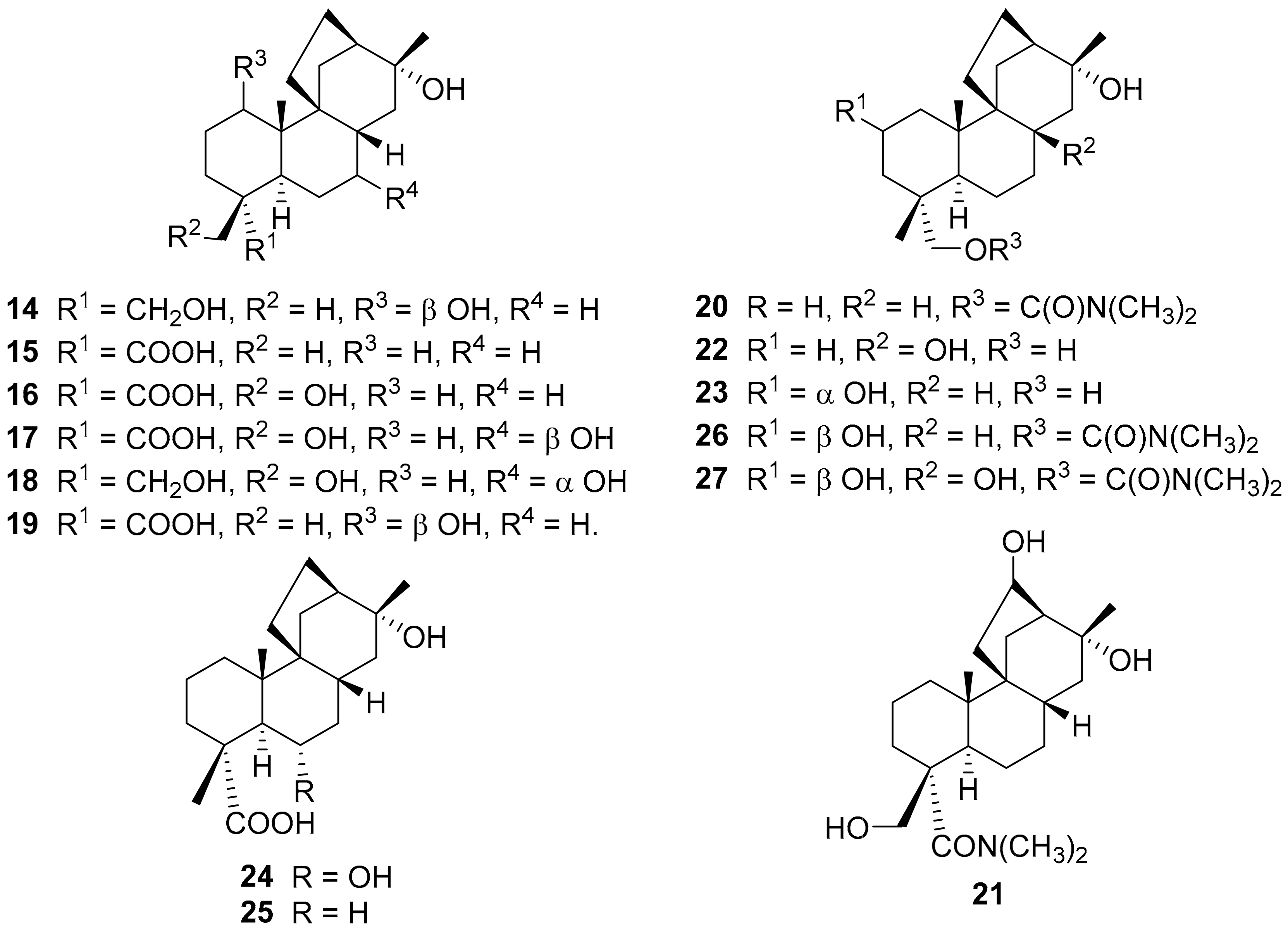
2. Structure
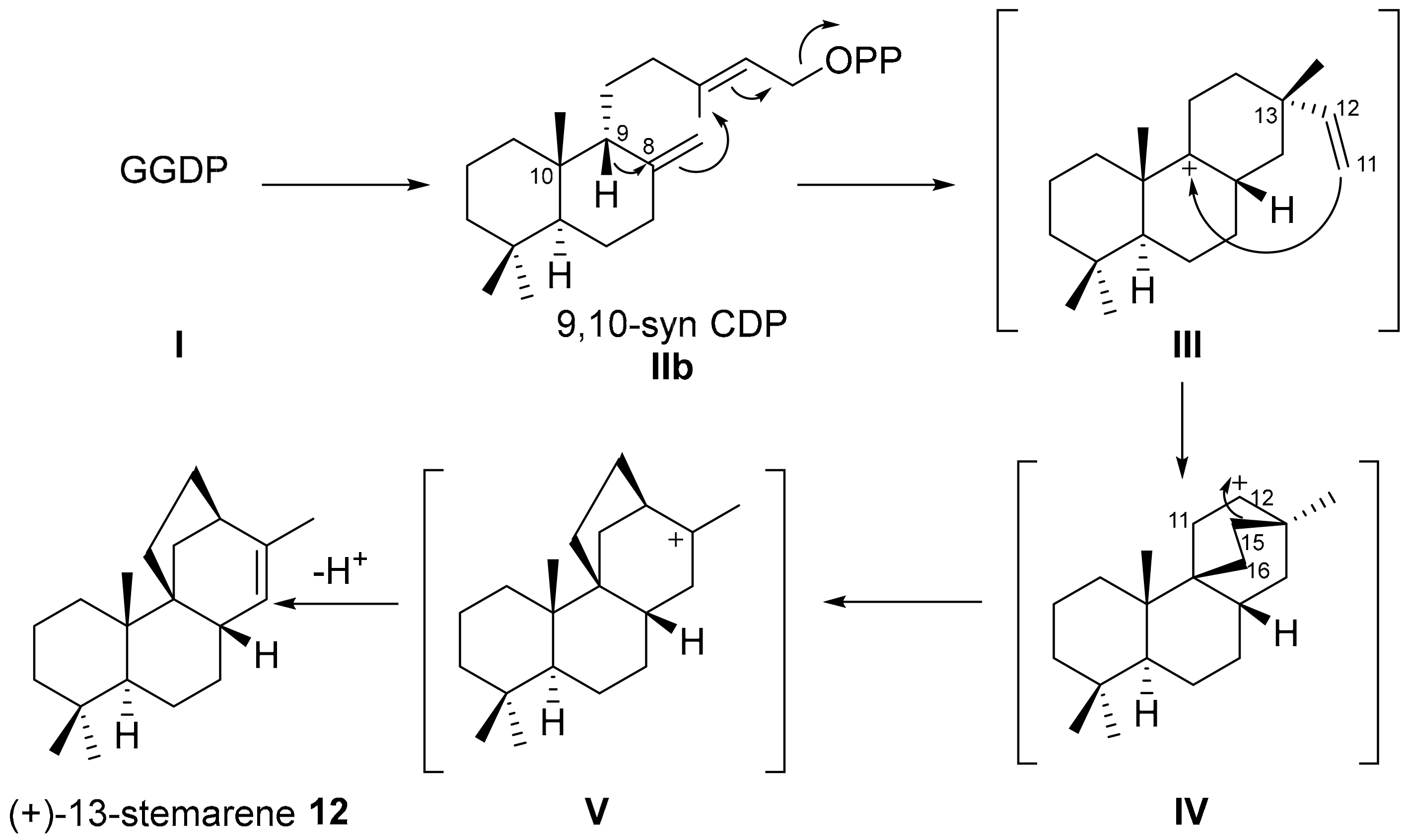
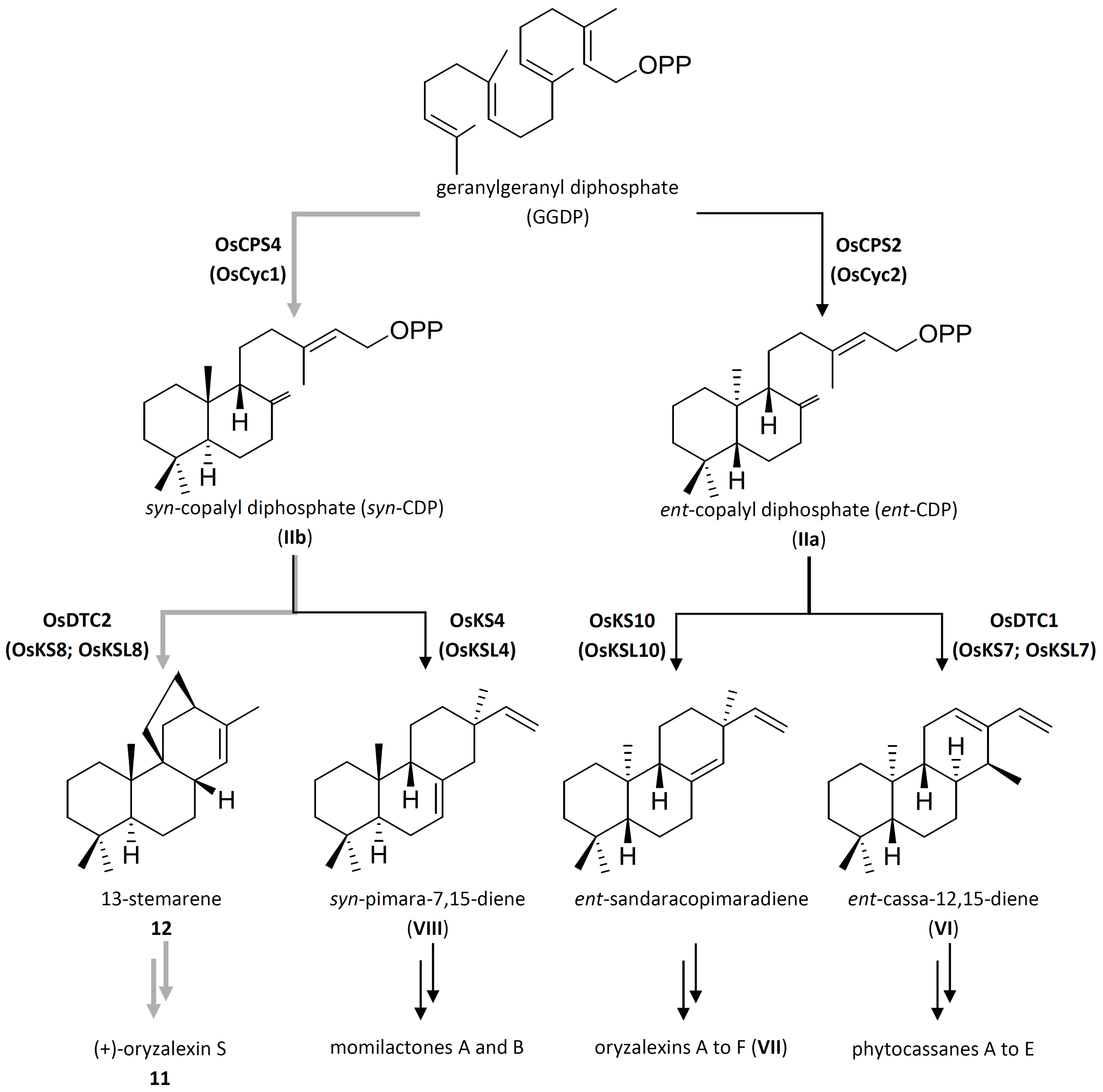
3. Biogenesis
4. Biosynthesis
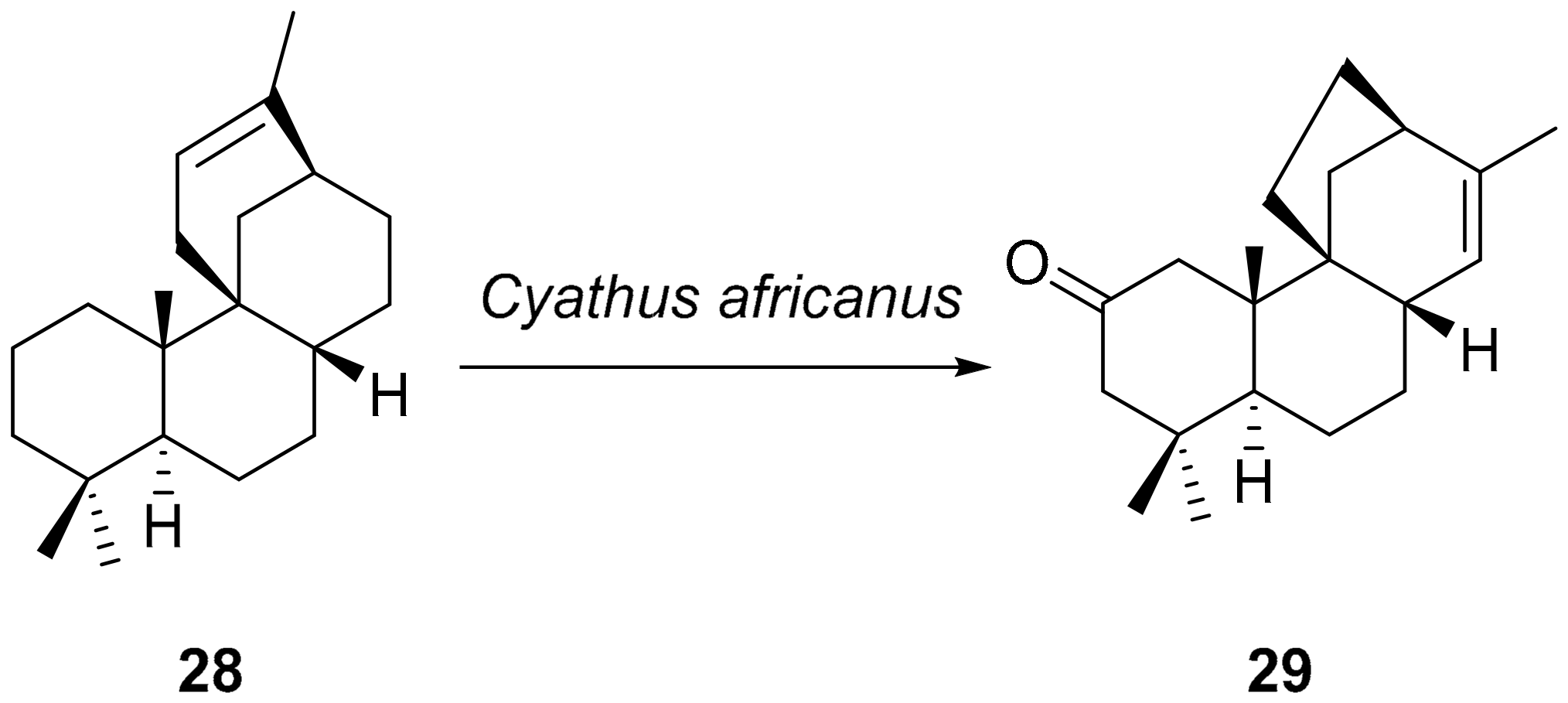
5. Biotransformations
6. Biological Activity
7. Synthesis
- (a)
- (b)
- (c)
7.1. The (±)-Stemarin 1 Total Synthesis by Allene Photoaddition to a 9(11)-Podocarpen-12-one Intermediate
7.2. Approaches for Diastereoselective Syntheses of (+)-13-Stemarene 12 and (+)-18-Deoxystemarin 2 and (±)-Stemarin 1 by Allene Photoaddition to 9(11)-Podocarpen-12-one and 8(9)-Podocarpen-14-one
7.2.1. Approach A: Diastereoselective Synthesis of (+)-13-Stemarene 12 and (+)-18-Deoxystemarin 2 by Allene Addition to 9(11)-Podocarpen-12-one
7.2.2. Approach B: Attempted Diastereoselective Synthesis of (±)-Stemarin 1 by Allene Addition to a 8(9)-Podocarpen-14-one
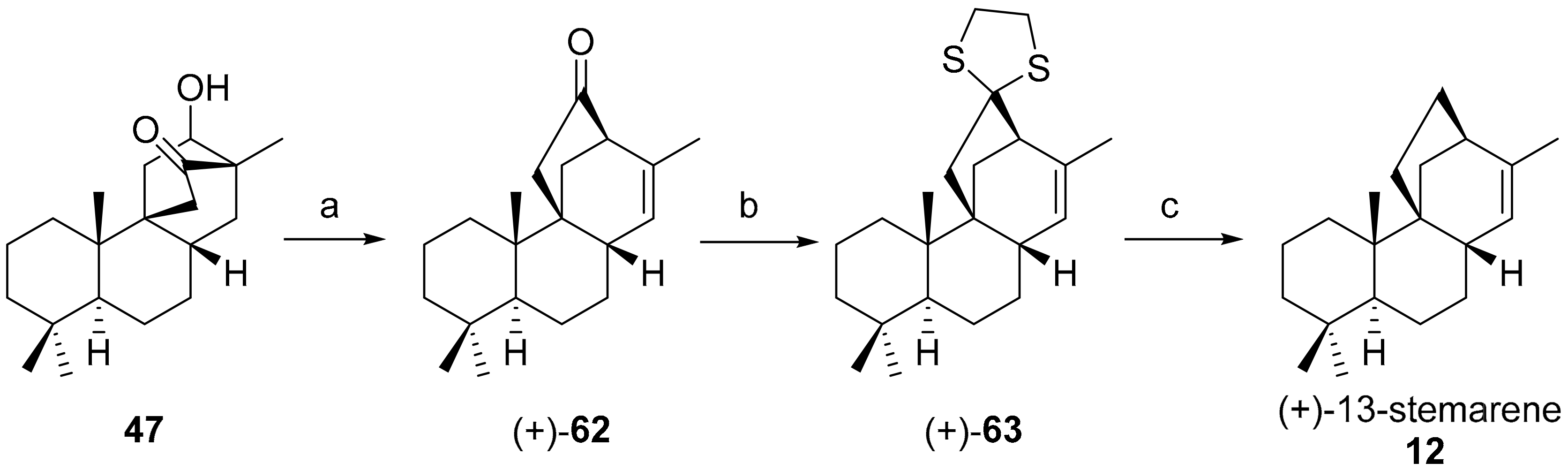
7.3. Regio- and Diastereoselective Synthesis of (+)-Stemar-13-ene 12 and (+)-18-Deoxystemarin 2 by the 6-Hydroxy-1-methylbicyclo[2.2.2]octan-2-one → 4-Methylbicyclo[3.2.1]oct-3-en-6-one Skeletal Rearrangement
7.4. Regio- and Diastereoselective Synthesis of (+)-2-Deoxyoryzalexin S 2 from (+)-Podocarpic Acid
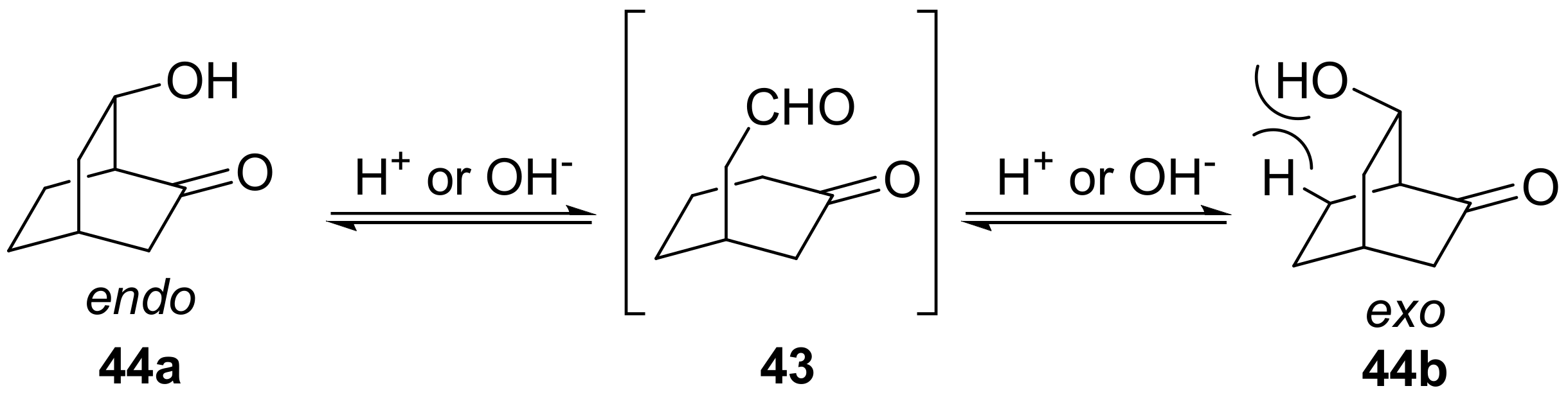
7.5. Other Strategies
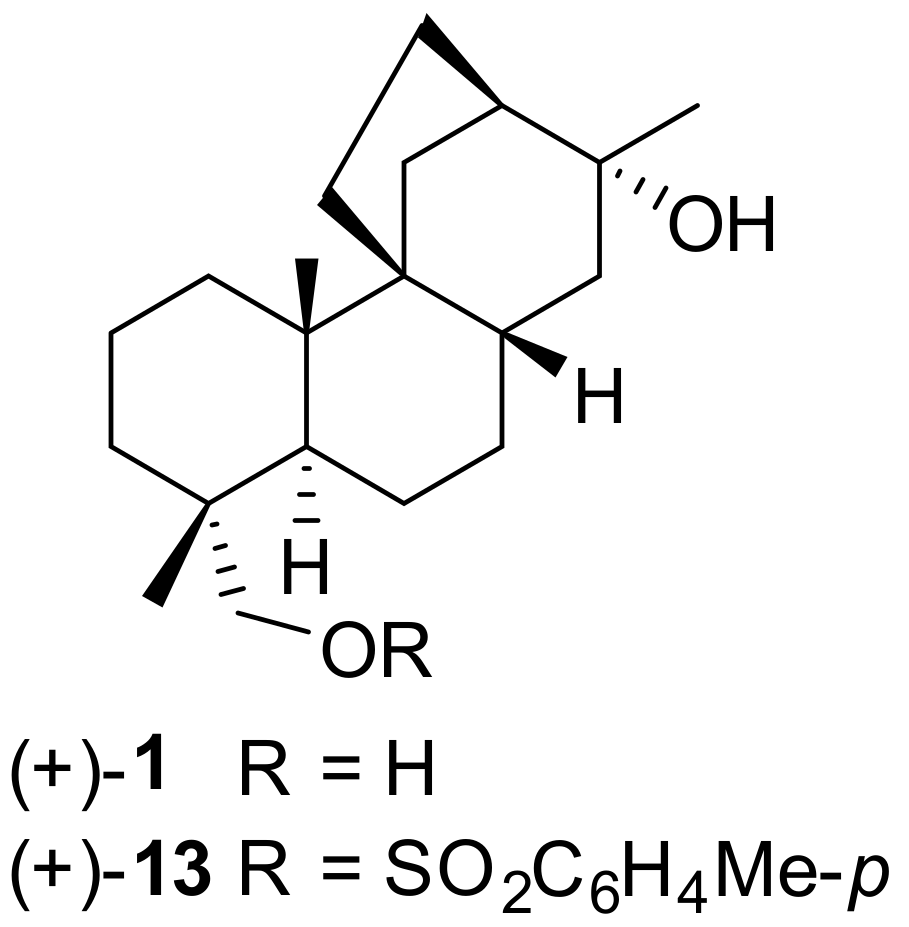
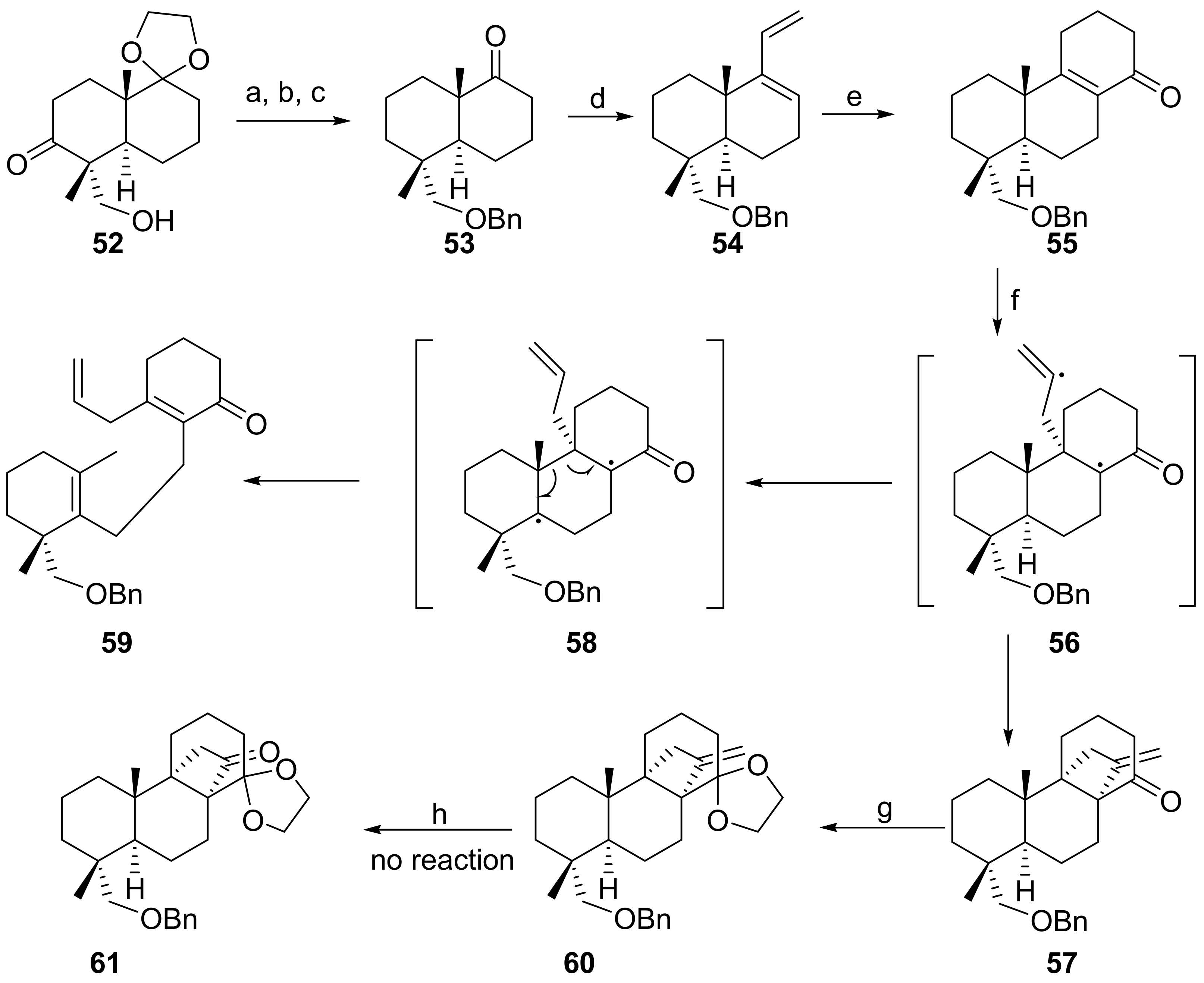
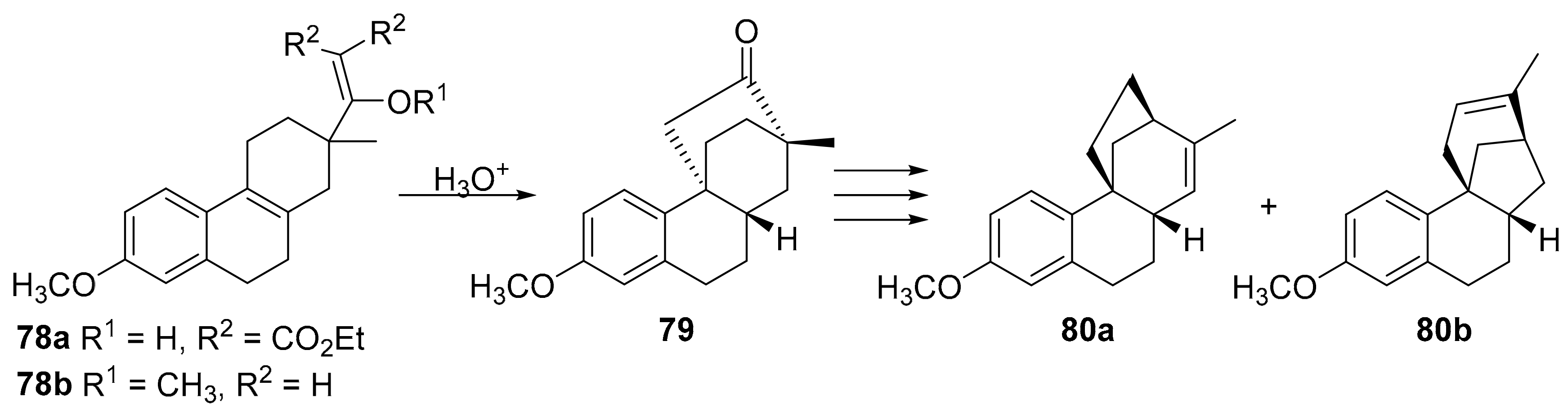
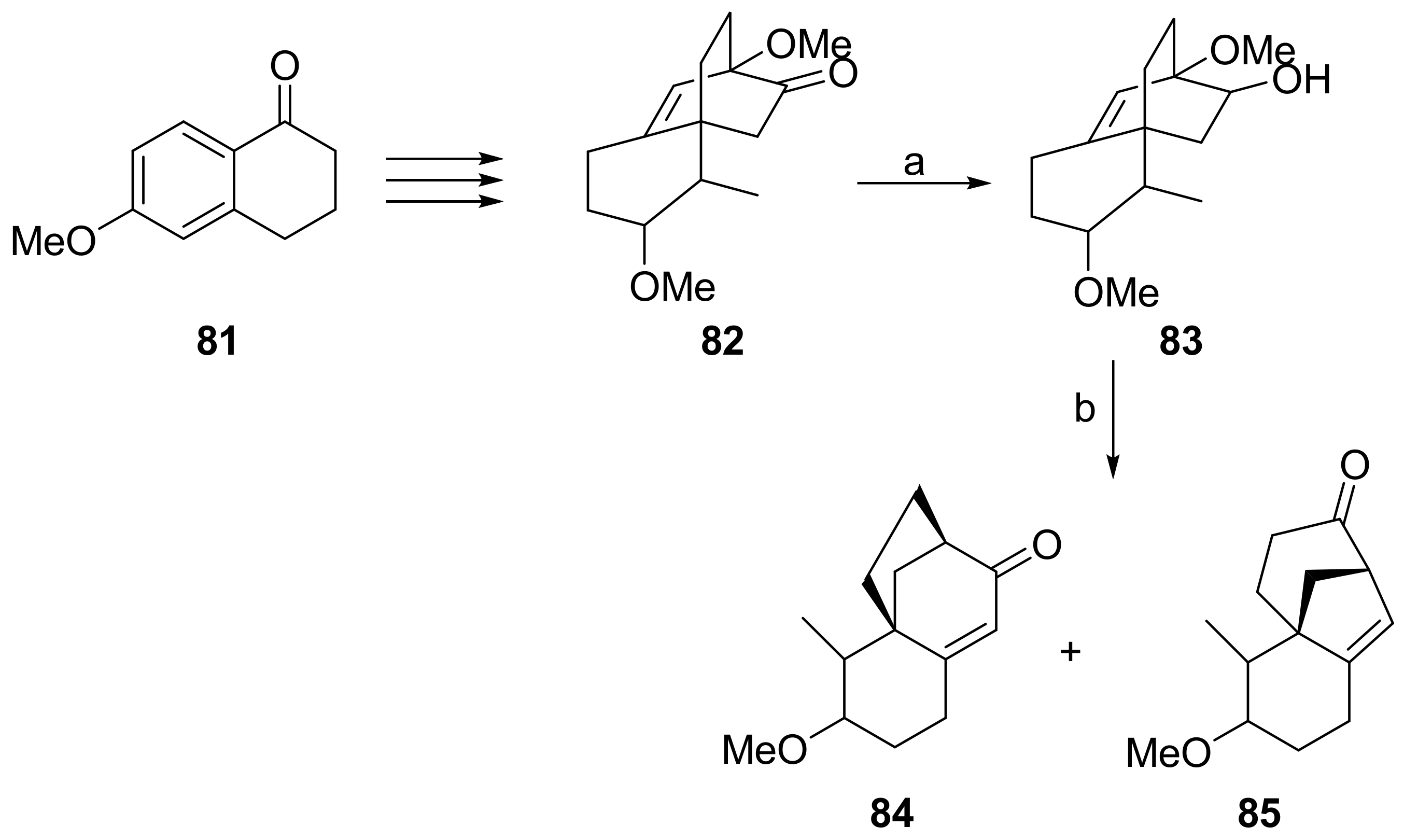
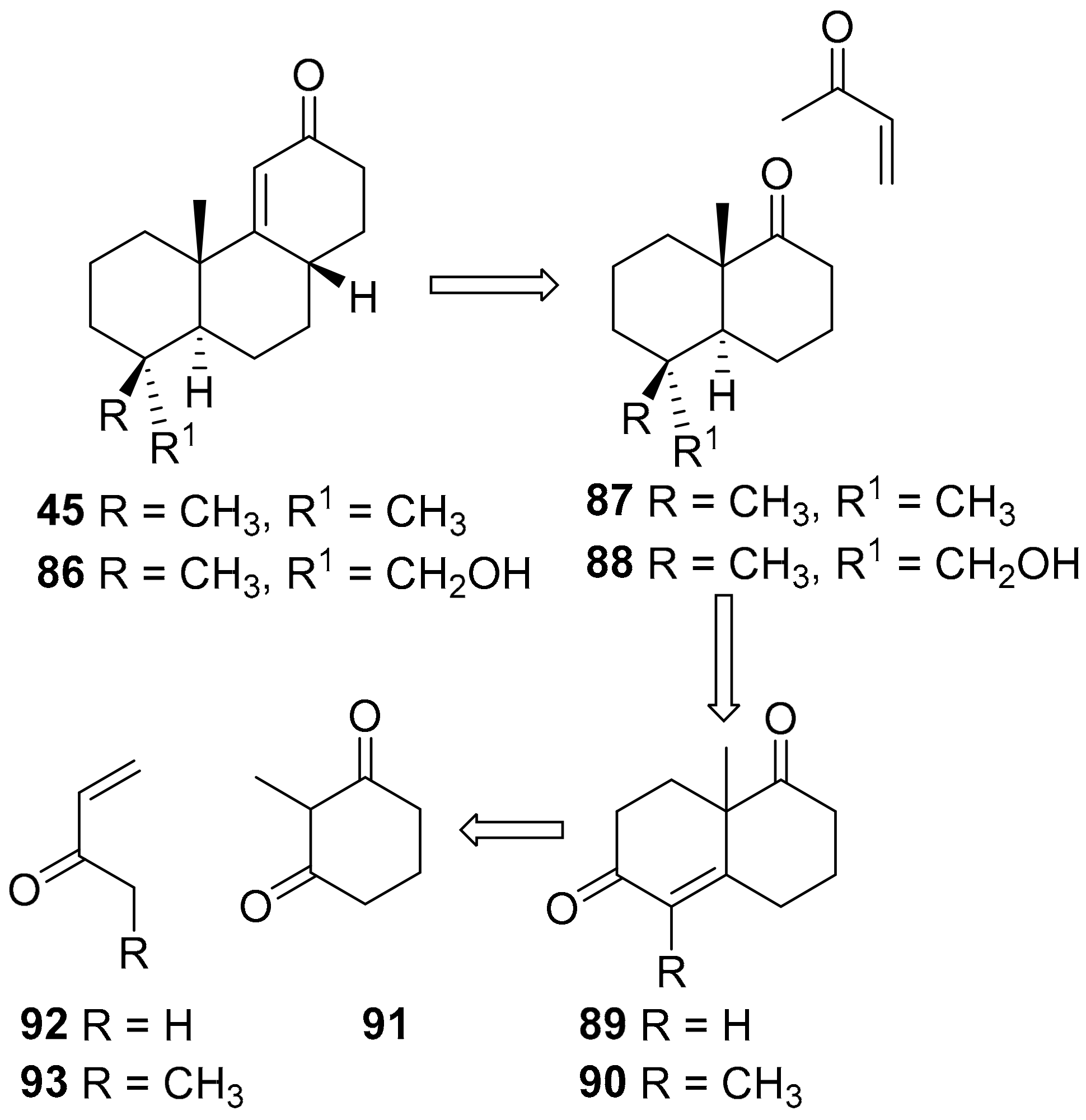
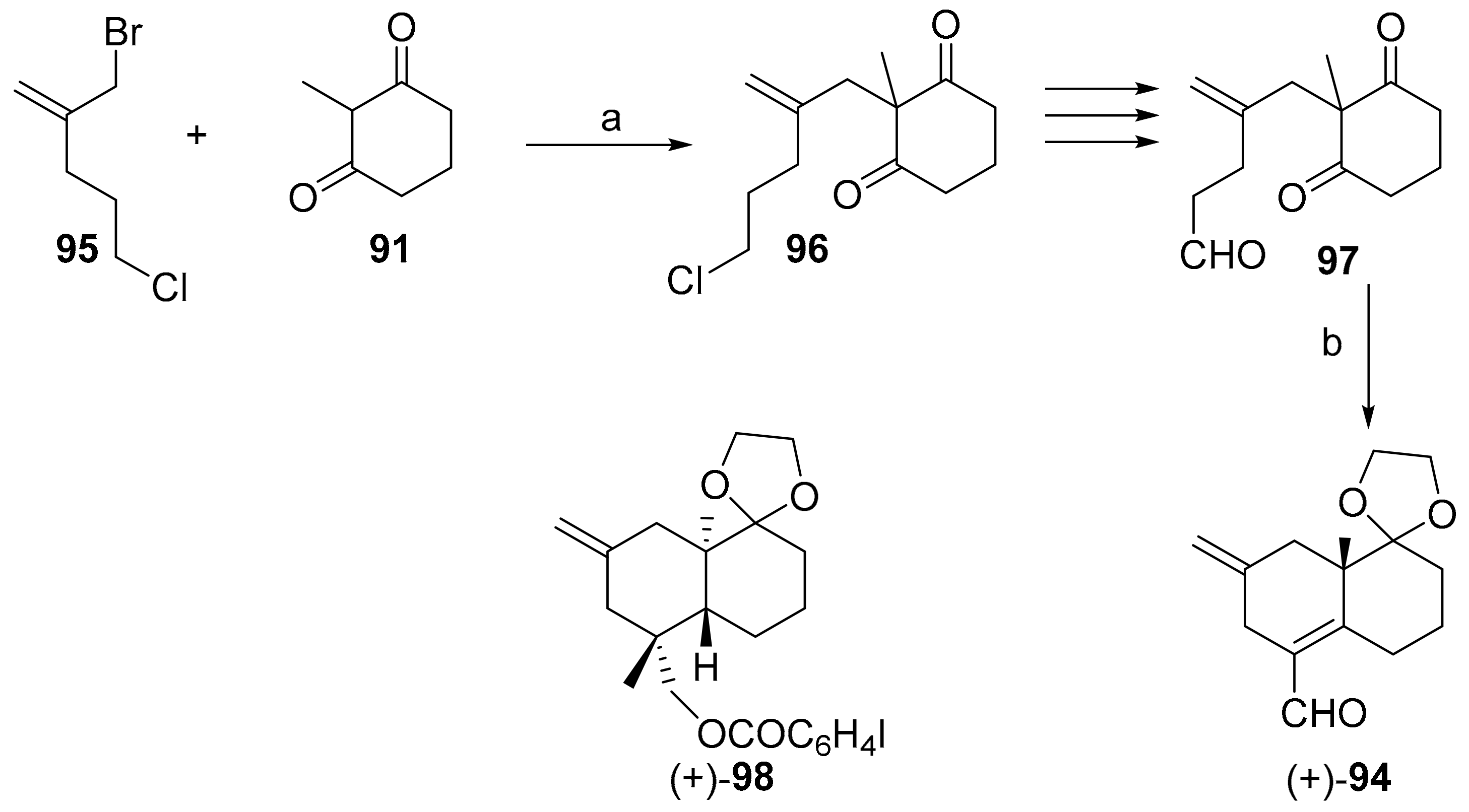
7.6. Enantioselective Synthesis of Stemarane Diterpenes and Diterpenoids via 9(11)-Podocarpen-12-ones
8. Conclusions
Funding
Conflicts of Interest
References
- Manchand, P.S.; Blount, J.F. X-ray Structure and Absolute Stereochemistry of Stemarin, a Diterpene with a New Skeleton. J. Chem. Soc. Chem. Commun. 1975, 894–895. [Google Scholar] [CrossRef]
- Chamy, M.C.; Piovano, M.; Garbarino, J.A.; Miranda, C.; Gambaro, V. Diterpenes from Calceolaria lepida. Phytochemistry 1990, 29, 2943–2946. [Google Scholar] [CrossRef]
- Garbarino, J.A.; Molinari, A. Diterpenes from Calceolaria latifolia. Phytochemistry 1990, 29, 3037–3039. [Google Scholar]
- Garbarino, J.A.; Molinari, A. Diterpenes from Calceolaria kingii. Phytochemistry 1990, 29, 3040–3041. [Google Scholar] [CrossRef]
- Chamy, M.C.; Piovano, M.; Garbarino, J.A.; Miranda, C.; Gambaro, V. Diterpenes from Calceolaria polifolia. Phytochemistry 1991, 30, 3365–3368. [Google Scholar] [CrossRef]
- Chamy, M.C.; Piovano, M.; Garbarino, J.A.; Vargas, C. Diterpenoids from Calceolaria dentata. Phytochemistry 1995, 40, 1751–1754. [Google Scholar] [CrossRef]
- Chamy, M.C.; Piovano, M.; Garbarino, J.A.; Mendoza, J. Diterpenoids from Calceolaria glabrata. Bol. Soc. Chil. Quim. 2001, 46, 223–225. [Google Scholar] [CrossRef]
- Chamy, M.C.; Piovano, M.; Garbarino, J.A.; Espinoza, L. Diterpenoids from Calceolaria paralia. J. Chil. Chem. Soc. 2006, 51, 779–780. [Google Scholar] [CrossRef]
- Kodama, O.; Li, W.X.; Tamogami, S.; Akatsuka, T. Oryzalexin S a novel stemarane-type diterpene rice phytoalexin. Biosci. Biotech. Biochem. 1992, 56, 1002–1003. [Google Scholar] [CrossRef]
- Dillon, V.M.; Overton, J.; Grayer, R.J.; Harborne, J.B. Differences in Phytoalexin Response Among Rice Cultivars of Different Resistance to Blast. Phytochemistry 1997, 44, 599–603. [Google Scholar] [CrossRef]
- Oikawa, H.; Ohashi, S.; König, W.A.; Kenmoku, H.; Sassa, T. Diversity of diterpene hydrocarbons in fungus Phoma betae. Tetrahedron Lett. 2001, 42, 2329–2332. [Google Scholar] [CrossRef]
- Leonelli, F.; Latini, V.; Trombetta, A.; Bartoli, G.; Ceccacci, F.; La Bella, A.; Sferrazza, A.; Lamba, D.; Migneco, L.M.; Marini Bettolo, R. Regio- and Diastereoselective Synthesis and X-ray Structure Determination of (+)-2-Deoxyoryzalexin S from (+)-Podocarpic Acid. Structural Non-identity with Its Nominal Natural Isolated Enantiomer. J. Nat. Prod. 2012, 75, 1944–1950. [Google Scholar] [PubMed]
- Tamogami, S.; Mitani, M.; Kodama, O.; Akatsuka, T. Oryzalexin structure: A new stemarane-type diterpene rice phytoalexin and its biogenesis. Tetrahedron 1993, 49, 2025–2032. [Google Scholar] [CrossRef]
- Berettoni, M.; Marini Bettolo, R.; Montanari, V.; Prencipe, T.; Romeo, S. Studies for a Diastereoselective Synthesis of the Tetracyclic Diol Stemarin: A Model Study for a New Preparation of the Key Intermediate and the Synthesis of (+)-18-Deoxystemarin. Helv. Chim. Acta 1991, 74, 1671–1678. [Google Scholar] [CrossRef]
- Mohan, R.S.; Yee, N.K.N.; Coates, R.M.; Ren, Y.-Y.; Stamenkovic, P.; Mendez, I.; West, C.A. Biosynthesis of Cyclic Diterpene Hydrocarbons in Rice Cell Suspensions: Conversion of 9,10-syn-Labda-8(17),13-dienyl Diphosphate to 9-Pimara-7,15-diene and Stemar-13-ene. Arch. Biochem. Biophys. 1996, 330, 33–47. [Google Scholar] [CrossRef]
- Nemoto, T.; Cho, E.-M.; Okada, A.; Okada, K.; Otomo, K.; Kanno, Y.; Toyomasu, T.; Mitsuhashi, W.; Sassa, T.; Minami, E.; Shibuya, N.; Nishiyama, M.; Nojiri, H.; Yamane, H. Stemar-13-ene synthase, a diterpene cyclase involved in the biosynthesis of the phytoalexin oryzalexin S in rice. FEBS Lett. 2004, 571, 182–186. [Google Scholar] [CrossRef] [Green Version]
- Nemoto, T.; Okada, A.; Okada, K.; Shibuya, N.; Toyomasu, T.; Nojiri, H.; Yamane, H. Promoter analysis of the rice stemar-13-ene synthase gene OsDTC2, which is involved in the biosynthesis of the phytoalexin S. Biochim. Biophys. Acta Gene Struct. Expr. 2007, 1769, 678–683. [Google Scholar]
- Xu, M.; Wilderman, P.R.; Morrone, D.; Xu, J.; Roy, A.; Margis-Pinheiro, M.; Upadhyaya, M.; Coates, R.M.; Peters, R.J. Functional characterization of the rice kaurene synthase-like gene family. Phytochemistry 2007, 68, 312–326. [Google Scholar] [CrossRef] [PubMed]
- Akatsuka, T.; Kodama, O.; Sekido, H.; Kono, Y.; Takeuchi, S. Novel phytoalexins (oryzalexins A, B and C) isolated from rice blast leaves infected with Pyricularia oryzae. Part I: Isolation, characterization and biological activities of oryzalexins. Agric. Biol. Chem. 1985, 49, 1689–1694. [Google Scholar] [CrossRef]
- Koga, J.; Shimura, M.; Oshima, K.; Ogawa, N.; Yamauchi, T.; Ogasawara, N. Phytocassanes A, B, C and D, novel diterpene phytoalexins from rice, Oryza sativa L. Tetrahedron 1995, 51, 7907–7918. [Google Scholar] [CrossRef]
- Koga, J.; Ogawa, N.; Yamauchi, T.; Kikuchi, N.; Ogasawara, N.; Shimura, M. Functional moiety for the antifungal activity of phytocassane E, a diterpene phytoalexin from rice. Phytochemistry 1997, 44, 249–253. [Google Scholar] [CrossRef]
- Cartwright, D.W.; Langcake, P.; Pryce, R.J.; Leworthy, D.P.; Ride, J.P. Isolation and characterization of two phytoalexins from rice as momilactones A and B. Phytochemistry 1981, 20, 535–537. [Google Scholar] [CrossRef]
- Kato, H.; Kodama, O.; Akatsuka, T. Oryzalexin E, a diterpene phytoalexin from UV-irradiated rice leaves. Phytochemistry 1993, 33, 79–81. [Google Scholar]
- Kato, H.; Kodama, O.; Akatsuka, T. Oryzalexin F, a diterpene phytoalexin from UV-irradiated rice leaves. Phytochemistry 1994, 36, 299–301. [Google Scholar]
- Zi, J.; Mafu, S.; Peters, R.J. To gibberellins and beyond! Surveying the evolution of (di) terpenoid metabolism. Annu. Rev. Plant Biol. 2014, 65, 259–286. [Google Scholar]
- Yajima, A.; Mori, K. Diterpenoid total synthesis, XXXII Synthesis and absolute configuration of (−)-phytocassane D, a diterpene phytoalexin isolated from the rice plant, Oryza sativa. Eur. J. Org. Chem. 2000, 2000, 4079–4091. [Google Scholar]
- Yajima, A.; Mori, K.; Yabuta, G. Total synthesis of ent-cassa-12,15-diene, a putative precursor of rice phytoalexins, phytocassanes A–E. Tetrahedron Lett. 2004, 45, 167–169. [Google Scholar]
- Kato, T.; Kabuto, C.; Sasaki, N.; Tsunagawa, M.; Aizawa, H.; Fujita, K.; Kato, Y.; Kitahara, Y.; Takahashi, N. Momilactones, growth inhibitors from rice, Oryza sativa L. Tetrahedron Lett. 1973, 14, 3861–3864. [Google Scholar]
- Davis, E.M.; Croteau, R. Cyclization enzymes in the biosynthesis of monoterpenes, sesquiterpenes, and diterpenes. In Biosynthesis; Springer: Berlin/Heidelberg, Germany, 2000; pp. 53–95. [Google Scholar]
- Cho, E.M.; Okada, A.; Kenmoku, H.; Otomo, K.; Toyomasu, T.; Mitsuhashi, W.; Sassa, T.; Yajima, A.; Yabuta, G.; Mori, K.; et al. Molecular cloning and characterization of a cDNA encoding ent-cassa-12, 15-diene synthase, a putative diterpenoid phytoalexin biosynthetic enzyme, from suspension-cultured rice cells treated with a chitin elicitor. Plant J. 2004, 37, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, H.; Nakamura, K.; Toshima, H.; Toyomasu, T.; Sassa, T. Proposed Mechanism for the Reaction Catalyzed by a Diterpene Cyclase, Aphidicolan-16â-ol Synthase: Experimental Results on Biomimetic Cyclization and Examination of the Cyclization Pathway by ab Initio Calculations. J. Am. Chem. Soc. 2002, 124, 9145–9153. [Google Scholar]
- Hong, Y.J.; Tantillo, D.J. A Maze of Dyotropic Rearrangements and Triple Shifts: Carbocation Rearrangements Connecting Stemarene, Stemodene, Betaerdene, Aphidicolene, and Scopadulanol. J. Org. Chem. 2018, 83, 3780–3793. [Google Scholar] [CrossRef]
- Buchanan, G.O.; Reese, P.B. Biotransformation of diterpenes and diterpene derivatives by Beauveria bassiana ATCC 7159. Phytochemistry 2001, 56, 141–151. [Google Scholar] [CrossRef]
- Chen, A.R.M.; Reese, P.B. Biotransformation of terpenes from Stemodia maritima by Aspergillus niger ATCC 9142. Phytochemistry 2002, 59, 57–62. [Google Scholar] [CrossRef]
- Chen, A.R.M.; Ruddock, P.L.D.; Lamm, A.S.; Reynolds, W.F.; Reese, P.B. Stemodane and stemarane diterpenoid hydroxylation by Mucor plumbeus and Whetzelinia sclerotiorum. Phytochemistry 2005, 66, 1898–1902. [Google Scholar]
- De Oliveira Silva, E.; Furtado, N.A.J.C.; Aleu, J.; Gonzáles Collado, I. Terpenoid biotransformations by Mucor species. Phytochem. Rev. 2013, 12, 857–876. [Google Scholar] [CrossRef]
- Lamm, A.S.; Reynolds, W.F.; Reese, P.B. Bioconversion of Stemodia maritima diterpenes and derivatives by Cunninghamella echinulata varelegans and Phanerochaete chrysosporium. Phytochemistry 2006, 66, 1088–1093. [Google Scholar] [CrossRef]
- McCook, K.P.; Chen, A.R.M.; Reynolds, W.F.; Reese, P.B. The potential of Cyathus africanus for tranformation of terpenes substrates. Phytochemistry 2012, 82, 61–66. [Google Scholar] [CrossRef]
- Manchand, P.S.; White, J.D.; Wright, H.; Clardy, J. Structures of Stemodin and Stemodinone. J. Am. Chem. Soc. 1973, 95, 2705–2706. [Google Scholar] [CrossRef]
- Hufford, C.D.; Guerrero, R.O.; Doorembos, N.J. Two new diterpenes from Stemodia maritima L. J. Pharm. Sci. 1976, 65, 778–780. [Google Scholar] [CrossRef]
- Austin, D.F. Florida Ethnobotany; CRC Press: Boca Raton, FL, USA, 2004; 162p. [Google Scholar]
- Garbarino, J.A.; Chamy, M.C.; Piovano, M. Chemistry of the Calceolaria Genus. Structural and Biological Aspects. Molecules 2000, 5, 302–303. [Google Scholar] [CrossRef]
- Arriaga, A.M.C.; Rodrigues, F.E.A.; Lemos, T.L.G.; de Oliveira, M. da C.F.; Lima, J.Q.; Santiago, G.M.P.; Braz-Filho, R.; Mafezoli, J. Composition and larvicidal activity of essential oil from Stemodia maritima L. Nat. Prod. Commun. 2007, 2, 1237–1239. [Google Scholar] [CrossRef]
- Da Silva, F.R.L.; Rodrigues, F.E.A.; Gomes, A.R.S.; Arriaga, A.M.C.; Mafezoli, J.; Lemos, T.L.G.; de Almeida, M.C.S.; Santiago, G.M.P.; Braz-Filho, R.; da Costa, J.G.M.; et al. Phytochemical study, antioxidant and antibacterial activities of Stemodia maritima. Quím. Nova 2014, 37, 1474–1478. [Google Scholar]
- Teixeira, A.H.; de Oliveira Freire, J.M.; de Sousa, L.H.T.; Parente, A.T.; de Sousa, N.A.; Arriaga, A.M.C.; Lopes da Silva, F.R.; Melo, I.M.; Castro da Silva, I.I.; Pereira, K.M.A.; et al. Stemodia maritima L. Extract Decreases Inflammation, Oxidative Stress, and Alveolar Bone Loss in an Experimental Periodontitis Rat Model. Front. Physiol. 2017, 8, 988. [Google Scholar]
- Schneider, W.G.; Valenta, Z. Karel František Wiesner. 25 November 1919–28 November 1986. Biogr. Mem. Fellows Royal Soc. 1991, 37, 462–490. [Google Scholar]
- Wiesner, K.; Tsai, T.Y.R.; Huber, K.; Bolton, S.E.; Vlahov, R. Total synthesis of talatisamine, a delphinine type alkaloid. J. Am. Chem. Soc. 1974, 96, 4990–4992. [Google Scholar] [CrossRef]
- Tsai, T.Y.R.; Tsai, C.S.J.; Sy, W.W.; Shanbag, M.N.; Liu, W.C.; Lee, S.F.; Wiesner, K. A Stereospecific Total Synthesis of Chasmanine. Heterocycles 1977, 7, 217–226. [Google Scholar]
- Wiesner, K. Centenary Lecture. Systematic Development of Strategy in the Synthesis of Polycyclic Polysubstituted Natural Products: The Aconite Alkaloids. Chem. Soc. Rev. 1977, 6, 413–430. [Google Scholar]
- Wiesner, K. Total Synthesis of Delphinine-Type Alkaloids by Simple, Fourth Generation Methods. Pure Appl. Chem. 1979, 51, 689–703. [Google Scholar]
- Atwal, K.S.; Marini Bettolo, R.; Sanchez, I.H.; Tsai, T.Y.R.; Wiesner, K. On the construction of the C/D ring systems of chasmanine and napelline by diene addition. Can. J. Chem. 1978, 56, 1102–1113. [Google Scholar]
- Tsai, T.Y.R.; Nambiar, K.P.; Krikorian, D.; Botta, M.; Marini Bettolo, R.; Wiesner, K. A new synthesis of chasmanine and 13-desoxydelphonine: A preferred route to the aromatic intermediate. Can. J. Chem. 1979, 57, 2124–2134. [Google Scholar] [CrossRef]
- Sethi, S.P.; Atwal, K.S.; Marini Bettolo, R.; Tsai, T.Y.R.; Wiesner, K. A stereospecific synthesis of napelline. Can. J. Chem. 1980, 58, 1889–1891. [Google Scholar] [CrossRef]
- Ciamician, G.; Silber, P. Chemische Lichtwirkungen. Ber. Dtsch. Chem. Ges 1908, 41, 1928–1935. [Google Scholar] [CrossRef] [Green Version]
- Buchi, G.; Goldman, I.M. Photochemical Reactions. VII.1 the Intramolecular Cyclization of Carvone to Carvonecamphor. J. Am. Chem. Soc. 1957, 79, 4741–4748. [Google Scholar] [CrossRef]
- Corey, E.J.; Bass, J.; Dolf, R.; Mitra, R.B. A Study of the Photochemical Reactions of 2-Cyclohexenones with Substituted Olefins. J. Am. Chem. Soc. 1964, 86, 5570–5583. [Google Scholar] [CrossRef]
- Wiesner, K. On the stereochemistry of photoaddition between α,β-unsaturated ketones and olefins. Tetrahedron 1975, 31, 1658. [Google Scholar] [CrossRef]
- Marini Bettolo, G.; Sahoo, S.P.; Poulton, G.A.; Tsai, T.Y.R.; Wiesner, K. On the stereochemistry of photoaddition between α,β-unsaturated ketones and olefins—II. Tetrahedron 1980, 36, 719–721. [Google Scholar] [CrossRef]
- Blount, J.F.; Gray, G.D.; Atwal, K.S.; Tsai, T.Y.R.; Wiesner, K. On the stereochemistry of photoaddition between α,β-unsaturated ketones and olefins. III. Tetrahedron Lett. 1980, 21, 4413–4416. [Google Scholar] [CrossRef]
- Valenta, K.; Grein, F. Excited states of acrolein: Ab initio model studies on α,β-unsaturated carbonyl compounds. Can. J. Chem. 1982, 60, 601–606. [Google Scholar] [CrossRef]
- Bell, R.A.; Ireland, R.E. The construction of the C/D ring system present in the diterpenoid alkaloids atisine and garryfoline. Tetrahedron Lett. 1963, 4, 269–273. [Google Scholar] [CrossRef] [Green Version]
- Migneco, L.M.; Leonelli, F.; Marini Bettolo, R. The intramolecular aldol condensation of 3-oxocyclo-hexaneacetaldehydes: A useful tool in the synthesis of natural products. Arkivoc 2004, 7, 253–265. [Google Scholar]
- Walborsky, H.M.; Baum, M.E.; Youssef, A.A. Acetolysis of Bicyclo[2.2.2]octyl-2 p-Bromobenzenesulfonate and the Absolute Configurations of Bicyclo[2.2.2]octanol-2 and cis- and trans-Bicyclo[3.2.1]octanol-2. J. Am. Chem. Soc. 1961, 83, 988–993. [Google Scholar] [CrossRef]
- Goering, H.L.; Sloan, M.F. Ionic Reactions in Bicyclic Systems. II. Carbonium Ion Reactions in Bicyclo[2.2.2]octane and Bicyclo[3.2.1]octane Derivatives. J. Am. Chem. Soc. 1961, 83, 1397–1401. [Google Scholar] [CrossRef]
- Goering, H.L.; Sloan, M.F. Ionic Reactions in Bicyclic Systems. III. Solvolysis of Bicycloöctanyl and Bicycloöctenyl p-Toluenesulfonates. J. Am. Chem. Soc. 1961, 83, 1992–1999. [Google Scholar] [CrossRef]
- Kraus, H.L.; Chassin, C.; Chassin, R.; Schmutte, P. Bicyclische Verbindungen, XVIII Solvolytische und reduktive Umlagerung von Bicyclo[2.2.2]octyltosylaten. Liebigs Ann. Chem. 1970, 738, 97–112. [Google Scholar] [CrossRef]
- Kelly, R.B.; Harley, M.L.; Alward, S.J. A total synthesis of (+)-stemarin, a diterpenoid with a unique bicyclic C/D ring system. Can. J. Chem. 1980, 58, 755–756. [Google Scholar] [CrossRef]
- Spencer, T.A.; Weaver, T.D.; Villarica, R.M.; Friary, R.J.; Posler, J.; Schwartz, M.A. Syntheses of methyl deisopropyldehydroabietate. Diterpenoid synthesis by the AB → ABC approach. J. Org. Chem. 1968, 33, 712–719. [Google Scholar] [CrossRef]
- Bravetti, D.; Marini Bettolo, R.; Lupi, A. On the Construction of the C/D-Ring Systems of Aphidicolin and Stemodin. A regio and Stereospecific Synthesis of 17-Noraphidicolan-16-one and 17-Norstemodan-16-one. Helv. Chim. Acta 1982, 65, 371–376. [Google Scholar] [CrossRef]
- Marini Bettolo, R.; Tagliatesta, P.; Lupi, A.; Bravetti, D. A Stereoselective Total Synthesis of (±)-Maritimol, (±)-2-Deoxystemodinone, (±)-Stemodinone and (±)-Stemodin. Helv. Chim. Acta 1983, 66, 760–770. [Google Scholar] [CrossRef]
- Lupi, A.; Patamia, M.; Grgurina, I.; Marini Bettolo, R.; Di Leo, O.; Gioia, P.; Antonaroli, S. Biogenetic-Type Total Synthesis of (+)-2-Deoxystemodinone. Helv. Chim. Acta 1984, 67, 2261–2263. [Google Scholar] [CrossRef]
- Marini Bettolo, R.; Tagliatesta, P.; Lupi, A.; Bravetti, D. A Total Synthesis of Aphidicolin: Stereospecific Synthesis of (±)-3α,18-Dihydroxy-17-noraphidicolan-16-one. Helv. Chim. Acta 1983, 66, 1922–1928. [Google Scholar] [CrossRef]
- Lupi, A.; Patamia, M.; Marini Bettolo, R. A Total Synthesis of (±)-Aphidicolin: Regio and Stereoselective Conversion of 3α,18-Di-O-benzyl-17-nor-14-aphidicolen-16-one into (±)-Aphidicolin. Helv. Chim. Acta 1988, 71, 872–875. [Google Scholar] [CrossRef]
- De Santis, B.; Iamiceli, A.L.; Marini Bettolo, R.; Migneco, L.M.; Scarpelli, R.; Cerichelli, G.; Fabrizi, G.; Lamba, D. On the Diastereoselectivity of the Aqueous-Acid-Catalyzed Intramolecular Aldol Condensation of 3-Oxocyclohexaneacetaldehydes. Helv. Chim. Acta 1998, 81, 2375–2387. [Google Scholar] [CrossRef]
- La Bella, A.; Leonelli, F.; Migneco, L.M.; Marini Bettolo, R. (+)-Podocarpic Acid as Chiral Template in the Synthesis of Aphidicolane, Stemodane and Stemarane Diterpenoids. Molecules 2016, 21, 1197. [Google Scholar] [CrossRef] [PubMed]
- Marini Bettolo, R.; Migneco, L.M.; Moretti, P.; Scarpelli, R. Improved Conversion of 6-endo-Tosyloxybicyclo[2.2.2]octan-2-ones into 6-exo-Acetoxy and 6-exo-Benzoyloxy-bicyclo[2.2.2]octan-2-ones. J. Prakt. Chem. 1999, 341, 687–690. [Google Scholar]
- Di Stefano, S.; Leonelli, F.; Garofalo, B.; Mandolini, L.; Marini Bettolo, R.; Migneco, L.M. Elusive 6-exo-Hydroxybicyclo[2.2.2]octan-2-ones from the Corresponding Acetates by Methanolysis in the Presence of CH3ONa/La(OTf)3. Org. Lett. 2002, 4, 2783–2785. [Google Scholar] [CrossRef]
- Steiweiswer, A., Jr.; Walsh, T.D.; Wolfe, J.R., Jr. Stereochemistry of Acetolysis of Alkyl Sulfonates. J. Am. Chem. Soc. 1965, 87, 3682–3685. [Google Scholar]
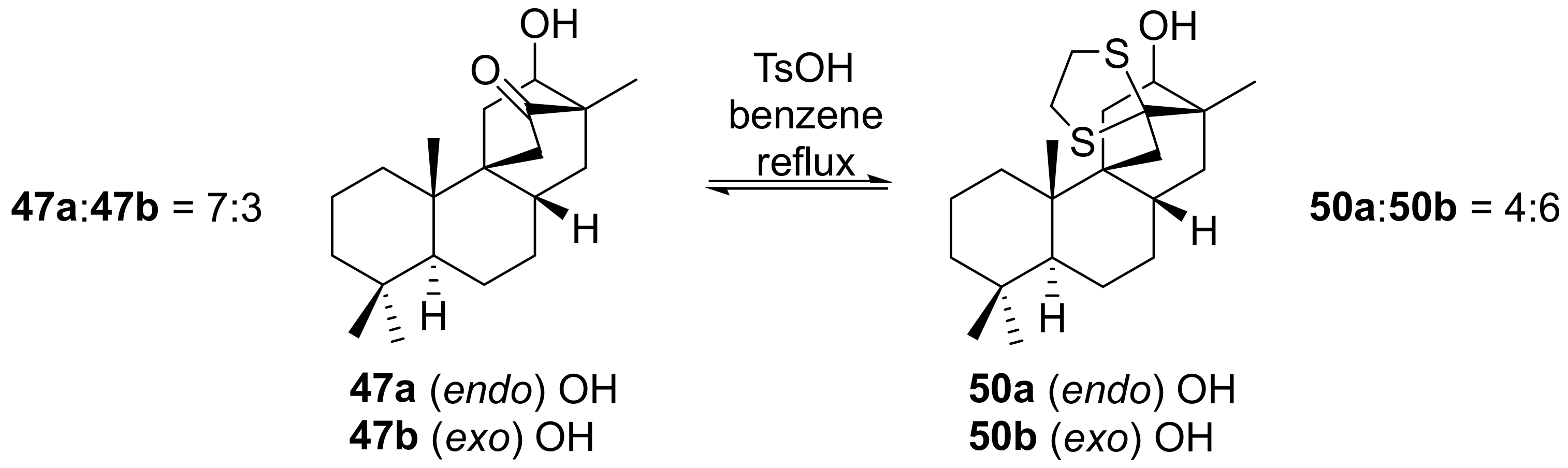
- Leonelli, F.; Caschera, B.; Silvestri, L.; Prastaro, A.; Corso, G.; Ceccacci, F.; La Bella, A.; Migneco, L.M.; Marini Bettolo, R. Synthesis of (+)-13-Stemarene and (+)-18-Deoxystemarin: Expeditious Preparation of the Key 6-exo-Hydroxybicyclo[2.2.2]octan-2-one Ethylene Dithioacetal. Helv. Chim. Acta 2008, 91, 598–607. [Google Scholar] [CrossRef]
- Antonaroli, S.; Berettoni, M.; Cifarelli, G.; Lupi, A.; Marini Bettolo, R.; Romeo, S. Studies for a Diastereoselective Synthesis of the Tetracyclic Diol Stemarin. An Improved Procedure to the Synthesis of 8(9)-Podocarpen-14-ones: The Total Synthesis of 18-benzyloxy-8(9)-Podocarpen-14-one and Related Studies. Gazz. Chim. Ital. 1992, 122, 55–59. [Google Scholar] [CrossRef]
- Bartoletti, A.; Berettoni, M.; Catteruccia, F.; De Chiara, G.; Marini Bettolo, R.; Mastrangeli, C.; Scarpelli, R.; Tozzi, C.; Lamba, D. Studies for a Diastereoselective Synthesis of the Tetracyclic Diterpenic Diol Stemarin. A Model Work for the Preparation, from an 8(9)podocarpen-14-one, of a Key Intermediate by the Wiesner Photochemical Method. Gazz. Chim Ital. 1996, 126, 223–226. [Google Scholar] [CrossRef]
- Trost, B.M.; Nishimura, Y.; Yamamoto, K.; McElvain, S.S. A Total Synthesis of Aphidicolin. J. Am. Chem. Soc. 1979, 101, 1328–1330. [Google Scholar] [CrossRef]
- McMurry, J.E.; Andrus, A.; Ksander, G.M.; Musser, J.H.; Johnson, M.A. Stereospecific Total Synthesis of Aphidicolin. J. Am. Chem. Soc. 1979, 101, 1330–1332. [Google Scholar] [CrossRef]
- Berettoni, M.; Cocchi, F.; Marini Bettolo, R.; Montagnini di Mirabello, L.; Romeo, S. Unprecedented Outcome of the Allene Photoaddition to a Fused α,β-Unsaturated Ketone. Tetrahedron Lett. 1993, 34, 715–716. [Google Scholar] [CrossRef]
- Srikrishna, A.; Satyanarayana, G.; Ravi Kumar, P. Enantiospecific synthesis of tricyclo[5.2.1.04,8]decanes via acid catalysed rearrangement of isotwistanes. Tetrahedron Lett. 2006, 47, 363–366. [Google Scholar] [CrossRef]
- Leonelli, F.; Blesi, F.; Dirito, P.; Trombetta, A.; Ceccacci, F.; La Bella, A.; Migneco, L.M.; Marini Bettolo, R. Diastereoselective Total Synthesis of (+)-13-Stemarene by Fourth Generation Methods: A Formal Total Synthesis of (+)-18-Deoxystemarin. J. Org. Chem. 2011, 76, 6871–6876. [Google Scholar] [CrossRef] [PubMed]
- Kanjilal, P.R.; Sarkar, M.; Patra, S.K.; Ghosh, S.; Ghatak, U.R. Synthetic Studies toward Complex Diterpenoids. 16.1 A Novel SyntheticRoute to the Carbocyclic Skeleta of Stemodin and Stemarin byAcid-Catalyzed Intramolecular C-Alkylation and Rearrangement Reactions. J. Org. Chem. 1985, 50, 857–863. [Google Scholar] [CrossRef]
- Kaliappan, K.; Subba Rao, G.S.R. An Expedient Route to the Preparation of Key Intermediates for the Total Synthesis of Aphidicolin, Stemodin and Oryzalexin, S. Tetrahedron Lett. 1996, 37, 8429–8430. [Google Scholar] [CrossRef]
- Leonelli, F.; Borocci, S.; Migneco, L.M.; Marini Bettolo, R.; Lamba, D. The Formation of 8-Epipodocarp-9(11)-en-12-one in the Course of the Preparation of Podocarp-9(11)-en-12-one from O-Methylpodocarpane and Related Studies. Helv. Chim. Acta 2002, 85, 2817–2826. [Google Scholar] [CrossRef]
- Hajos, Z.G.; Parrish, D.R. German Patent. DE 2102623, 29 July 1971. [Google Scholar]
- Hajos, Z.G.; Parrish, D.R. Stereocontrolled synthesis of trans-hydrindan steroidal intermediates. J. Org. Chem. 1973, 38, 3239–3243. [Google Scholar] [CrossRef]
- Hajos, Z.G.; Parrish, D.R. Asymmetric synthesis of bicyclic intermediates of natural product chemistry. J. Org. Chem. 1974, 39, 1615–1621. [Google Scholar] [CrossRef]
- Hagiwara, H.; Uda, H.J. Optically pure (4aS)-(+)- or (4aR)-(-)-1,4a-dimethyl-4,4a,7,8-tetrahydronaphthalene-2,5(3H,6H)-dione and its use in the synthesis of an inhibitor of steroid biosynthesis. J. Org. Chem. 1988, 53, 2308–2311. [Google Scholar] [CrossRef]
- Leonelli, F.; Garofalo, B.; Migneco, L.M.; Marini Bettolo, R.; Colais, F.; Sinibaldi, M. Chiral HPLC Resolution of the Wieland-Miescher Ketone and Derivatives. J. Liq. Chromatogr. Relat. Technol. 2003, 3, 409–424. [Google Scholar] [CrossRef]
- Smith, A.B., III; Mewshaw, R. An Efficient Approach to Chiral, Nonracemic trans-Decahydro-5,8a-dimethyl-1,6-naphth alenedione Derivatives: Total Synthesis of (+)-Pallescensin, A. J. Org. Chem. 1984, 49, 3685–3689. [Google Scholar] [CrossRef]
- Leonelli, F.; Trombetta, A.; La Bella, A.; Lucarelli, G.; Demitri, N.; Lamba, D.; Migneco, L.M.; Marini Bettolo, R. Enantioselective Synthesis and X-ray Structure of (+)((4aS,5S,8aS)-5,8a-Dimethyl-7-methyleneoctahydro-2Hspiro[naphthalene-1,2′-[1,3]dioxolan]-5-yl)methyl-4-iodobenzoate. Eur. J. Org. Chem. 2019, 2019, 1594–1599. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leonelli, F.; Valletta, A.; Migneco, L.M.; Marini Bettolo, R. Stemarane Diterpenes and Diterpenoids. Int. J. Mol. Sci. 2019, 20, 2627. https://doi.org/10.3390/ijms20112627
Leonelli F, Valletta A, Migneco LM, Marini Bettolo R. Stemarane Diterpenes and Diterpenoids. International Journal of Molecular Sciences. 2019; 20(11):2627. https://doi.org/10.3390/ijms20112627
Chicago/Turabian StyleLeonelli, Francesca, Alessio Valletta, Luisa Maria Migneco, and Rinaldo Marini Bettolo. 2019. "Stemarane Diterpenes and Diterpenoids" International Journal of Molecular Sciences 20, no. 11: 2627. https://doi.org/10.3390/ijms20112627
APA StyleLeonelli, F., Valletta, A., Migneco, L. M., & Marini Bettolo, R. (2019). Stemarane Diterpenes and Diterpenoids. International Journal of Molecular Sciences, 20(11), 2627. https://doi.org/10.3390/ijms20112627





