Cytomegalovirus Infections after Hematopoietic Stem Cell Transplantation: Current Status and Future Immunotherapy
Abstract
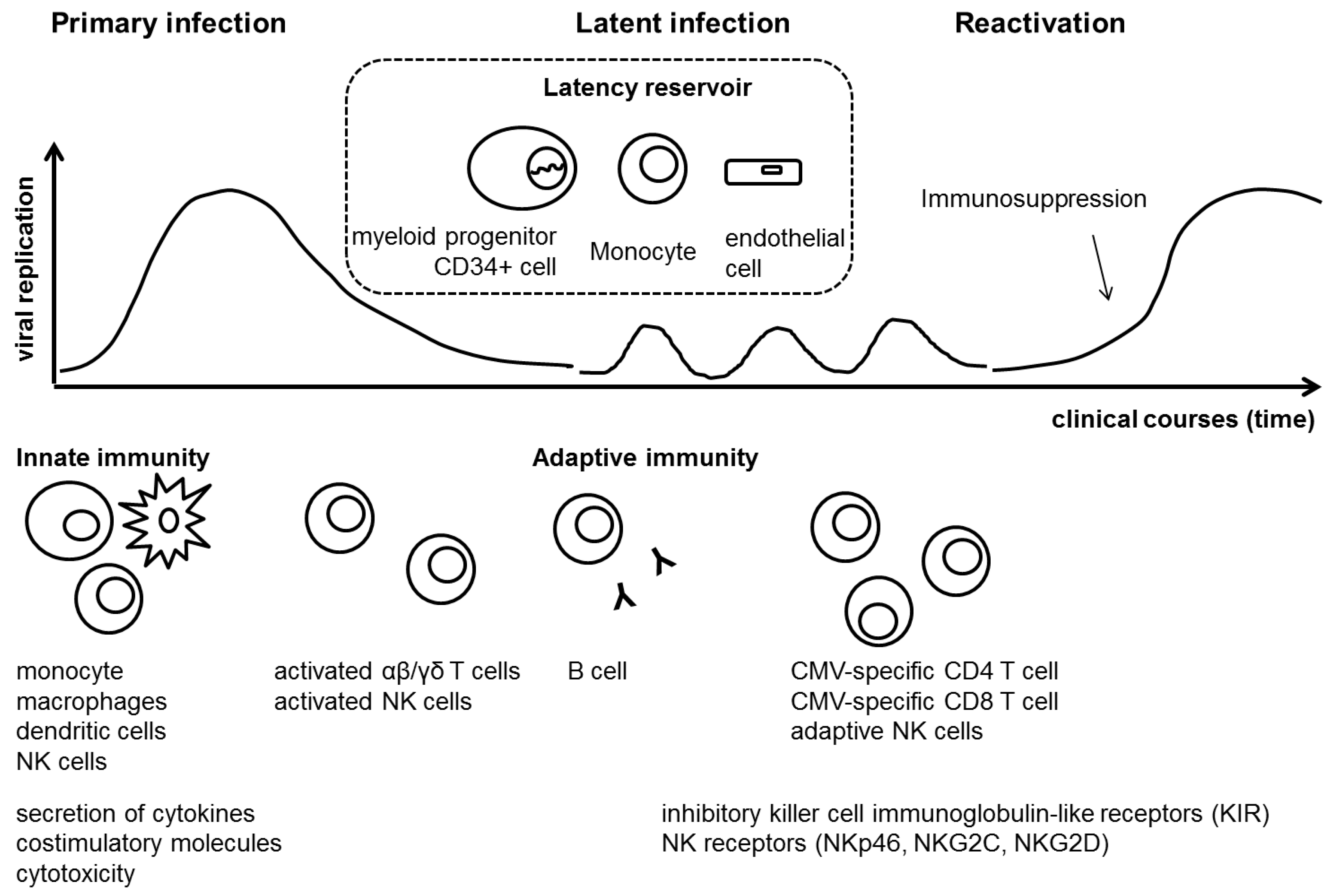
:1. Cytomegalovirus Infection after Hematopoietic Stem Cell Transplantation
2. Current Therapeutic Approaches
3. New Antiviral Agents
4. Cytomegalovirus and Host Immune Response
5. Vaccine Trials and Immunogenicity
6. Immunotherapy for Cytomegalovirus
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADCC | Antibody dependent cell mediated cytotoxicity |
| ATG | Antithymocyte globulin |
| BAK | Benzalkonium chloride |
| CMI | Cell-mediated immunity |
| CMV | Cytomegalovirus |
| CTL | Cytotoxic T lymphocyte |
| EBV | Epstein-Barr virus |
| ELISpot | Enzyme-linked immunospot |
| gB | Glycoprotein B |
| GVHD | Graft-versus-host disease |
| GVL | Graft-versus-leukemic |
| HLA | Human leukocyte antigen |
| HSCT | Hematopoietic stem cell transplantation |
| ICC | Intracellular cytokine staining |
| IFN-γ | Interferon-γ |
| MHC | Major histocompatibility complex |
| PBMC | Peripheral blood mononuclear cell |
| pp65 | Phosphoprotein 65 |
| SOT | Solid organ transplantation |
| Th1 | T helper 1 type |
| TNF-α | Tumor necrosis factor-α |
References
- Cho, S.Y.; Lee, H.J.; Lee, D.G. Infectious complications after hematopoietic stem cell transplantation: Current status and future perspectives in Korea. Korean J. Intern. Med. 2018, 33, 256–276. [Google Scholar] [CrossRef] [PubMed]
- Wingard, J.R.; Hsu, J.; Hiemenz, J.W. Hematopoietic stem cell transplantation: An overview of infection risks and epidemiology. Hematol. Oncol. Clin. North. Am. 2011, 25, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Tomblyn, M.; Chiller, T.; Einsele, H.; Gress, R.; Sepkowitz, K.; Storek, J.; Wingard, J.R.; Young, J.A.; Boeckh, M.J. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: A global perspective. Biol. Blood Marrow Transpl. 2009, 15, 1143–1238. [Google Scholar] [CrossRef]
- Safdar, A.; Armstrong, D. Infections in patients with hematologic neoplasms and hematopoietic stem cell transplantation: Neutropenia, humoral, and splenic defects. Clin. Infect. Dis. 2011, 53, 798–806. [Google Scholar] [CrossRef]
- Boeckh, M.; Geballe, A.P. Cytomegalovirus: Pathogen, paradigm, and puzzle. J. Clin. Invest. 2011, 121, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Maertens, J.; Lyon, S. Current and future Current and future options for cytomegalovirus reactivation in hematopoietic cell transplantation patients. Future Microbiol. 2017, 12, 839–842. [Google Scholar] [CrossRef]
- Jeon, S.; Lee, W.K.; Lee, Y.; Lee, D.G.; Lee, J.W. Risk factors for cytomegalovirus retinitis in patients with cytomegalovirus viremia after hematopoietic stem cell transplantation. Ophthalmology 2012, 119, 1892–1898. [Google Scholar] [CrossRef]
- Schmidt-Hieber, M.; Labopin, M.; Beelen, D.; Volin, L.; Ehninger, G.; Finke, J.; Socié, G.; Schwerdtfeger, R.; Kröger, N.; Ganser, A.; et al. CMV serostatus still has an important prognostic impact in de novo acute leukemia patients after allogeneic stem cell transplantation: A report from the Acute Leukemia Working Party of EBMT. Blood 2013, 122, 3359–3364. [Google Scholar] [CrossRef] [PubMed]
- Styczynski, J.; Czyzewski, K.; Wysocki, M.; Gryniewicz-Kwiatkowska, O.; Kolodziejczyk-Gietka, A.; Salamonowicz, M.; Hutnik, L.; Zajac-Spychala, O.; Zaucha-Prazmo, A.; Chelmecka-Wiktorczyk, L.; et al. Polish Society of Paediatric Oncology and Haematology. Increased risk of infections and infection-related mortality in children undergoing haematopoietic stem cell transplantation compared to conventional anticancer therapy: A multicentre nationwide study. Clin. Microbiol. Infect. 2016, 179, e1–e10. [Google Scholar]
- Teira, P.; Battiwalla, M.; Ramanathan, M.; Barrett, A.J.; Ahn, K.W.; Chen, M.; Green, J.S.; Saad, A.; Antin, J.H.; Savani, B.N.; et al. Early cytomegalovirus reactivation remains associated with increased transplant-related mortality in the current era: A CIBMTR analysis. Blood 2016, 127, 2427–2438. [Google Scholar] [CrossRef] [PubMed]
- Green, M.L.; Leisenring, W.; Xie, H.; Mast, T.C.; Cui, Y.; Sandmaier, B.M.; Sorror, M.L.; Goyal, S.; Özkök, S.; Yi, J.; et al. Cytomegalovirus viral load and mortality after haemopoietic stem cell transplantation in the era of pre-emptive therapy: A retrospective cohort study. Lancet Haematol. 2016, 3, e119–e127. [Google Scholar] [CrossRef]
- Schuster, M.G.; Cleveland, A.A.; Dubberke, E.R.; Kauffman, C.A.; Avery, R.K.; Husain, S.; Paterson, D.L.; Silveira, F.P.; Chiller, T.M.; Benedict, K.; et al. Infections in hematopoietic cell transplant recipients: Results from the organ transplant infection project, a multicenter, prospective, cohort study. Open Forum Infect. Dis 2017, 4, ofx050. [Google Scholar] [CrossRef]
- Ljungman, P.; Boeckh, M.; Hirsch, H.H.; Josephson, F.; Lundgren, J.; Nichols, G.; Pikis, A.; Razonable, R.R.; Miller, V.; Griffiths, P.D. Disease Definitions Working Group of the Cytomegalovirus Drug Development Forum. Definitions of Cytomegalovirus Infection and Disease in Transplant Patients for Use in Clinical Trials. Clin. Infect. Dis. 2017, 64, 87–91. [Google Scholar] [PubMed]
- Ljungman, P.; Reusser, P.; de la Camara, R.; Einsele, H.; Engelhard, D.; Ribaud, P.; Ward, K. European Group for Blood and Marrow Transplantation. Management of CMV infections: Recommendations from the infectious diseases working party of the EBMT. Bone Marrow Transpl. 2004, 33, 1075–1081. [Google Scholar] [CrossRef]
- Boeckh, M.; Leisenring, W.; Riddell, S.R.; Bowden, R.A.; Huang, M.L.; Myerson, D.; Stevens-Ayers, T.; Flowers, M.E.; Cunningham, T.; Corey, L. Late cytomegalovirus disease and mortality in recipients of allogeneic hematopoietic stem cell transplants: Importance of viral load and T-cell immunity. Blood 2003, 101, 407–414. [Google Scholar] [CrossRef]
- Lee, H.Y.; Rhee, C.K.; Choi, J.Y.; Lee, H.Y.; Lee, J.W.; Lee, D.G. Diagnosis of cytomegalovirus pneumonia by quantitative polymerase chain reaction using bronchial washing fluid from patients with hematologic malignancies. Oncotarget 2017, 8, 39736. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.; Estey, E.; Raad, I.; Rolston, K.; Kantarjian, H.; Jacobson, K.; Konoplev, S.; Ghosh, S.; Luna, M.; Tarrand, J.; et al. Cytomegalovirus pneumonia in adults with leukemia: An emerging problem. Clin. Infect. Dis. 2001, 32, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Erard, V.; Guthrie, K.A.; Seo, S.; Smith, J.; Huang, M.; Chien, J.; Flowers, M.E.; Corey, L.; Boeckh, M. Reduced mortality of cytomegalovirus pneumonia after hematopoietic cell transplantation due to antiviral therapy and changes in transplantation practices. Clin. Infect. Dis. 2015, 61, 31–39. [Google Scholar] [CrossRef]
- Boeckh, M.; Ljungman, P. How I treat cytomegalovirus in hematopoietic cell transplant recipients. Blood 2009, 113, 5711–5719. [Google Scholar] [CrossRef] [PubMed]
- Boeckh, M.; Murphy, W.J.; Peggs, K.S. Recent advances in cytomegalovirus: An update on pharmacologic and cellular therapies. Biol. Blood Marrow Transpl. 2015, 21, 24–29. [Google Scholar] [CrossRef]
- Hammerstrom, A.E.; Lombardi, L.R.; Pingali, S.R.; Rondon, G.; Chen, J.; Milton, D.R.; Chemaly, R.F.; Champlin, R.E.; Gulbis, A.; Ciurea, S.O. Prevention of Cytomegalovirus reactivation in haploidentical stem cell transplantation. Biol. Blood Marrow Transpl. 2018, 24, 353–358. [Google Scholar] [CrossRef]
- Boeckh, M.; Nichols, W.G.; Chemaly, R.F.; Papanicolaou, G.A.; Wingard, J.R.; Xie, H.; Syrjala, K.L.; Flowers, M.E.; Stevens-Ayers, T.; Jerome, K.R.; et al. Valganciclovir for the prevention of complications of late cytomegalovirus infection after allogeneic hematopoietic cell transplantation: A randomized trial. Ann. Intern. Med. 2015, 162, 1–10. [Google Scholar] [CrossRef]
- De Clercq, E.; Li, G. Approved antiviral drugs over the past 50 years. Clin. Microbiol. Rev. 2016, 29, 695–747. [Google Scholar] [CrossRef]
- Park, S.H.; Choi, S.M.; Lee, D.G.; Choi, J.H.; Yoo, J.H.; Kim, S.H.; Kim, H.J.; Cho, S.G.; Eom, K.S.; Lee, J.W.; et al. Infectious complications associated with alemtuzumab use for allogeneic hematopoietic stem cell transplantation: Comparison with anti-thymocyte globulin. Transpl. Infect. Dis. 2009, 11, 413–423. [Google Scholar] [CrossRef]
- Chaer, E.F.; Shah, D.P.; Chemaly, R.F. How I treat resistant cytomegalovirus infection in hematopoietic cell transplantation recipients. Blood 2016, 128, 2624–2636. [Google Scholar] [CrossRef] [Green Version]
- Hantz, S.; Garnier-Geoffroy, F.; Mazeron, M.C.; Garrigue, I.; Merville, P.; Mengelle, C.; Rostaing, L.; Saint Marcoux, F.; Essig, M.; Rerolle, J.P.; et al. French CMV Resistance Survey Study Group. Drug-resistant cytomegalovirus in transplant recipients: A French cohort study. J. Antimicrob. Chemother. 2010, 65, 2628–2640. [Google Scholar] [CrossRef]
- Allice, T.; Busca, A.; Locatelli, F.; Falda, M.; Pittaluga, F.; Ghisetti, V. Valganciclovir as pre-emptive therapy for cytomegalovirus infection post- allogenic stem cell transplantation: Implications for the emergence of drug-resistant cytomegalovirus. J. Antimicrob. Chemother. 2009, 63, 600–608. [Google Scholar] [CrossRef]
- Lurain, N.S.; Chou, S. Antiviral drug resistance of human cytomegalovirus. Clin. Microbiol. Rev. 2010, 23, 689–712. [Google Scholar] [CrossRef]
- van der Beek, M.T.; Marijt, E.W.; Vossen, A.C.; van der Blij-de Brouwer, C.S.; Wolterbeek, R.; Halkes, C.J.; Claas, E.C.; Kroes, A.C. Failure of pre-emptive treatment of cytomegalovirus infections and antiviral resistance in stem cell transplant recipients. Antivir. Ther. 2012, 17, 45–51. [Google Scholar] [CrossRef]
- Shmueli, E.; Or, R.; Shapira, M.Y.; Resnick, I.B.; Caplan, O.; Bdolah-Abram, T.; Wolf, D.G. High rate of cytomegalovirus drug resistance among patients receiving preemptive antiviral treatment after haploidentical stem cell transplantation. J. Infect. Dis. 2014, 209, 557–561. [Google Scholar] [CrossRef]
- Seggewiss, R.; Einsele, H. Immune reconstitution after allogeneic transplantation and expanding options for immunomodulation: An update. Blood 2010, 115, 3861–3868. [Google Scholar] [CrossRef]
- Dziedzic, M.; Sadowska-Krawczenko, I.; Styczynski, J. Risk factors for cytomegalovirus infection after allogeneic hematopoietic cell transplantation in malignancies: Proposal for classification. Anticancer Res. 2017, 37, 6551–6556. [Google Scholar]
- Styczynski, J. Who Is the patient at risk of CMV recurrence: A review of the current scientific evidence with a focus on hematopoietic cell transplantation. Infect. Dis. Ther. 2018, 7, 1–16. [Google Scholar] [CrossRef]
- Emery, V.; Zuckerman, M.; Jackson, G.; Aitken, C.; Osman, H.; Pagliuca, A.; Potter, M.; Peggs, K.; Clark, A. British Committee for Standards in Haematology; British Society of Blood and Marrow Transplantation; UK Virology Network. Management of cytomegalovirus infection in haemopoietic stem cell transplantation. Br. J. Haematol. 2013, 162, 25–39. [Google Scholar] [CrossRef]
- Ljungman, P.; Engelhard, D.; Link, H.; Biron, P.; Brandt, L.; Cordonnier, C.; Debusscher, L.; de Laurenzi, A.; Kolb, H.J.; Messina, C.; et al. Treatment of interstitial pneumonitis due to cytomegalovirus with ganciclovir and intravenous immune globulin: Experience of European Bone Marrow Transplant Group. Clin. Infect. Dis. 1992, 14, 831–835. [Google Scholar] [CrossRef]
- Sokos, D.R.; Berger, M.; Lazarus, H.M. Intravenous immunoglobulin: Appropriate indications and uses in hematopoietic stem cell transplantation. Biol. Blood Marrow Transpl. 2002, 8, 117–130. [Google Scholar] [CrossRef]
- Piret, J.; Boivin, G. Clinical development of letermovir and maribavir: Overview of human cytomegalovirus drug resistance. Antiviral Res. 2019, 163, 91–105. [Google Scholar] [CrossRef]
- Chemaly, R.F.; Ullmann, A.J.; Stoelben, S.; Richard, M.P.; Bornhäuser, M.; Groth, C.; Einsele, H.; Silverman, M.; Mullane, K.M.; Brown, J.; et al. Letermovir for cytomegalovirus prophylaxis in hematopoietic-cell transplantation. N. Engl. J. Med. 2014, 370, 1781–1789. [Google Scholar] [CrossRef]
- Marty, F.M.; Ljungman, P.; Chemaly, R.F.; Maertens, J.; Dadwal, S.S.; Duarte, R.F.; Haider, S.; Ullmann, A.J.; Katayama, Y.; Brown, J.; et al. Letermovir Prophylaxis for Cytomegalovirus in Hematopoietic-Cell Transplantation. N. Engl. J. Med. 2017, 377, 2433–2444. [Google Scholar] [CrossRef]
- Goldner, T.; Hempel, C.; Ruebsamen-Schaeff, H.; Zimmermann, H.; Lischka, P. Geno- and phenotypic characterization of human cytomegalovirus mutants selected in vitro after letermovir (AIC246) exposure. Antimicrob. Agents Chemother. 2014, 58, 610–613. [Google Scholar] [CrossRef]
- Chow, S. Rapid in vitro Evolution of Human Cytomegalovirus UL56 Mutations That Confer Letermovir Resistance. Antimicrob. Agents Chemother. 2015, 59, 6588–6593. [Google Scholar] [CrossRef] [PubMed]
- Lischka, P.; Michel, D.; Zimmermann, H. Characterization of Cytomegalovirus Breakthrough Events in a Phase 2 Prophylaxis Trial of Letermovir (AIC246, MK 8228). J. Infect. Dis. 2016, 213, 23–30. [Google Scholar] [CrossRef]
- Williams, S.L.; Hartline, C.B.; Kushner, N.L.; Harden, E.A.; Bidanset, D.J.; Drach, J.C.; Townsend, L.B.; Underwood, M.R.; Biron, K.K.; Kern, E.R. In vitro activities of benzimidazole D- and L-ribonucleosides against herpesviruses. Antimicrob. Agents Chemother. 2003, 47, 2186–2192. [Google Scholar] [CrossRef] [PubMed]
- Drew, W.L.; Miner, R.C.; Marousek, G.I.; Chou, S. Maribavir sensitivity of cytomegalovirus isolates resistant to ganciclovir, cidofovir or foscarnet. J. Clin. Virol. 2006, 37, 124–127. [Google Scholar] [CrossRef]
- Trofe, J.; Pote, L.; Wade, E.; Blumberg, E.; Bloom, R.D. Maribavir: A novel antiviral agent with activity against cytomegalovirus. Ann. Pharmacother. 2008, 42, 1447–1457. [Google Scholar] [CrossRef]
- Winston, D.J.; Young, J.A.; Pullarkat, V.; Papanicolaou, G.A.; Vij, R.; Vance, E.; Alangaden, G.J.; Chemaly, R.F.; Petersen, F.; Chao, N.; et al. Maribavir prophylaxis for prevention of cytomegalovirus infection in allogeneic stem cell transplant recipients: A multicenter, randomized, double-blind, placebo-controlled, dose-ranging study. Blood 2008, 111, 5403–5410. [Google Scholar] [CrossRef]
- Marty, F.M.; Ljungman, P.; Papanicolaou, G.A.; Winston, D.J.; Chemaly, R.F.; Strasfeld, L.; Young, J.A.; Rodriguez, T.; Maertens, J.; Schmitt, M.; et al. Maribavir prophylaxis for prevention of cytomegalovirus disease in recipients of allogeneic stem-cell transplants: A phase 3, double-blind, placebo-controlled, randomised trial. Lancet. Infect. Dis. 2011, 11, 284–292. [Google Scholar] [CrossRef]
- Marty, F.M.; Boeckh, M. Maribavir and human cytomegalovirus-what happened in the clinical trials and why might the drug have failed? Curr. Opin. Virol. 2011, 1, 555–562. [Google Scholar] [CrossRef]
- Chemaly, R.F.; Hill, J.A.; Voigt, S.; Peggs, K.S. In vitro comparison of currently available and investigational antiviral agents against pathogenic human double-stranded DNA viruses: A systematic literature review. Antiviral Res. 2019, 163, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Bright, P.; Gompels, M.; Donati, M.; Johnston, S. Successful oral treatment of ganciclovir resistant cytomegalovirus with maribavir in the context of primary immunodeficiency: First case report and review. J. Clin. Virol. 2017, 87, 12–16. [Google Scholar] [CrossRef]
- Papanicolaou, G.A.; Silveira, F.P.; Langston, A.A.; Pereira, M.R.; Avery, R.K.; Uknis, M.; Wijatyk, A.; Wu, J.; Boeckh, M.; Marty, F.M.; et al. Maribavir for refractory or resistant cytomegalovirus infections in hematopoietic-cell or solid-organ transplant recipients: A randomized, dose-ranging, double-blind, phase 2 Study. Clin. Infect. Dis. 2018. [Google Scholar] [CrossRef]
- Gagelmann, N.; Ljungman, P.; Styczynski, J.; Kröger, N. Comparative efficacy and safety of different antiviral agents for cytomegalovirus prophylaxis in allogeneic hematopoietic cell transplantation: A systematic review and meta-analysis. Biol. Blood Marrow Transpl. 2018, 24, 2101–2109. [Google Scholar] [CrossRef]
- Ramsay, I.D.; Attwood, C.; Irish, D.; Griffiths, P.D.; Kyriakou, C.; Lowe, D.M. Disseminated adenovirus infection after allogeneic stem cell transplant and the potential role of brincidofovir—Case series and 10 year experience of management in an adult transplant cohort. J. Clin. Virol. 2017, 96, 73–79. [Google Scholar] [CrossRef]
- Marty, F.M.; Winston, D.J.; Rowley, S.D.; Vance, E.; Papanicolaou, G.A.; Mullane, K.M.; Brundage, T.M.; Robertson, A.T.; Godkin, S.; Momméja-Marin, H.; et al. CMX001 to prevent cytomegalovirus disease in hematopoietic-cell transplantation. N. Engl. J. Med. 2013, 369, 1227–1236. [Google Scholar] [CrossRef]
- Marty, F.M.; Winston, D.J.; Chemaly, R.F.; Mullane, K.M.; Shore, T.B.; Papanicolaou, G.A.; Chittick, G.; Brundage, T.M.; Wilson, C.; Morrison, M.E.; et al. A randomized, double-blind, placebo-controlled phase 3 trial of oral brincidofovir for cytomegalovirus prophylaxis in allogeneic hematopoietic cell transplantation. Biol. Blood Marrow Transpl. 2019, 25, 369–381. [Google Scholar] [CrossRef]
- Crumpacker, C.S. Cytomegalovirus. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases; Bennett, J.E., Dolin, R., Blaser, M.J., Eds.; Elsevier: Philadelphia, PA, USA, 2014. [Google Scholar]
- Ciáurriz, M.; Zabalza, A.; Beloki, L.; Mansilla, C.; Pérez-Valderrama, E.; Lachén, M.; Bandrés, E.; Olavarría, E.; Ramírez, N. The immune response to cytomegalovirus in allogeneic hematopoietic stem cell transplant recipients. Cell Mol. Life Sci. 2015, 72, 4049–4062. [Google Scholar] [CrossRef]
- Ljungman, P. Vaccination of immunocompromised hosts. In Plotkin’s Vaccines; Plotkin, S.A., Orenstein, W.A., Offit, P.A., Edwards, K.M., Eds.; Elsevier: Philadelphia, PA, USA, 2018. [Google Scholar]
- Lacey, S.F.; Diamond, D.J.; Zaia, J.A. Assessment of cellular immunity to human cytomegalovirus in recipients of allogeneic stem cell transplants. Biol. Blood Marrow Transplant. 2004, 10, 433–447. [Google Scholar] [CrossRef] [Green Version]
- Ljungman, P.; Hakki, M.; Boeckh, M. Cytomegalovirus in hematopoietic stem cell transplant recipients. Hematol. Oncol. Clin. North. Am. 2011, 25, 151–169. [Google Scholar] [CrossRef]
- Król, L.; Stuchlý, J.; Hubáček, P.; Keslová, P.; Sedláček, P.; Starý, J.; Hrušák, O.; Kalina, T. Signature profiles of CMV-specific T-cells in patients with CMV reactivation after hematopoietic SCT. Bone Marrow Transpl. 2011, 46, 1089–1098. [Google Scholar] [CrossRef]
- Blyth, E.; Withers, B.; Clancy, L.; Gottlieb, D. CMV-specific immune reconstitution following allogeneic stem cell transplantation. Virulence 2016, 7, 967–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lilleri, D.; Gerna, G.; Zelini, P.; Chiesa, A.; Rognoni, V.; Mastronuzzi, A.; Giorgiani, G.; Zecca, M.; Locatelli, F. Monitoring of human cytomegalovirus and virus-specific T-cell response in young patients receiving allogeneic hematopoietic stem cell transplantation. PLoS ONE 2012, 7, e41648. [Google Scholar] [CrossRef]
- Hakki, M.; Riddell, S.R.; Storek, J.; Carter, R.A.; Stevens-Ayers, T.; Sudour, P.; White, K.; Corey, L.; Boeckh, M. Immune reconstitution to cytomegalovirus after allogeneic hematopoietic stem cell transplantation: Impact of host factors, drug therapy, and subclinical reactivation. Blood 2003, 102, 3060–3067. [Google Scholar] [CrossRef]
- Widmann, T.; Sester, U.; Gärtner, B.C.; Schubert, J.; Pfreundschuh, M.; Köhler, H.; Sester, M. Levels of CMV specific CD4 T cells are dynamic and correlate with CMV viremia after allogeneic stem cell transplantation. PLoS ONE 2008, 3, e3634. [Google Scholar] [CrossRef]
- Quinnan, G.V., Jr.; Kirmani, N.; Rook, A.H.; Manischewitz, J.F.; Jackson, L.; Moreschi, G.; Santos, G.W.; Saral, R.; Burns, W.H. Cytotoxic T cells in cytomegalovirus infection: HLA-restricted T-lymphocyte and non-T-lymphocyte cytotoxic responses correlate with recovery from cytomegalovirus infection in bone-marrow-transplant recipients. N. Engl. J. Med. 1982, 307, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Ogonek, J.; Varanasi, P.; Luther, S.; Schweier, P.; Kühnau, W.; Göhring, G.; Dammann, E.; Stadler, M.; Ganser, A.; Borchers, S.; et al. Possible impact of cytomegalovirus-specific CD8+ T cells on immune reconstitution and conversion to complete donor chimerism after allogeneic stem cell transplantation. Biol. Blood Marrow Transpl. 2017, 23, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Litjens, N.H.R.; van der Wagen, L.; Kuball, J.; Kwekkeboom, J. Potential Beneficial Effects of Cytomegalovirus Infection after Transplantation. Front. Immunol. 2018, 9, 389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, J.H.; Lee, S.; Kim, H.J.; Jeon, Y.W.; Lee, S.E.; Cho, B.S.; Lee, D.G.; Eom, K.S.; Kim, Y.J.; Min, C.K.; et al. Impact of cytomegalovirus reactivation on relapse and survival in patients with acute leukemia who received allogeneic hematopoietic stem cell transplantation in first remission. Oncotarget 2016, 7, 17230–17241. [Google Scholar] [CrossRef] [PubMed]
- Elmaagacli, A.H.; Koldehoff, M. Cytomegalovirus replication reduces the relapse incidence in patients with acute myeloid leukemia. Blood 2016, 128, 456–459. [Google Scholar] [CrossRef] [Green Version]
- Jang, J.E.; Kim, S.J.; Cheong, J.W.; Hyun, S.Y.; Kim, Y.D.; Kim, Y.R.; Kim, J.S.; Min, Y.H. Early CMV replication and subsequent chronic GVHD have a significant anti-leukemic effect after allogeneic HSCT in acute myeloid leukemia. Ann. Hematol. 2015, 94, 275–282. [Google Scholar] [CrossRef]
- Alexander, W.S. Suppressors of cytokine signalling (SOCS) in the immune system. Nat. Rev. Immunol. 2002, 2, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Hill, G.R.; Kuns, R.D.; Raffelt, N.C.; Don, A.L.; Olver, S.D.; Markey, K.A.; Wilson, Y.A.; Tocker, J.; Alexander, W.S.; Clouston, A.D.; et al. SOCS3 regulates graft-versus-host disease. Blood 2010, 116, 287–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, T.H.; Lee, J.Y.; Park, S.; Shin, S.H.; Yahng, S.A.; Yoon, J.H.; Lee, S.E.; Cho, B.S.; Kim, Y.J.; Lee, S.; et al. Expression of SOCS1 and SOCS3 genes in human graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Blood Res. 2013, 48, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Lee, J.Y.; Lee, T.H.; Park, S.H.; Yahng, S.A.; Yoon, J.H.; Lee, S.E.; Cho, B.S.; Lee, D.G.; Kim, Y.J.; et al. SOCS1 and SOCS3 are expressed in mononuclear cells in human cytomegalovirus viremia after allogeneic hematopoietic stem cell transplantation. Blood Res. 2015, 50, 40–45. [Google Scholar] [CrossRef]
- Yong, M.K.; Cameron, P.U.; Slavin, M.; Morrissey, C.O.; Bergin, K.; Spencer, A.; Ritchie, D.; Cheng, A.C.; Samri, A.; Carcelain, G.; et al. Identifying cytomegalovirus complications using the quantiferon-CMV assay after allogeneic hematopoietic stem cell transplantation. J. Infect. Dis. 2017, 215, 1684–1694. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Lee, H.J.; Kim, S.M.; Kang, Y.A.; Lee, Y.S.; Chong, Y.P.; Sung, H.; Lee, S.O.; Choi, S.H.; Kim, Y.S.; et al. Diagnostic usefulness of dynamic changes of CMV-specific T-cell responses in predicting CMV infections in HCT recipients. J. Clin. Virol. 2017, 87, 5–11. [Google Scholar] [CrossRef]
- Wloch, M.K.; Smith, L.R.; Boutsaboualoy, S.; Reyes, L.; Han, C.; Kehler, J.; Smith, H.D.; Selk, L.; Nakamura, R.; Brown, J.M.; et al. Safety and immunogenicity of a bivalent cytomegalovirus DNA vaccine in healthy adult subjects. J. Infect. Dis. 2008, 197, 1634–1642. [Google Scholar] [CrossRef] [PubMed]
- Schleiss, M.R. Cytomegalovirus vaccines under clinical development. J. Virus Erad. 2016, 2, 198–207. [Google Scholar]
- Kharfan-Dabaja, M.A.; Boeckh, M.; Wilck, M.B.; Langston, A.A.; Chu, A.H.; Wloch, M.K.; Guterwill, D.F.; Smith, L.R.; Rolland, A.P.; Kenney, R.T. A novel therapeutic cytomegalovirus DNA vaccine in allogeneic haemopoietic stem-cell transplantation: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Infect. Dis. 2012, 12, 290–299. [Google Scholar] [CrossRef]
- Smith, L.R.; Wloch, M.K.; Chaplin, J.A.; Gerber, M.; Rolland, A.P. Clinical development of a cytomegalovirus DNA vaccine: From product concept to pivotal phase 3 trial. Vaccines 2013, 1, 398–414. [Google Scholar] [CrossRef]
- Griffiths, P.D.; Stanton, A.; McCarrell, E.; Smith, C.; Osman, M.; Harber, M.; Davenport, A.; Jones, G.; Wheeler, D.C.; O’Beirne, J.; et al. Cytomegalovirus glycoprotein-B vaccine with MF59 adjuvant in transplant recipients: A phase 2 randomised placebo-controlled trial. Lancet 2011, 377, 1256–1263. [Google Scholar] [CrossRef]
- Dole, K.; Segal, F.P.; Feire, A.; Magnusson, B.; Rondon, J.C.; Vemula, J.; Yu, J.; Pang, Y.; Pertel, P. A first-in-human study to assess the safety and pharmacokinetics of monoclonal antibodies against human cytomegalovirus in healthy volunteers. Antimicrob. Agents Chemother. 2016, 60, 2881–2887. [Google Scholar] [CrossRef]
- Fuji, S.; Löffler, J.; Einsele, H.; Kapp, M. Immunotherapy for opportunistic infections: Current status and future perspectives. Virulence 2016, 7, 939–949. [Google Scholar] [CrossRef] [Green Version]
- Riddell, S.R.; Watanabe, K.S.; Goodrich, J.M.; Li, C.R.; Agha, M.E.; Greenberg, P.D. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science 1992, 257, 238–241. [Google Scholar] [CrossRef]
- Walter, E.A.; Greenberg, P.D.; Gilbert, M.J.; Finch, R.J.; Watanabe, K.S.; Thomas, E.D.; Riddell, S.R. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N. Engl. J. Med. 1995, 333, 1038–1044. [Google Scholar] [CrossRef]
- Leen, A.M.; Tripic, T.; Rooney, C.M. Challenges of T cell therapies for virus-associated diseases after hematopoietic stem cell transplantation. Expert Opin. Biol. Ther. 2010, 10, 337–351. [Google Scholar] [CrossRef] [Green Version]
- Kaeuferle, T.; Krauss, R.; Blaeschke, F.; Willier, S.; Feuchtinger, T. Strategies of adoptive T -cell transfer to treat refractory viral infections post allogeneic stem cell transplantation. J. Hematol. Oncol. 2019, 12, 13. [Google Scholar] [CrossRef]
- Einsele, H.; Roosnek, E.; Rufer, N.; Sinzger, C.; Riegler, S.; Löffler, J.; Grigoleit, U.; Moris, A.; Rammensee, H.G.; Kanz, L.; et al. Infusion of cytomegalovirus (CMV)-specific T cells for the treatment of CMV infection not responding to antiviral chemotherapy. Blood 2002, 99, 3916–3922. [Google Scholar] [CrossRef]
- Peggs, K.; Verfuerth, S.; Mackinnon, S. Induction of cytomegalovirus (CMV)-specific T-cell responses using dendritic cells pulsed with CMV antigen: A novel culture system free of live CMV virions. Blood 2001, 97, 994–1000. [Google Scholar] [CrossRef]
- Rooney, C.M.; Smith, C.A.; Ng, C.Y.; Loftin, S.K.; Sixbey, J.W.; Gan, Y.; Srivastava, D.K.; Bowman, L.C.; Krance, R.A.; Brenner, M.K.; et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood 1998, 92, 1549–1555. [Google Scholar]
- Fuji, S.; Einsele, H.; Kapp, M. Cytomegalovirus disease in hematopoietic stem cell transplant patients: Current and future therapeutic options. Curr. Opin. Infect. Dis 2017, 30, 372–376. [Google Scholar] [CrossRef]
- Peggs, K.S.; Thomson, K.; Samuel, E.; Dyer, G.; Armoogum, J.; Chakraverty, R.; Pang, K.; Mackinnon, S.; Lowdell, M.W. Directly selected cytomegalovirus-reactive donor T cells confer rapid and safe systemic reconstitution of virus-specific immunity following stem cell transplantation. Clin. Infect. Dis. 2011, 52, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Feuchtinger, T.; Opherk, K.; Bethge, W.A.; Topp, M.S.; Schuster, F.R.; Weissinger, E.M.; Mohty, M.; Or, R.; Maschan, M.; Schumm, M.; et al. Adoptive transfer of pp65-specific T cells for the treatment of chemorefractory cytomegalovirus disease or reactivation after haploidentical and matched unrelated stem cell transplantation. Blood 2010, 116, 4360–4367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, N.; Nam, Y.S.; Im, K.I.; Lim, J.Y.; Jeon, Y.W.; Song, Y.; Lee, J.W.; Cho, S.G. Robust Production of Cytomegalovirus pp65-Specific T Cells using a fully automated IFN-γ cytokine capture system. Transfus. Med. Hemother. 2018, 45, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Neuenhahn, M.; Albrecht, J.; Odendahl, M.; Schlott, F.; Dössinger, G.; Schiemann, M.; Lakshmipathi, S.; Martin, K.; Bunjes, D.; Harsdorf, S.; et al. Transfer of minimally manipulated CMV-specific T cells from stem cell or third-party donors to treat CMV infection after allo-HSCT. Leukemia 2017, 31, 2161–2171. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.Y.; Zhao, X.Y.; Chang, Y.J.; Liu, J.; Xu, L.P.; Wang, Y.; Zhang, X.H.; Han, W.; Chen, Y.H.; Huang, X.J. Cytomegalovirus-specific T-cell transfer for refractory cytomegalovirus infection after haploidentical stem cell transplantation: The quantitative and qualitative immune recovery for cytomegalovirus. J. Infect. Dis. 2017, 216, 945. [Google Scholar] [CrossRef]

| Drugs | Mechanism | Dosing Regimens | Main Adverse Events and Considerations |
|---|---|---|---|
| Ganciclovir | Inhibits DNA polymerase (encoded by UL54 gene, Needs to be phosphorylated by viral phosphotransferase (encoded by UL97 gene) | Induction: 5 mg/kg IV every 12 h for at least 7–14 days Maintenance: 5 mg/kg IV once daily until test is negative Note: Minimum total induction and maintenance treatment is 2 weeks when 14 days of induction is used, and 3 weeks when a 7-day induction course is used. | Myelosuppression, Nephrotoxicity |
| Valganciclovir | Inhibits DNA polymerase, orally bioavailable formulation prodrug of ganciclovir | (Persons ≥40 kg with good oral intake) Induction: 900 mg PO twice daily for at least 14 days Maintenance: 900 mg PO once daily for 1–2 weeks until test is negative Note: Minimum treatment course is 14 days regardless of drug used | Myelosuppression, Nephrotoxicity |
| Foscarnet | Inhibits DNA polymerase UL54 directly by this pyrophosphate analogue | Induction: 60 mg/kg IV every 8 h or 90 mg/kg every 12 h for 2–3 weeks Maintenance: 90 to 120 mg/kg once daily | Nephrotoxicity, Electrolyte imbalance, Myelosuppression |
| Cidofovir | Nucleotide analogue that inhibits DNA polymerase UL54 | Induction: 5 mg/kg IV every weekly for 2 weeks Maintenance: 5 mg/kg IV every 2 weeks | Nephrotoxicity, Myelosuppression; Hydration and probenecid required to reduce nephrotoxicity |
| Leflunomide | Inhibits virion assembly, frequently used as add-on therapy | Loading dose: 100 mg orally once daily for 3 days only for patients at low risk for hepatotoxicity or myelosuppression Maintenance dose: 20 mg orally once daily; may reduce dose to 10 mg orally once daily if higher dose not tolerated | Liver cytolysis, Myelosuppression |
| CMV Ig/Polyclonal Ig | Increase CMV antibody levels | Varies among different studies and disease status | Infusion reactions |
| Drugs | Mechanisms | Indication or Primary Endpoint in Clinical Trials, Dosing Regimens if Possible | Main Adverse Events and Considerations |
|---|---|---|---|
| Letermovir | CMV terminase inhibitor that targets the UL56 viral subunit | Prophylaxis of CMV infection and disease in CMV-seropositive recipients of an allogeneic HSCT 480 mg PO or IV once daily through 100 days post transplantation (coadministration with cyclosporine: If cyclosporine is initiated after starting letermovir, decrease the next letermovir dose to 240 mg once daily; if cyclosporine is discontinued after starting letermovir, increase the next letermovir dose to 480 mg once daily; if cyclosporine is interrupted due to high cyclosporine levels, no dose adjustment of letermovir is needed) | Nausea, vomiting Has not been studied as an agent for treatment; has multiple drug interactions; lacks activity against other herpesviruses including HSV and VZV |
| Maribavir | UL97 viral protein kinase inhibitor | Not approved yet Clinical trials for treatment in transplant recipients with CMV infections that are refractory or resistant to treatment with ganciclovir, valganciclovir, foscarnet, or cidofovir; preemptive treatment in adult transplanted patients presenting with asymptomatic viremia | Taste disturbance Lower risk of hematotoxicity and absence of clear nephrotoxicity |
| Brincidofovir | Inhibits DNA polymerase, orally bioavailable formuation prodrug of cidofovir | Not approved yet Clinical trials for prophylactic or preemptive treatment of CMV infections; can be administered twice a week due to a long half-life | Diarrhea No excessive risk of nephro- and hematotoxocity |
| CMV vaccine | Stimulate CMV specific T cell immunity | Not approved yet Clinical trials for prophylaxis of CMV infection or reactivation in CMV seropositive patients undergoing allogeneic HSCT (ASP0113) | Minimal differences between the vaccine and placebo groups (ASP0113) |
| Passive CMV immune therapy | Monoclonal antibodies that block gB and others | Not approved yet Clinical trials for preventing of CMV infections in patients undergoing allogeneic HSCT recipients (CSJ148) | Nausea, Diarrhea, Vomiting, Stomatitis, and pyrexia (CSJ148) |
| Cell therapy | Adoptive transfer of CMV specific cytotoxic T lymphocytes | Not approved yet Clinical trials for treatment of persistent or refractory CMV infection after allogeneic HSCT | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, S.-Y.; Lee, D.-G.; Kim, H.-J. Cytomegalovirus Infections after Hematopoietic Stem Cell Transplantation: Current Status and Future Immunotherapy. Int. J. Mol. Sci. 2019, 20, 2666. https://doi.org/10.3390/ijms20112666
Cho S-Y, Lee D-G, Kim H-J. Cytomegalovirus Infections after Hematopoietic Stem Cell Transplantation: Current Status and Future Immunotherapy. International Journal of Molecular Sciences. 2019; 20(11):2666. https://doi.org/10.3390/ijms20112666
Chicago/Turabian StyleCho, Sung-Yeon, Dong-Gun Lee, and Hee-Je Kim. 2019. "Cytomegalovirus Infections after Hematopoietic Stem Cell Transplantation: Current Status and Future Immunotherapy" International Journal of Molecular Sciences 20, no. 11: 2666. https://doi.org/10.3390/ijms20112666
APA StyleCho, S.-Y., Lee, D.-G., & Kim, H.-J. (2019). Cytomegalovirus Infections after Hematopoietic Stem Cell Transplantation: Current Status and Future Immunotherapy. International Journal of Molecular Sciences, 20(11), 2666. https://doi.org/10.3390/ijms20112666




