Regulation of Dual-Specificity Phosphatase (DUSP) Ubiquitination and Protein Stability
Abstract
:1. The DUSP Family Phosphatases
2. Negative Regulation of DUSPs by Lys48-Linked Ubiquitination and Proteasomal Degradation
3. Other Post-Translational Regulations of DUSP Ubiquitination and/or Stability
3.1. Phosphorylation
3.2. Oxidation
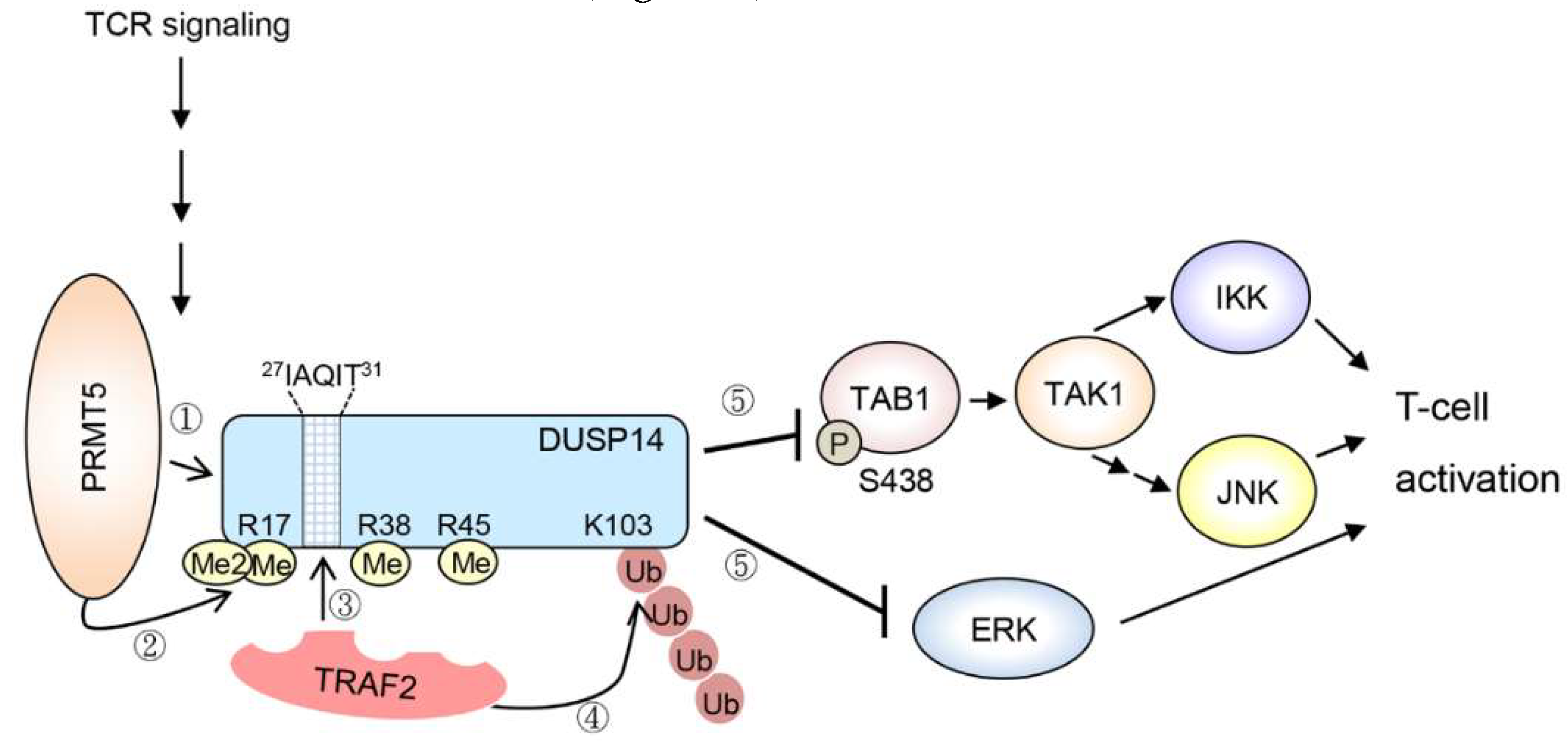
3.3. Methylation
4. Dysregulation of DUSPs in Diseases
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Ub | ubiquitination |
| DUSP | dual-specificity phosphatase |
| MKP | MAP kinase phosphatase |
| MAPK | mitogen-activated protein kinase |
| KIM | kinase-interacting motif |
| Skp2 | S-phase kinase-associated protein |
| Cks1 | cyclin-dependent kinase regulatory subunit 1 |
| FoxM1 | forkhead box M1 |
| STAT1 | signal transducer and activator of transcription 1 |
| LncRNA | long non-coding RNA |
| EGF | epidermal growth factor |
| PRMT5 | protein arginine methyltransferase 5 |
| TFH | T follicular helper cell |
| EAE | experimental autoimmune encephalomyelitis |
| SLE | systemic lupus erythematosus |
| SPOP | Speckle-type POZ protein |
| PDGF-BB | platelet-derived growth factor-B chains |
References
- Widmann, C.; Gibson, S.; Jarpe, M.B.; Johnson, G.L. Mitogen-activated protein kinase: Conservation of a three-kinase module from yeast to human. Physiol. Rev. 1999, 79, 143–180. [Google Scholar] [CrossRef]
- Raman, M.; Chen, W.; Cobb, M.H. Differential regulation and properties of MAPKs. Oncogene 2007, 26, 3100–3112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.R.; Meyer, C.F.; Tan, T.H. Persistent activation of c-Jun N-terminal kinase 1 (JNK1) in γ radiation-induced apoptosis. J. Biol. Chem. 1996, 271, 631–634. [Google Scholar] [CrossRef]
- Chen, Y.R.; Wang, X.; Templeton, D.; Davis, R.J.; Tan, T.H. The role of c-Jun N-terminal kinase (JNK) in apoptosis induced by ultraviolet C and γ radiation: Duration of JNK activation may determine cell death and proliferation. J. Biol. Chem. 1996, 271, 31929–31936. [Google Scholar] [CrossRef]
- MacCorkle, R.A.; Tan, T.H. Mitogen-activated protein kinases in cell-cycle control. Cell Biochem. Biophys. 2005, 43, 451–461. [Google Scholar] [CrossRef]
- Cargnello, M.; Roux, P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.R.; Tan, T.H. The c-Jun N-terminal kinase pathway and apoptotic signaling. Int. J. Oncol. 2000, 16, 651–662. [Google Scholar] [CrossRef]
- Marshall, C.J. MAP kinase kinase kinase, MAP kinase kinase and MAP kinase. Curr. Opin. Genet. Dev. 1994, 4, 82–89. [Google Scholar] [CrossRef]
- Wada, T.; Penninger, J.M. Mitogen-activated protein kinases in apoptosis regulation. Oncogene 2004, 23, 2838–2849. [Google Scholar] [CrossRef] [Green Version]
- Turjanski, A.G.; Vaque, J.P.; Gutkind, J.S. MAP kinases and the control of nuclear events. Oncogene 2007, 26, 3240–3253. [Google Scholar] [CrossRef] [Green Version]
- Kyriakis, J.M.; Avruch, J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 2001, 81, 807–869. [Google Scholar] [CrossRef]
- Dhillon, A.S.; Hagan, S.; Rath, O.; Kolch, W. MAP kinase signalling pathways in cancer. Oncogene 2007, 26, 3279–3290. [Google Scholar] [CrossRef] [Green Version]
- Caunt, C.J.; Keyse, S.M. Dual-specificity MAP kinase phosphatases (MKPs): Shaping the outcome of MAP kinase signalling. FASEB J. 2013, 280, 489–504. [Google Scholar] [CrossRef]
- Farooq, A.; Zhou, M.M. Structure and regulation of MAPK phosphatases. Cell. Signal. 2004, 16, 769–779. [Google Scholar] [CrossRef]
- Huang, C.Y.; Tan, T.H. DUSPs, to MAP kinases and beyond. Cell Biosci. 2012, 2, 24. [Google Scholar] [CrossRef]
- Mandl, M.; Slack, D.N.; Keyse, S.M. Specific inactivation and nuclear anchoring of extracellular signal-regulated kinase 2 by the inducible dual-specificity protein phosphatase DUSP5. Mol. Cell. Biol. 2005, 25, 1830–1845. [Google Scholar] [CrossRef]
- Masuda, K.; Shima, H.; Watanabe, M.; Kikuchi, K. MKP-7, a novel mitogen-activated protein kinase phosphatase, functions as a shuttle protein. J. Biol. Chem. 2001, 276, 39002–39011. [Google Scholar] [CrossRef]
- Karlsson, M.; Mathers, J.; Dickinson, R.J.; Mandl, M.; Keyse, S.M. Both nuclear-cytoplasmic shuttling of the dual specificity phosphatase MKP-3 and its ability to anchor MAP kinase in the cytoplasm are mediated by a conserved nuclear export signal. J. Biol. Chem. 2004, 279, 41882–41891. [Google Scholar] [CrossRef] [PubMed]
- Tanoue, T.; Adachi, M.; Moriguchi, T.; Nishida, E. A conserved docking motif in MAP kinases common to substrates, activators and regulators. Nat. Cell Biol. 2000, 2, 110–116. [Google Scholar] [CrossRef]
- Welchman, R.L.; Gordon, C.; Mayer, R.J. Ubiquitin and ubiquitin-like proteins as multifunctional signals. Nat. Rev. Mol. Cell Biol. 2005, 6, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Dikic, I.; Wakatsuki, S.; Walters, K.J. Ubiquitin-binding domains -from structures to functions. Nat. Rev. Mol. Cell Biol. 2009, 10, 659–671. [Google Scholar] [CrossRef]
- Malynn, B.A.; Ma, A. Ubiquitin makes its mark on immune regulation. Immunity 2010, 33, 843–852. [Google Scholar] [CrossRef]
- Kwon, Y.T.; Ciechanover, A. The ubiquitin code in the ubiquitin-proteasome system and autophagy. Trends Biochem. Sci. 2017, 42, 873–886. [Google Scholar] [CrossRef] [PubMed]
- Slack, D.N.; Seternes, O.M.; Gabrielsen, M.; Keyse, S.M. Distinct binding determinants for ERK2/p38α and JNK map kinases mediate catalytic activation and substrate selectivity of map kinase phosphatase-1. J. Biol. Chem. 2001, 276, 16491–16500. [Google Scholar] [CrossRef] [PubMed]
- Patterson, K.I.; Brummer, T.; O’Brien, P.M.; Daly, R.J. Dual-specificity phosphatases: Critical regulators with diverse cellular targets. Biochem. J. 2009, 418, 475–489. [Google Scholar] [CrossRef]
- Ma, R.Y.; Tong, T.H.; Cheung, A.M.; Tsang, A.C.; Leung, W.Y.; Yao, K.M. Raf/MEK/MAPK signaling stimulates the nuclear translocation and transactivating activity of FOXM1c. J. Cell Sci. 2005, 118, 795–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.W.; Chuang, S.M.; Yang, J.L. ERK1/2 achieves sustained activation by stimulating MAPK phosphatase-1 degradation via the ubiquitin-proteasome pathway. J. Biol. Chem. 2003, 278, 21534–21541. [Google Scholar] [CrossRef] [PubMed]
- Calvisi, D.F.; Pinna, F.; Meloni, F.; Ladu, S.; Pellegrino, R.; Sini, M.; Daino, L.; Simile, M.M.; De Miglio, M.R.; Virdis, P.; et al. Dual-specificity phosphatase 1 ubiquitination in extracellular signal-regulated kinase-mediated control of growth in human hepatocellular carcinoma. Cancer Res. 2008, 68, 4192–4200. [Google Scholar] [CrossRef] [PubMed]
- Major, M.L.; Lepe, R.; Costa, R.H. Forkhead box M1B transcriptional activity requires binding of Cdk-cyclin complexes for phosphorylation-dependent recruitment of p300/CBP coactivators. Mol. Cell. Biol. 2004, 24, 2649–2661. [Google Scholar] [CrossRef]
- Wang, I.C.; Chen, Y.J.; Hughes, D.; Petrovic, V.; Major, M.L.; Park, H.J.; Tan, Y.; Ackerson, T.; Costa, R.H. Forkhead box M1 regulates the transcriptional network of genes essential for mitotic progression and genes encoding the SCF (Skp2-Cks1) ubiquitin ligase. Mol. Cell. Biol. 2005, 25, 10875–10894. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.H.; Hur, E.M.; Lee, J.H.; Jun, D.J.; Kim, K.T. Protein kinase Cδ-mediated proteasomal degradation of MAP kinase phosphatase-1 contributes to glutamate-induced neuronal cell death. J. Cell Sci. 2006, 119, 1329–1340. [Google Scholar] [CrossRef] [PubMed]
- Geetha, N.; Mihaly, J.; Stockenhuber, A.; Blasi, F.; Uhrin, P.; Binder, B.R.; Freissmuth, M.; Breuss, J.M. Signal integration and coincidence detection in the mitogen-activated protein kinase/extracellular signal-regulated kinase (ERK) cascade: Concomitant activation of receptor tyrosine kinases and of LRP-1 leads to sustained ERK phosphorylation via down-regulation of dual specificity phosphatases (DUSP1 and -6). J. Biol. Chem. 2011, 286, 25663–25674. [Google Scholar] [PubMed]
- Xie, P.; Guo, S.; Fan, Y.; Zhang, H.; Gu, D.; Li, H. Atrogin-1/MAFbx enhances simulated ischemia/reperfusion-induced apoptosis in cardiomyocytes through degradation of MAPK phosphatase-1 and sustained JNK activation. J. Biol. Chem. 2009, 284, 5488–5496. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, Y.; Zhang, H.; Zhao, Q.; Liu, Z.; Xu, Y. USP49 inhibits ischemia-reperfusion-induced cell viability suppression and apoptosis in human AC16 cardiomyocytes through DUSP1-JNK1/2 signaling. J. Cell. Physiol. 2019, 234, 6529–6538. [Google Scholar] [CrossRef]
- Qin, X.Y.; Zhang, Y.L.; Chi, Y.F.; Yan, B.; Zeng, X.J.; Li, H.H.; Liu, Y. Angiotensin II regulates Th1 T cell differentiation through angiotensin II type 1 receptor-PKA-mediated activation of proteasome. Cell. Physiol. Biochem. 2018, 45, 1366–1376. [Google Scholar] [CrossRef]
- Chen, P.; Hutter, D.; Yang, X.; Gorospe, M.; Davis, R.J.; Liu, Y. Discordance between the binding affinity of mitogen-activated protein kinase subfamily members for MAP kinase phosphatase-2 and their ability to activate the phosphatase catalytically. J. Biol. Chem. 2001, 276, 29440–29449. [Google Scholar] [CrossRef]
- Torres, C.; Francis, M.K.; Lorenzini, A.; Tresini, M.; Cristofalo, V.J. Metabolic stabilization of MAP kinase phosphatase-2 in senescence of human fibroblasts. Exp. Cell Res. 2003, 290, 195–206. [Google Scholar] [CrossRef]
- Gomez, N.V.; Gorostizaga, A.B.; Mori Sequeiros Garcia, M.M.; Brion, L.; Acquier, A.; Gonzalez-Calvar, S.I.; Mendez, C.F.; Podesta, E.J.; Paz, C. MAPK phosphatase-2 (MKP-2) is induced by hCG and plays a role in the regulation of CYP11A1 expression in MA-10 Leydig cells. Endocrinology 2013, 154, 1488–1500. [Google Scholar] [CrossRef]
- Kucharska, A.; Rushworth, L.K.; Staples, C.; Morrice, N.A.; Keyse, S.M. Regulation of the inducible nuclear dual-specificity phosphatase DUSP5 by ERK MAPK. Cell. Signal. 2009, 21, 1794–1805. [Google Scholar] [CrossRef]
- Kidger, A.M.; Rushworth, L.K.; Stellzig, J.; Davidson, J.; Bryant, C.J.; Bayley, C.; Caddye, E.; Rogers, T.; Keyse, S.M.; Caunt, C.J. Dual-specificity phosphatase 5 controls the localized inhibition, propagation, and transforming potential of ERK signaling. Proc. Natl. Acad. Sci. USA 2017, 114, E317–E326. [Google Scholar] [CrossRef] [Green Version]
- Groom, L.A.; Sneddon, A.A.; Alessi, D.R.; Dowd, S.; Keyse, S.M. Differential regulation of the MAP, SAP and RK/p38 kinases by Pyst1, a novel cytosolic dual-specificity phosphatase. EMBO J. 1996, 15, 3621–3632. [Google Scholar] [CrossRef] [PubMed]
- Muda, M.; Boschert, U.; Dickinson, R.; Martinou, J.C.; Martinou, I.; Camps, M.; Schlegel, W.; Arkinstall, S. MKP-3, a novel cytosolic protein-tyrosine phosphatase that exemplifies a new class of mitogen-activated protein kinase phosphatase. J. Biol. Chem. 1996, 271, 4319–4326. [Google Scholar] [CrossRef]
- Chan, D.W.; Liu, V.W.; Tsao, G.S.; Yao, K.M.; Furukawa, T.; Chan, K.K.; Ngan, H.Y. Loss of MKP3 mediated by oxidative stress enhances tumorigenicity and chemoresistance of ovarian cancer cells. Carcinogenesis 2008, 29, 1742–1750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.H.; Choi, Y.W.; Han, J.H.; Lee, J.; Soh, E.Y.; Park, S.H.; Kim, J.H.; Park, T.J. TSH signaling overcomes B-RafV600E-induced senescence in papillary thyroid carcinogenesis through regulation of DUSP6. Neoplasia 2014, 16, 1107–1120. [Google Scholar] [CrossRef]
- Martin, M.J.; Hayward, R.; Viros, A.; Marais, R. Metformin accelerates the growth of BRAF V600E-driven melanoma by upregulating VEGF-A. Cancer Discov. 2012, 2, 344–355. [Google Scholar] [CrossRef]
- Lonne, G.K.; Masoumi, K.C.; Lennartsson, J.; Larsson, C. Protein kinase Cδ supports survival of MDA-MB-231 breast cancer cells by suppressing the ERK1/2 pathway. J. Biol. Chem. 2009, 284, 33456–33465. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Ci, W.; Karmakar, S.; Chen, K.; Dhar, R.; Fan, Z.; Guo, Z.; Zhang, J.; Ke, Y.; Wang, L.; et al. SPOP promotes tumorigenesis by acting as a key regulatory hub in kidney cancer. Cancer Cell 2014, 25, 455–468. [Google Scholar] [CrossRef]
- Cotsiki, M.; Oehrl, W.; Samiotaki, M.; Theodosiou, A.; Panayotou, G. Phosphorylation of the M3/6 dual-specificity phosphatase enhances the activation of JNK by arsenite. Cell. Signal. 2012, 24, 664–676. [Google Scholar] [CrossRef]
- Muda, M.; Theodosiou, A.; Rodrigues, N.; Boschert, U.; Camps, M.; Gillieron, C.; Davies, K.; Ashworth, A.; Arkinstall, S. The dual specificity phosphatases M3/6 and MKP-3 are highly selective for inactivation of distinct mitogen-activated protein kinases. J. Biol. Chem. 1996, 271, 27205–27208. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.R.; Shrivastava, A.; Tan, T.H. Down-regulation of the c-Jun N-terminal kinase (JNK) phosphatase M3/6 and activation of JNK by hydrogen peroxide and pyrrolidine dithiocarbamate. Oncogene 2001, 20, 367–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbacid, M.; Vazquez, D. (3H)anisomycin binding to eukaryotic ribosomes. J. Mol. Biol. 1974, 84, 603–623. [Google Scholar] [CrossRef]
- Theodosiou, A.; Ashworth, A. Differential effects of stress stimuli on a JNK-inactivating phosphatase. Oncogene 2002, 21, 2387–2397. [Google Scholar] [CrossRef] [Green Version]
- Jiapaer, Z.; Li, G.; Ye, D.; Bai, M.; Li, J.; Guo, X.; Du, Y.; Su, D.; Jia, W.; Chen, W.; et al. LincU preserves naive pluripotency by restricting ERK activity in embryonic stem cells. Stem Cell Reports 2018, 11, 395–409. [Google Scholar] [CrossRef]
- Lin, Y.W.; Yang, J.L. Cooperation of ERK and SCFSkp2 for MKP-1 destruction provides a positive feedback regulation of proliferating signaling. J. Biol. Chem. 2006, 281, 915–926. [Google Scholar] [CrossRef]
- Moosavi, S.M.; Prabhala, P.; Ammit, A.J. Role and regulation of MKP-1 in airway inflammation. Respir. Res. 2017, 18, 154. [Google Scholar] [CrossRef]
- Brondello, J.M.; Pouyssegur, J.; McKenzie, F.R. Reduced MAP kinase phosphatase-1 degradation after p42/p44MAPK-dependent phosphorylation. Science 1999, 286, 2514–2517. [Google Scholar] [CrossRef]
- Crowell, S.; Wancket, L.M.; Shakibi, Y.; Xu, P.; Xue, J.; Samavati, L.; Nelin, L.D.; Liu, Y. Post-translational regulation of mitogen-activated protein kinase phosphatase (MKP)-1 and MKP-2 in macrophages following lipopolysaccharide stimulation: The role of the C termini of the phosphatases in determining their stability. J. Biol. Chem. 2014, 289, 28753–28764. [Google Scholar] [CrossRef]
- Liu, R.; Zheng, H.Q.; Zhou, Z.; Dong, J.T.; Chen, C. KLF5 promotes breast cell survival partially through fibroblast growth factor-binding protein 1-pERK-mediated dual specificity MKP-1 protein phosphorylation and stabilization. J. Biol. Chem. 2009, 284, 16791–16798. [Google Scholar] [CrossRef]
- Chen, P.; Li, J.; Barnes, J.; Kokkonen, G.C.; Lee, J.C.; Liu, Y. Restraint of proinflammatory cytokine biosynthesis by mitogen-activated protein kinase phosphatase-1 in lipopolysaccharide-stimulated macrophages. J. Immunol. 2002, 169, 6408–6416. [Google Scholar] [CrossRef]
- Kassel, O.; Sancono, A.; Kratzschmar, J.; Kreft, B.; Stassen, M.; Cato, A.C. Glucocorticoids inhibit MAP kinase via increased expression and decreased degradation of MKP-1. EMBO J. 2001, 20, 7108–7116. [Google Scholar] [CrossRef] [Green Version]
- Swantek, J.L.; Cobb, M.H.; Geppert, T.D. Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) is required for lipopolysaccharide stimulation of tumor necrosis factor α (TNF-α) translation: Glucocorticoids inhibit TNF-α translation by blocking JNK/SAPK. Mol. Cell. Biol. 1997, 17, 6274–6282. [Google Scholar] [CrossRef]
- Kontoyiannis, D.; Pasparakis, M.; Pizarro, T.T.; Cominelli, F.; Kollias, G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: Implications for joint and gut-associated immunopathologies. Immunity 1999, 10, 387–398. [Google Scholar] [CrossRef]
- Jacob, A.; Smolenski, A.; Lohmann, S.M.; Begum, N. MKP-1 expression and stabilization and cGK Iα prevent diabetes-associated abnormalities in VSMC migration. Am. J. Physiol. Cell Physiol. 2004, 287, C1077–C1086. [Google Scholar] [CrossRef]
- Ciccarelli, M.; Rusciano, M.R.; Sorriento, D.; Basilicata, M.F.; Santulli, G.; Campiglia, P.; Bertamino, A.; De Luca, N.; Trimarco, B.; Iaccarino, G.; et al. CaMKII protects MKP-1 from proteasome degradation in endothelial cells. Cell. Signal. 2014, 26, 2167–2174. [Google Scholar] [CrossRef]
- Perander, M.; Al-Mahdi, R.; Jensen, T.C.; Nunn, J.A.; Kildalsen, H.; Johansen, B.; Gabrielsen, M.; Keyse, S.M.; Seternes, O.M. Regulation of atypical MAP kinases ERK3 and ERK4 by the phosphatase DUSP2. Sci. Rep. 2017, 7, 43471. [Google Scholar] [CrossRef]
- Brondello, J.M.; Brunet, A.; Pouyssegur, J.; McKenzie, F.R. The dual specificity mitogen-activated protein kinase phosphatase-1 and -2 are induced by the p42/p44MAPK cascade. J. Biol. Chem. 1997, 272, 1368–1376. [Google Scholar] [CrossRef]
- Peng, D.J.; Zhou, J.Y.; Wu, G.S. Post-translational regulation of mitogen-activated protein kinase phosphatase-2 (MKP-2) by ERK. Cell Cycle 2010, 9, 4650–4655. [Google Scholar] [Green Version]
- Hijiya, N.; Tsukamoto, Y.; Nakada, C.; Tung Nguyen, L.; Kai, T.; Matsuura, K.; Shibata, K.; Inomata, M.; Uchida, T.; Tokunaga, A.; et al. Genomic loss of DUSP4 contributes to the progression of intraepithelial neoplasm of pancreas to invasive carcinoma. Cancer Res. 2016, 76, 2612–2625. [Google Scholar] [CrossRef]
- Cadalbert, L.C.; Sloss, C.M.; Cunningham, M.R.; Al-Mutairi, M.; McIntire, A.; Shipley, J.; Plevin, R. Differential regulation of MAP kinase activation by a novel splice variant of human MAP kinase phosphatase-2. Cell. Signal. 2010, 22, 357–365. [Google Scholar] [CrossRef]
- Marchetti, S.; Gimond, C.; Chambard, J.C.; Touboul, T.; Roux, D.; Pouyssegur, J.; Pages, G. Extracellular signal-regulated kinases phosphorylate mitogen-activated protein kinase phosphatase 3/DUSP6 at serines 159 and 197, two sites critical for its proteasomal degradation. Mol. Cell. Biol. 2005, 25, 854–864. [Google Scholar] [CrossRef]
- Queipo, M.J.; Gil-Redondo, J.C.; Morente, V.; Ortega, F.; Miras-Portugal, M.T.; Delicado, E.G.; Perez-Sen, R. P2X7 nucleotide and EGF receptors exert dual modulation of the dual-specificity phosphatase 6 (MKP-3) in granule neurons and astrocytes, contributing to negative feedback on ERK signaling. Front. Mol. Neurosci. 2017, 10, 448. [Google Scholar] [CrossRef]
- Feng, B.; Jiao, P.; Yang, Z.; Xu, H. MEK/ERK pathway mediates insulin-promoted degradation of MKP-3 protein in liver cells. Mol. Cell. Endocrinol. 2012, 361, 116–123. [Google Scholar] [CrossRef] [Green Version]
- Bermudez, O.; Marchetti, S.; Pages, G.; Gimond, C. Post-translational regulation of the ERK phosphatase DUSP6/MKP3 by the mTOR pathway. Oncogene 2008, 27, 3685–3691. [Google Scholar] [CrossRef] [Green Version]
- Jurek, A.; Amagasaki, K.; Gembarska, A.; Heldin, C.H.; Lennartsson, J. Negative and positive regulation of MAPK phosphatase 3 controls platelet-derived growth factor-induced Erk activation. J. Biol. Chem. 2009, 284, 4626–4634. [Google Scholar] [CrossRef]
- Benavides-Serrato, A.; Anderson, L.; Holmes, B.; Cloninger, C.; Artinian, N.; Bashir, T.; Gera, J. mTORC2 modulates feedback regulation of p38 MAPK activity via DUSP10/MKP5 to confer differential responses to PP242 in glioblastoma. Genes Cancer 2014, 5, 393–406. [Google Scholar] [Green Version]
- Tanoue, T.; Yamamoto, T.; Maeda, R.; Nishida, E. A novel MAPK phosphatase MKP-7 acts preferentially on JNK/SAPK and p38 α and β MAPKs. J. Biol. Chem. 2001, 276, 26629–26639. [Google Scholar] [CrossRef]
- Katagiri, C.; Masuda, K.; Urano, T.; Yamashita, K.; Araki, Y.; Kikuchi, K.; Shima, H. Phosphorylation of Ser-446 determines stability of MKP-7. J. Biol. Chem. 2005, 280, 14716–14722. [Google Scholar] [CrossRef]
- Masuda, K.; Shima, H.; Katagiri, C.; Kikuchi, K. Activation of ERK induces phosphorylation of MAPK phosphatase-7, a JNK specific phosphatase, at Ser-446. J. Biol. Chem. 2003, 278, 32448–32456. [Google Scholar] [CrossRef]
- Kamata, H.; Honda, S.; Maeda, S.; Chang, L.; Hirata, H.; Karin, M. Reactive oxygen species promote TNFα-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell 2005, 120, 649–661. [Google Scholar] [CrossRef]
- Bai, L.; Xu, X.; Wang, Q.; Xu, S.; Ju, W.; Wang, X.; Chen, W.; He, W.; Tang, H.; Lin, Y. A superoxide-mediated mitogen-activated protein kinase phosphatase-1 degradation and c-Jun NH2-terminal kinase activation pathway for luteolin-induced lung cancer cytotoxicity. Mol. Pharmacol. 2012, 81, 549–555. [Google Scholar] [CrossRef]
- Kim, H.S.; Ullevig, S.L.; Zamora, D.; Lee, C.F.; Asmis, R. Redox regulation of MAPK phosphatase 1 controls monocyte migration and macrophage recruitment. Proc. Natl. Acad. Sci. USA 2012, 109, E2803–E2812. [Google Scholar] [CrossRef]
- Tephly, L.A.; Carter, A.B. Differential expression and oxidation of MKP-1 modulates TNF-α gene expression. Am. J. Respir. Cell Mol. Biol. 2007, 37, 366–374. [Google Scholar] [CrossRef]
- Barajas-Espinosa, A.; Basye, A.; Jesse, E.; Yan, H.; Quan, D.; Chen, C.A. Redox activation of DUSP4 by N-acetylcysteine protects endothelial cells from Cd2+-induced apoptosis. Free Radic. Biol. Med. 2014, 74, 188–199. [Google Scholar] [CrossRef]
- Marti, F.; Krause, A.; Post, N.H.; Lyddane, C.; Dupont, B.; Sadelain, M.; King, P.D. Negative-feedback regulation of CD28 costimulation by a novel mitogen-activated protein kinase phosphatase, MKP6. J. Immunol. 2001, 166, 197–206. [Google Scholar] [CrossRef]
- Yang, C.Y.; Li, J.P.; Chiu, L.L.; Lan, J.L.; Chen, D.Y.; Chuang, H.C.; Huang, C.Y.; Tan, T.H. Dual-specificity phosphatase 14 (DUSP14/MKP6) negatively regulates TCR signaling by inhibiting TAB1 activation. J. Immunol. 2014, 192, 1547–1557. [Google Scholar] [CrossRef]
- Yang, C.Y.; Chiu, L.L.; Tan, T.H. TRAF2-mediated Lys63-linked ubiquitination of DUSP14/MKP6 is essential for its phosphatase activity. Cell. Signal. 2016, 28, 145–151. [Google Scholar] [CrossRef]
- Yang, C.Y.; Chiu, L.L.; Chang, C.C.; Chuang, H.C.; Tan, T.H. Induction of DUSP14 ubiquitination by PRMT5-mediated arginine methylation. FASEB J. 2018, 32, 6760–6770. [Google Scholar] [CrossRef]
- Bermudez, O.; Pages, G.; Gimond, C. The dual-specificity MAP kinase phosphatases: Critical roles in development and cancer. Am. J. Physiol. Cell Physiol. 2010, 299, C189–C202. [Google Scholar] [CrossRef]
- Hsu, W.C.; Chen, M.Y.; Hsu, S.C.; Huang, L.R.; Kao, C.Y.; Cheng, W.H.; Pan, C.H.; Wu, M.S.; Yu, G.Y.; Hung, M.S.; et al. DUSP6 mediates T cell receptor-engaged glycolysis and restrains TFH cell differentiation. Proc. Natl. Acad. Sci. USA 2018, 115, E8027–E8036. [Google Scholar] [CrossRef]
- Ruan, J.W.; Statt, S.; Huang, C.T.; Tsai, Y.T.; Kuo, C.C.; Chan, H.L.; Liao, Y.C.; Tan, T.H.; Kao, C.Y. Dual-specificity phosphatase 6 deficiency regulates gut microbiome and transcriptome response against diet-induced obesity in mice. Nat. Microbiol. 2016, 2, 16220. [Google Scholar] [CrossRef]
- Okudela, K.; Yazawa, T.; Woo, T.; Sakaeda, M.; Ishii, J.; Mitsui, H.; Shimoyamada, H.; Sato, H.; Tajiri, M.; Ogawa, N.; et al. Down-regulation of DUSP6 expression in lung cancer: Its mechanism and potential role in carcinogenesis. Am. J. Pathol. 2009, 175, 867–881. [Google Scholar] [CrossRef]
- Furukawa, T.; Sunamura, M.; Motoi, F.; Matsuno, S.; Horii, A. Potential tumor suppressive pathway involving DUSP6/MKP-3 in pancreatic cancer. Am. J. Pathol. 2003, 162, 1807–1815. [Google Scholar] [CrossRef]
- Hou, P.C.; Li, Y.H.; Lin, S.C.; Lin, S.C.; Lee, J.C.; Lin, B.W.; Liou, J.P.; Chang, J.Y.; Kuo, C.C.; Liu, Y.M.; et al. Hypoxia-induced downregulation of DUSP-2 phosphatase drives colon cancer stemness. Cancer Res. 2017, 77, 4305–4316. [Google Scholar] [CrossRef]
- Wang, J.Y.; Yeh, C.L.; Chou, H.C.; Yang, C.H.; Fu, Y.N.; Chen, Y.T.; Cheng, H.W.; Huang, C.Y.; Liu, H.P.; Huang, S.F.; et al. Vaccinia H1-related phosphatase is a phosphatase of ErbB receptors and is down-regulated in non-small cell lung cancer. J. Biol. Chem. 2011, 286, 10177–10184. [Google Scholar] [CrossRef]
- Chen, Y.R.; Chou, H.C.; Yang, C.H.; Chen, H.Y.; Liu, Y.W.; Lin, T.Y.; Yeh, C.L.; Chao, W.T.; Tsou, H.H.; Chuang, H.C.; et al. Deficiency in VHR/DUSP3, a suppressor of focal adhesion kinase, reveals its role in regulating cell adhesion and migration. Oncogene 2017, 36, 6509–6517. [Google Scholar] [CrossRef]
- Shin, S.H.; Park, S.Y.; Kang, G.H. Down-regulation of dual-specificity phosphatase 5 in gastric cancer by promoter CpG island hypermethylation and its potential role in carcinogenesis. Am. J. Pathol. 2013, 182, 1275–1285. [Google Scholar] [CrossRef]
- Togel, L.; Nightingale, R.; Wu, R.; Chueh, A.C.; Al-Obaidi, S.; Luk, I.; Davalos-Salas, M.; Chionh, F.; Murone, C.; Buchanan, D.D.; et al. DUSP5 is methylated in CIMP-high colorectal cancer but is not a major regulator of intestinal cell proliferation and tumorigenesis. Sci. Rep. 2018, 8, 1767. [Google Scholar] [CrossRef]
- Li, J.P.; Yang, C.Y.; Chuang, H.C.; Lan, J.L.; Chen, D.Y.; Chen, Y.M.; Wang, X.; Chen, A.J.; Belmont, J.W.; Tan, T.H. The phosphatase JKAP/DUSP22 inhibits T-cell receptor signalling and autoimmunity by inactivating Lck. Nat. Commun. 2014, 5, 3618. [Google Scholar] [CrossRef] [Green Version]
- Chuang, H.C.; Chen, Y.M.; Hung, W.T.; Li, J.P.; Chen, D.Y.; Lan, J.L.; Tan, T.H. Downregulation of the phosphatase JKAP/DUSP22 in T cells as a potential new biomarker of systemic lupus erythematosus nephritis. Oncotarget 2016, 7, 57593–57605. [Google Scholar] [CrossRef] [Green Version]
- Melard, P.; Idrissi, Y.; Andrique, L.; Poglio, S.; Prochazkova-Carlotti, M.; Berhouet, S.; Boucher, C.; Laharanne, E.; Chevret, E.; Pham-Ledard, A.; et al. Molecular alterations and tumor suppressive function of the DUSP22 (Dual Specificity Phosphatase 22) gene in peripheral T-cell lymphoma subtypes. Oncotarget 2016, 7, 68734–68748. [Google Scholar] [CrossRef]
- Feldman, A.L.; Dogan, A.; Smith, D.I.; Law, M.E.; Ansell, S.M.; Johnson, S.H.; Porcher, J.C.; Ozsan, N.; Wieben, E.D.; Eckloff, B.W.; et al. Discovery of recurrent t(6;7)(p25.3;q32.3) translocations in ALK-negative anaplastic large cell lymphomas by massively parallel genomic sequencing. Blood 2011, 117, 915–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gioeli, D.; Mandell, J.W.; Petroni, G.R.; Frierson, H.F., Jr.; Weber, M.J. Activation of mitogen-activated protein kinase associated with prostate cancer progression. Cancer Res. 1999, 59, 279–284. [Google Scholar] [PubMed]
- Hoshino, R.; Chatani, Y.; Yamori, T.; Tsuruo, T.; Oka, H.; Yoshida, O.; Shimada, Y.; Ari-i, S.; Wada, H.; Fujimoto, J.; et al. Constitutive activation of the 41-/43-kDa mitogen-activated protein kinase signaling pathway in human tumors. Oncogene 1999, 18, 813–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Classification | Gene Symbol | Alias | Domain Structure | MAPK Substrates | ||
|---|---|---|---|---|---|---|
| Typical DUSPs (also named MKPs) | DUSP1 | MKP1, CL100, VH1, HVH1, PTPN10 |  | JNK, p38 > ERK | ||
| DUSP4 | MKP2, VH2, HVH2, TYP |  | ERK, JNK > p38 | |||
| DUSP6 | MKP3, PYST1 |  | ERK | |||
| DUSP7 | PYST2, MKPX* |  | ERK | |||
| DUSP9 | MKP4 |  | ERK > p38 | |||
| DUSP10 | MKP5 |  | JNK, p38 | |||
| DUSP16 | MKP7 |  | JNK (p38?) | |||
| Typical DUSPs (not named as MKPs) | DUSP2 | PAC1 |  | ERK, JNK, p38 | ||
| DUSP5 | VH3, HVH3 |  | ERK | |||
| DUSP8 | HB5, VH5, HVH-5, HVH8, (Mouse: M3/6) |  | JNK (p38?) | |||
| Atypical DUSPs | DUSP3 | VHR |  | |||
| DUSP11 | PIR1 |  | ||||
| DUSP12 | YVH1 |  | ||||
| DUSP13 | DUSP13A, DUSP13B, BEDP, MDSP, SKRP4, TMDP |  | ||||
| DUSP15 | VHY |  | ||||
| DUSP18 | DUSP20, LMW-DSP20 |  | ||||
| DUSP19 | DUSP17, LMW-DSP3, SKRP1, TS-DSP1 |  | ||||
| DUSP21 | LMW-DSP21 |  | ||||
| DUSP22 | JKAP, JSP1, VHX, LMW-DSP2, MKPX* |  | ||||
| DUSP23 | DUSP25, VHZ, LDP-3, MOSP |  | ||||
| DUSP24 | STYXL1, MK-STYX |  | ||||
| DUSP27 |  | |||||
| DUSP28 | VHP, DUSP26# |  | ||||
| Atypical DUSPs (also named MKPs) | DUSP14 | MKP6, MKP-L |  | JNK > ERK > p38 | ||
| DUSP26 | MKP8, LDP-4, NATA1, SKRP3, NEAP, DUSP24# |  | p38 (ERK?) | |||
 |  |  |  |  |  |  |
| Cdc25-homology | Kinase-interacting motif (KIM) | Phosphatase | Phosphatase (inactive) | PEST | Disintegrin | Unknown |
| Stimuli | Stability | Modification | Modification Enzyme | Experimental Methods | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|
| Protein Level | Half-Life | Ubiquitination | Proteasome Inhibitor | ||||||
| DUSP1 | Serum | ↓ | Phosphorylation↑ (human Ser296 †/Ser323 †); Ubiquitination↑ | ERK; CUL1 | ✓ | ✓ | ✓ | LLnL; MG132 | [28,54] |
| Estradiol | ↑ | Phosphorylation↑ (human Ser359 †/Ser364 †) | ERK | ✓ | ✓ | ✓ | LLnL; MG132; Lactacystin | [56] | |
| LPS | ↑ | Phosphorylation↑ (human Ser359 †/Ser364 †) | ERK | ✓ | ✓ | ? | MG132; PS-341 | [57] | |
| Pb2+ | ↓ | Ubiquitination↑ | ✓ | ✓ | ✓ | LLnL; MG132 | [27] | ||
| Glutamate/ PKCδ | ↓ | Ubiquitination↑ | ✓ | ✓ | MG132; LLnL; Lactacystin | [31] | |||
| Atrogin-1 upregulation | ↓ | Ubiquitination↑ | Atrogin-1 | ✓ | ✓ | ✓ | MG132 | [33] | |
| USP49 upregulation | ↑ | Ubiquitination↓ | USP49 | ✓ | ✓ | MG132 | [34] | ||
| KLF5 upregulation | ↑ | Phosphorylation↑ (human Ser359 †/Ser364 †) | ERK | ✓ | ✓ | MG132 | [58] | ||
| LPS | ↑ | Phosphorylation↑ (human Ser359 †/Ser364 †) | ERK | ✓ | ✓ | [59] | |||
| Insulin | ↑ | Phosphorylation↑ | ✓ | MG132; Lactacystin | [63] | ||||
| Asbestos/ ROS | ↓ | Oxidation↑ | ✓ | MG132 | [82] | ||||
| TNFα/ ROS | ↓ | Oxidation↑ | ✓ | MG132 | [79] | ||||
| ROS | ↓ | S-glutathionylation↑ (human Cys258 †) | ✓ | MG132 | [81] | ||||
| Glucocorticoid | ↑ | ✓ | MG132; LLnL | [60] | |||||
| EGF plus Lactoferrin | ↓ | ✓ | MG132 | [32] | |||||
| Angiotensin II/ PKA | ↓ | ✓ | Bortezomib | [35] | |||||
| CaMKII inhibition | ↓ | ✓ | MG132 | [64] | |||||
| Luteolin/ Superoxide | ↓ | ✓ | ✓ | MG132 | [80] | ||||
| DUSP2 | ERK4 | ↑ | ✓ | ✓ | [65] | ||||
| DUSP4 | LPS | ↑ | Phosphorylation↑ (human Ser386 †/Ser391 †) | ERK | ✓ | ✓ | ? | MG132; PS-341 | [57] |
| ERK inhibitor | ↓ | Phosphorylation↓ (human Ser386 †/Ser391 †); Ubiquitination↑ | ERK | ✓ | ✓ | ✓ | MG132 | [67] | |
| Cd2+ / Oxidative stress | ↓ | Oxidation↑ | GSSG | ✓ | [83] | ||||
| 8-Bromo-cAMP | ↑ | ✓ | ✓ | MG132 | [38] | ||||
| Senescence | ↑ | ✓ | ✓ | MG132 | [37] | ||||
| DUSP5 | ERK2 binding | ↑ | Ubiquitination↓ | ✓ | ✓ | ✓ | MG132 | [39] | |
| DUSP6 | Serum | ↓ | Phosphorylation↑ (human Ser159 †/Ser197 †) Ubiquitination↑ | ERK | ✓ | ✓ | ✓ | LLnL; Lactacystin | [70,73] |
| ROS | ↓ | Phosphorylation↑ (human Ser159 †/Ser197 †); Ubiquitination↑ | ✓ | ✓ | MG132 | [43] | |||
| PDGF | ↓ | Phosphorylation↑ (human Ser174 †); Ubiquitination↑ | ✓ | ✓ | ✓ | MG132 | [74] | ||
| P2X7 nucleotide, EGF | ↓ | Phosphorylation↑ (human Ser197 †) | ERK | ✓ | ✓ | MG132 | [71] | ||
| Insulin | ↓ | Phosphorylation↑ (human Ser159 †/Ser197 †) | ERK | ✓ | ✓ | [72] | |||
| Amino acid, insulin, IGF-1/ mTOR | ↓ | Phosphorylation↑ (human Ser159 †) | ✓ | ✓ | [73] | ||||
| PKCδ downregulation | ↓ | ✓ | MG132 | [46] | |||||
| TSH | ↑ | ✓ | ✓ | MG132 | [44] | ||||
| Metformin/ AMP-activated protein kinase | ↓ | ✓ | MG132 | [45] | |||||
| EGF plus Lactoferrin | ↓ | ✓ | MG132 | [32] | |||||
| DUSP7 | Hypoxic stress/ HIFs | ↓ | Ubiquitination↑ | SPOP | ✓ | ✓ | MG132 | [47] | |
| DUSP8 | Anisomycin | ↓ | Phosphorylation↑; Ubiquitination↑ | JNK | ✓ | ✓ | ? | Lactacystin | [52] |
| DUSP9 | LincU upregulation | ↑ | Ubiquitination↓ | ✓ | ✓ | ✓ | MG132 | [53] | |
| DUSP10 | Insulin | ↑ | Phosphorylation↑ (human Ser224 †/Ser230 †) | mTORC2 | ✓ | ✓ | [75] | ||
| DUSP14 | TCR signaling | (Activity↑) | Methylation↑ (human Arg17 †/Arg38 †/Arg45); Ubiquitination↑ (human Lys103 †) | PRMT5; TRAF2 | ✓ | ✓ | [86,87] | ||
| DUSP16 | ERK upregulation | ↑ | Phosphorylation↑ (human Ser446 †); Ubiquitination↓ | ERK | ✓ | ✓ | ✓ | MG132; MG115 | [77,78] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.-F.; Chuang, H.-C.; Tan, T.-H. Regulation of Dual-Specificity Phosphatase (DUSP) Ubiquitination and Protein Stability. Int. J. Mol. Sci. 2019, 20, 2668. https://doi.org/10.3390/ijms20112668
Chen H-F, Chuang H-C, Tan T-H. Regulation of Dual-Specificity Phosphatase (DUSP) Ubiquitination and Protein Stability. International Journal of Molecular Sciences. 2019; 20(11):2668. https://doi.org/10.3390/ijms20112668
Chicago/Turabian StyleChen, Hsueh-Fen, Huai-Chia Chuang, and Tse-Hua Tan. 2019. "Regulation of Dual-Specificity Phosphatase (DUSP) Ubiquitination and Protein Stability" International Journal of Molecular Sciences 20, no. 11: 2668. https://doi.org/10.3390/ijms20112668
APA StyleChen, H.-F., Chuang, H.-C., & Tan, T.-H. (2019). Regulation of Dual-Specificity Phosphatase (DUSP) Ubiquitination and Protein Stability. International Journal of Molecular Sciences, 20(11), 2668. https://doi.org/10.3390/ijms20112668






