Abstract
Tuberculosis (TB) is one of the top infectious diseases causing numerous human deaths in the world. Despite enormous efforts, the physiology of the causative agent, Mycobacterium tuberculosis, is poorly understood. To contribute to better understanding the physiological capacity of these microbes, we have carried out extensive in silico analyses of the 1111 mycobacterial species genomes focusing on revealing the role of the orphan cytochrome P450 monooxygenase (CYP) CYP139 family. We have found that CYP139 members are present in 894 species belonging to three mycobacterial groups: M. tuberculosis complex (850-species), Mycobacterium avium complex (34-species), and non-tuberculosis mycobacteria (10-species), with all CYP139 members belonging to the subfamily “A”. CYP139 members have unique amino acid patterns at the CXG motif. Amino acid conservation analysis placed this family in the 8th among CYP families belonging to different biological domains and kingdoms. Biosynthetic gene cluster analyses have revealed that 92% of CYP139As might be associated with producing different secondary metabolites. Such enhanced secondary metabolic potentials with the involvement of CYP139A members might have provided mycobacterial species with advantageous traits in diverse niches competing with other microbial or viral agents, and might help these microbes infect hosts by interfering with the hosts’ metabolism and immune system.
1. Introduction
Tuberculosis (TB), a prehistoric disease, remains one of the top 10 causes of death and the leading cause from a single infectious agent, Mycobacterium tuberculosis, despite global efforts in disease control programs during the past 20 years [1]. TB is a global disease, found in every country in the world [1]. It became mankind’s oldest and worst enemy owing to its widespread nature across the world and developing resistance to known and available drugs [1]. In 2017, 10 million people developed TB, and an estimated 1.3 million deaths among human immunodeficiency virus (HIV)-negative people and an additional 300,000 deaths from TB among HIV-positive people occurred [1]. The latest data from the Statistics South Africa show that TB is one of the top killers in South Africa [2], suggesting an urgent need to understand M. tuberculosis physiology to be able to come up with novel drugs and drug targets.
Despite living in the most advanced medicine era, TB remains a major threat to human health [1]. After 21 years of M. tuberculosis genome sequencing [3], to date its physiology is poorly understood and many proteins remain orphans. Genome sequencing analysis of M. tuberculosis H37Rv revealed the presence of 20 cytochrome P450 monooxygenases (CYPs/P450s) in its genome [3]. P450s are mixed function oxidoreductases ubiquitously distributed across the biological kingdoms [4]. P450s are well known for their role in essential cellular anabolic and catabolic processes.
Among 20 P450s, to date, the role of only six M. tuberculosis H37Rv P450s in its physiology have been elucidated [5]. CYP51B1, highly conserved P450 family across microbes, has been found to catalyse the 14α-demethylation of lanosterol [6,7,8]; CYP121A1 catalyses oxidative crosslinking of the two tyrosines in a cyclodipeptide [9]; CYP125A1 and CYP142A1 catalyse the 26-hydroxylation of cholesterol and cholest-4-en-3-one [10,11]; CYP124A1 catalyses the terminal hydroxylation of methyl-branched hydrocarbons such as those of phytanic acid and farnesol [12], cholesterol and related sterols [10,13], and vitamin D3 and CYP128A1 is involved in oxidation of menaquinone MK9 [14].
Among M. tuberculosis H37Rv P450s, the CYP139A1 gene was found downstream of polyketide synthase genes (pks10, pks7, pks8, pks17, pks9 and pks11) and situated next to macrolide transport protein [15,16]. Two of the polyketide synthases, pks7 and pks8, were found to be essential for the survival of M. tuberculosis [17,18]. Polyketide synthases along with other genes were found to be part of biosynthetic gene clusters (BGCs). As per Medema et al. [19], a BGC can be defined as a physically clustered group of two or more genes in a particular genome that together encode a biosynthetic pathway for the production of a specialised metabolite (including its chemical variants). Bacteria, fungi and plants are known to possess different types of BGCs producing a variety of secondary metabolites that are beneficial to humans. Among the genes that are part of a BGC, P450s play a key role in contributing to the diversity of a secondary metabolite owing to their regio and stereo-specific oxidation [20]. Recently, comprehensive comparative analysis of P450s and those associated with secondary metabolism revealed a large number of P450s involved in the production of secondary metabolites in different bacterial species [21,22].
Based on CYP139A1 location, this P450 is assumed to be involved in oxidative tailoring of the macrolide structure. In the latest study, involving comprehensive comparative analysis of P450s in bacterial species belonging to the genera Mycobacterium and Streptomyces, CYP139 P450s were found to be dominantly located in different secondary metabolite BGCs [22]. This strongly indicates that CYP139 P450s are possibly involved in the synthesis of secondary metabolites. This study is aimed at using an in silico approach to unravel the CYP139 P450 family’s role in mycobacterial species physiology.
2. Results and Discussion
2.1. CYP139 P450s Are Present Only in Certain Mycobacterial Category Species
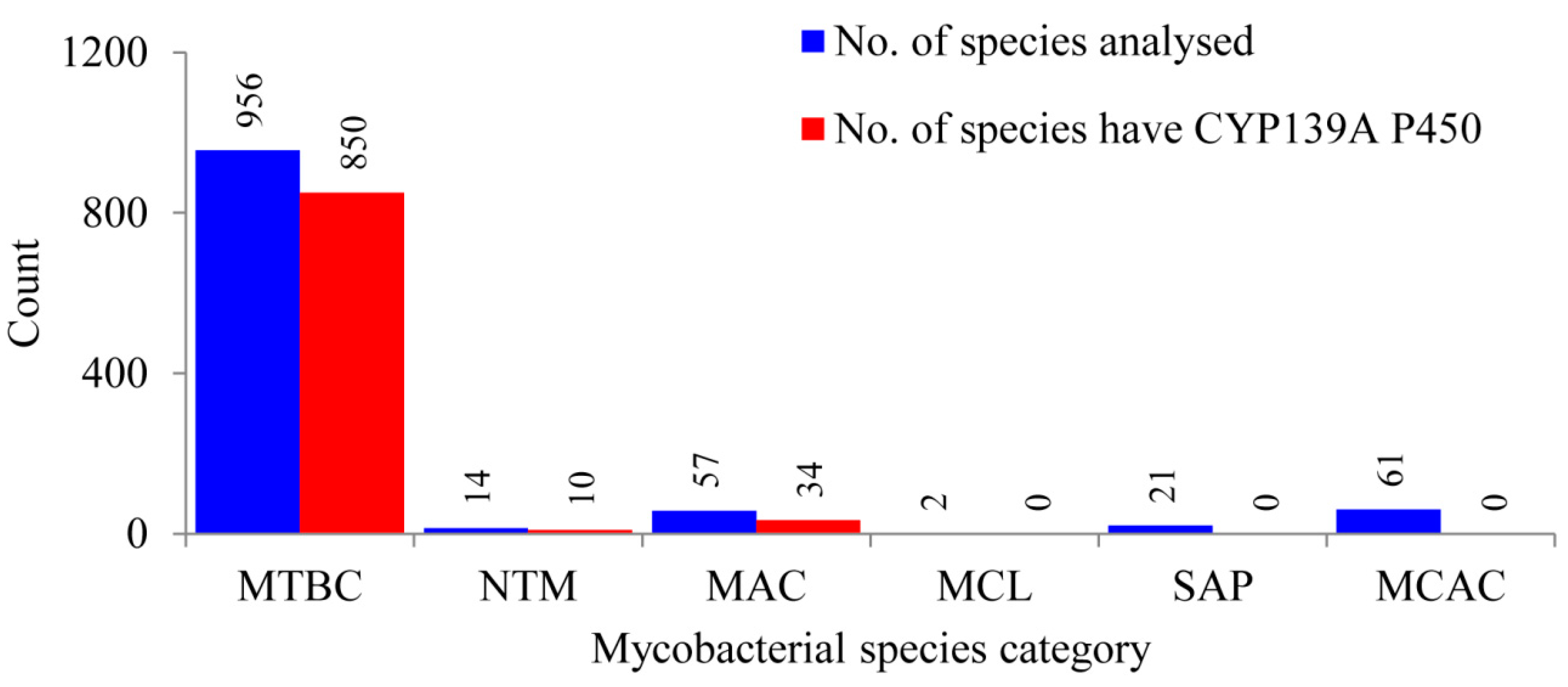
Comprehensive comparative analysis of CYP139 P450s in 1111 mycobacterial species belonging to six different categories (Table S1) revealed that CYP139 P450s are present in 894 mycobacterial species belonging to three categories, namely the Mycobacterium tuberculosis complex (MTBC), M. avium complex (MAV) and non-tuberculosis mycobacteria (NTM) (Figure 1 and Table S2). This phenomenon of identifying CYP139 P450s only in these three mycobacterial categories was also observed previously when 60 mycobacterial species were analysed [23]. Results from this study, which involved such a large data set, not only supported, but also confirmed that mycobacterial species belonging to categories such as Mycobacterium causing leprosy (MCL), Saprophytes (SAP) and the Mycobacterium chelonae-abscessus complex (MCAC) do not have CYP139 P450s in their genomes, as seen in Figure 1. Interestingly, not all mycobacterial species of MTBC, NTM and MAC categories have CYP139 P450 (Figure 1). Among 956 mycobacterial species, only 850 mycobacterial species of MTBC have CYP139 P450; 10 of 14 and 34 of 57 mycobacterial species of NTM and MAC, respectively, have this P450 (Figure 1 and Table S2). A detailed analysis of CYP139 P450s along with species names and protein ID is presented in Table S2 and the CYP139 P450 sequences are presented in Supplementary Dataset 1.
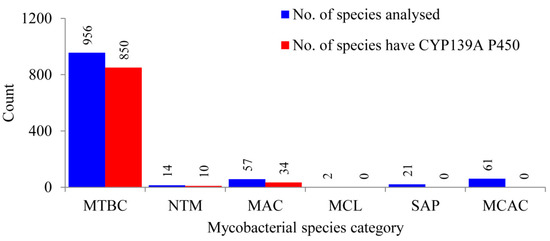
Figure 1.
Comparative analysis of CYP139A P450s in species belonging to six different mycobacterial categories. Abbreviations: MTBC, Mycobacterium tuberculosis complex; MAV, M. avium complex; NTM, non-tuberculosis mycobacteria; MCL, Mycobacterium causing leprosy; SAP, Saprophytes and MCAC, Mycobacterium chelonae-abscessus complex. Information on mycobacterial species and CYP139A P450s is presented in Supplementary Tables S1 and S2, respectively.
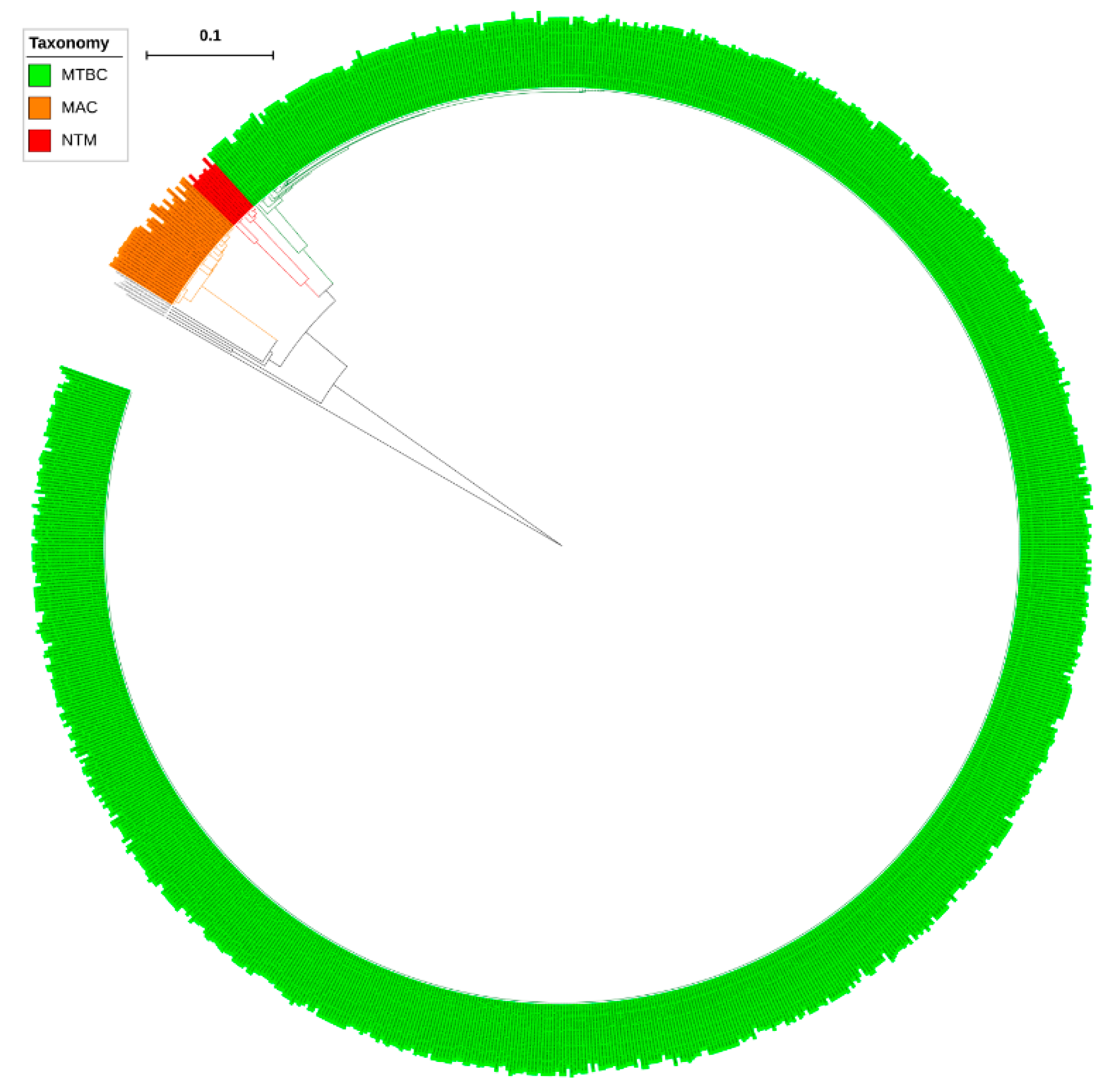
Analysis of CYP139 P450s in the genomes of mycobacterial species revealed that only a single copy of the CYP139 P450 gene is present in all mycobacterial species (Table S2). Furthermore, P450 subfamily analysis revealed that all CYP139 P450s found in 894 mycobacterial species belong to the subfamily “A” (Figure 2). Phylogenetic analysis of CYP139A P450s revealed that CYP139A P450s grouped per their mycobacterial category, indicating after speciation CYP139A P450s were subjected to amino acid changes specific to their category (Figure 1), similar to what was observed for other P450s described elsewhere [23,24]. However, four CYP139A P450s belonging to M. genavense ATCC 51234 and Mycobacterium sp. JDM601 of NTM and Mycobacterium sp. UM CSW and M. avium avium Env 77 of MAC were aligned separately, suggesting that these CYP139A P450s had deviated from their counterparts (Figure 2). Percentage identity among CYP139 P450s further confirmed that CYP139A P450s from these species have a low percentage identity with their counterparts (Supplementary Dataset 2). CYP139A P450s of Mycobacterium sp. UM CSW and M. avium avium Env 77 have an average of ~77% and ~63% identity, whereas CYP139A P450s of M. genavense ATCC 51234 and Mycobacterium sp. JDM601 have an average of 75% and 60% with their counterparts (Supplementary Dataset 2) suggesting these P450s have been subjected to significant amino acid changes. The phenomenon of P450s not grouping with their counterpart species was also observed in fungal species, where CYP53D1 has been subjected to extensive amino acid changes [24], the same as what was observed for the four CYP139A P450s identified in this study. Determining the effect of these amino acid changes on functional specificity of four CYP139A P450s, if any, will be interesting future work.
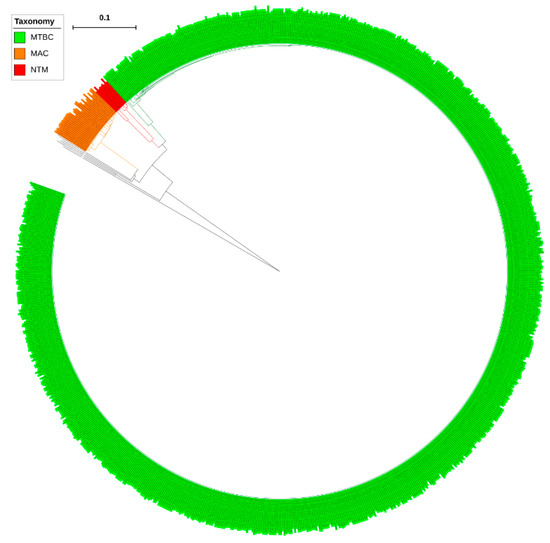
Figure 2.
Phylogenetic analysis of CYP139A P450s. Different mycobacterial categories were indicated in different colours. CYP51B1 from Mycobacterium tuberculosis H37Rv is used as an outgroup. Abbreviations: MTBC, Mycobacterium tuberculosis complex; MAV, M. avium complex; NTM, non-tuberculosis mycobacteria. A high-resolution phylogenetic tree is provided in Supplementary Figure S1.
2.2. CYP139 P450 Family Ranked among Top 10 P450 Families
Ranking of P450 families belonging to different biological kingdoms, based on the number of conserved amino acids in their protein sequence, placed the CYP139 P450 family in the twelfth rank [23,25]. While ranking the CYP139 P450 family, only 54 CYP139A P450s were used [23,25]. Identification of quite a large number of CYP139A P450s in this study necessitated re-analysis of the ranking of this P450 family. In order to identify the conservation rank, CYP139A P450s were subjected to PROfile Multiple Alignment with Local Structures and 3D constraints (PROMALS3D) [26] analysis (Supplementary Dataset 3). PROMALS3D analysis revealed the presence of 165 amino acids invariantly conserved in CYP139 P450s (Table 1). Comparative analysis with other P450 families from different biological kingdoms revealed that the CYP139 P450 family now occupies the eighth rank compared to the twelfth rank as assigned previously (Table 1).

Table 1.
Comparative amino acid conservation analysis of CYP139 P450 family with top 10 ranked P450 families [23,25]. The conservation index score is obtained as described in the section on materials and methods, following the procedure described elsewhere [27]. The conservation score (5–9) obtained via PROMALS3D is presented in the table, where the number 9 indicates invariantly conserved amino acids in P450 members. P450 families were arranged from the highest to the lowest number of amino acids conserved. CYP139 P450 family is indicated in bold.
2.3. CYP139 Family Has Unique Amino Acid Patterns at CXG Motif
In a study by Syed and Mashele [28], analysis of the P450 signature motifs, EXXR and CXG, among different P450 families led to the discovery of amino acid patterns characteristic of a P450 family. The authors proposed that “during the divergence of P450 families from a common ancestor, these amino acids patterns evolved and are retained in each P450 family as a signature of that family” [28]. However, in that study, the CYP139 P450 family is not included. Furthermore, identification of a large number of CYP139A P450s, in this study, gives us an opportunity to identify CYP139 P450 family characteristic amino acid patterns at EXXR and CXG motifs, if any.
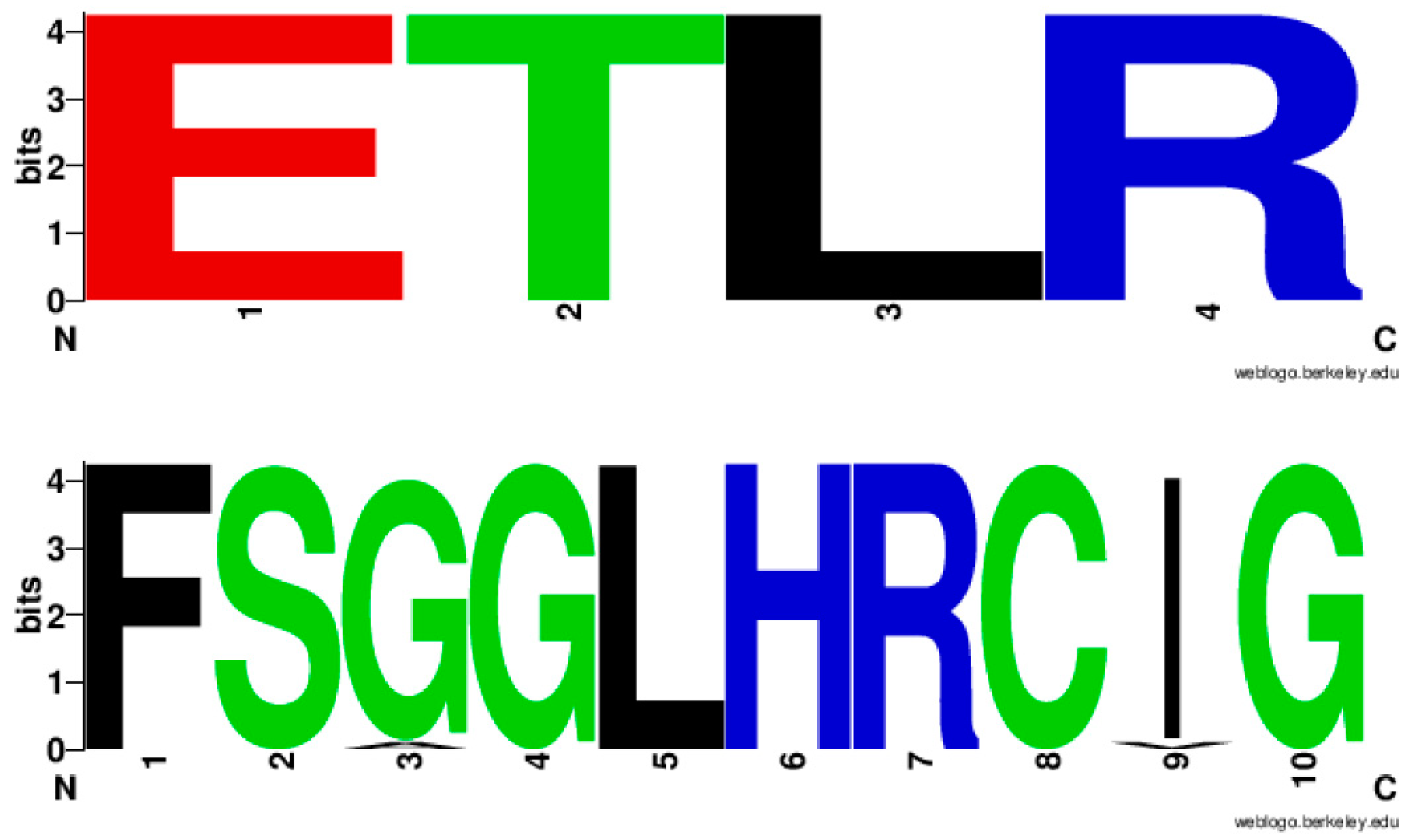
Analysis of EXXR and CXG motifs in 894 CYP139A P450s revealed that the CYP139 P450 family EXXR domain is absolutely conserved with amino acid patterns E-T-L-R, whereas, eight amino acids are invariantly conserved in CXG motifs with amino acid patterns of F-S-G(96%)/A(4%)-G-L-H-R-C-I(96%)/V(4%)-G (Figure 3). It is interesting to note that the CYP139 P450 family EXXR motif amino acid pattern absolutely matched with the CYP5 family [28] and amino acid patterns at the CXG motif were unique and not matched with any P450 families described in the literature [25,28,29]. The CYP139 P450 family amino acid patterns at the EXXR and CXG motifs further strongly support the above hypothesis proposed by Syed and Mashele [28].
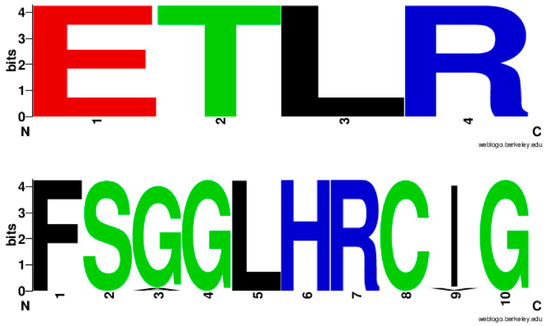
Figure 3.
Analysis of amino acid patterns at the EXXR and CXG motif in CYP139 P450 family. In total 894 CYP139 P450 sequences were analysed for EXXR and CXG signature sequences.
2.4. Most CYP139A P450s Are Part of Secondary Metabolite Biosynthetic Gene Clusters
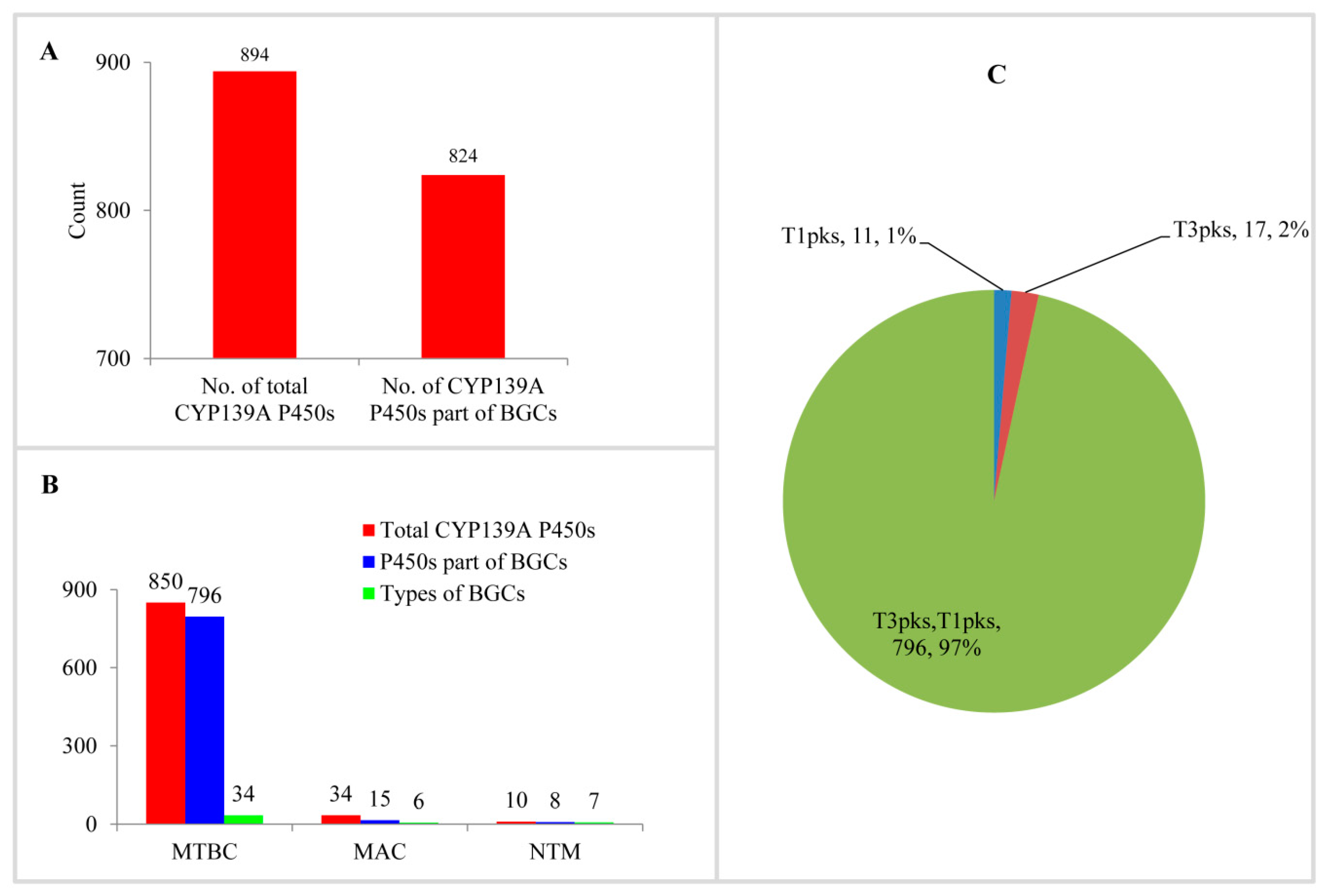
Analysis of CYP139A P450s as part of secondary metabolite BGCs in mycobacterial species revealed that most of the CYP139A P450s are part of different BGCs (Figure 4A and Table S2). Among 894 CYP139A P450s, 824 CYP139A P450s (92%) were found to be part of secondary metabolic BGCs (Figure 4A). This means 70 CYP139A P450s were not found to be part of any secondary metabolite BGCs. Comparison of CYP139A P450s that are part of BGCs in three categories revealed that most of the CYP139A P450s in MTBC and NTM species were part of BGCs, compared to species of MAC, where fewer than half of CYP139A P450s were part of secondary metabolite BGCs (Figure 4B).
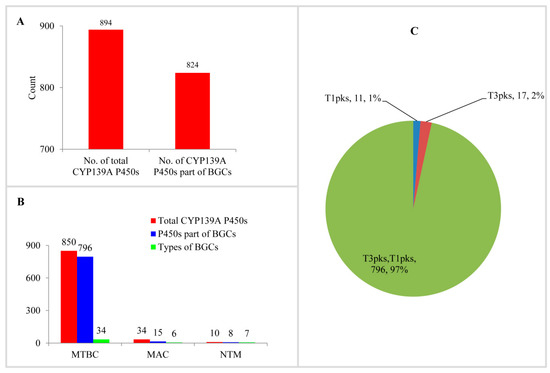
Figure 4.
CYP139A P450s secondary metabolite BGCs analysis in mycobacterial species. (A) Analysis of CYP139A P450s that are part of BGCs. (B) Comparative analysis of CYP139A P450s that are part of BGCs and types of BGCs in different mycobacterial categories. Abbreviations: MTBC, Mycobacterium tuberculosis complex; MAV, M. avium complex; NTM, non-tuberculosis mycobacteria. (C) Comparative analysis of CYP139A P450 cluster types. The type of cluster and the number of CYP139A P450s and their percentage in the total number of P450s were presented in the figure. Abbreviation: T1pks, Type 1 polyketide synthase; T2pks, Type 2 polyketide synthase.
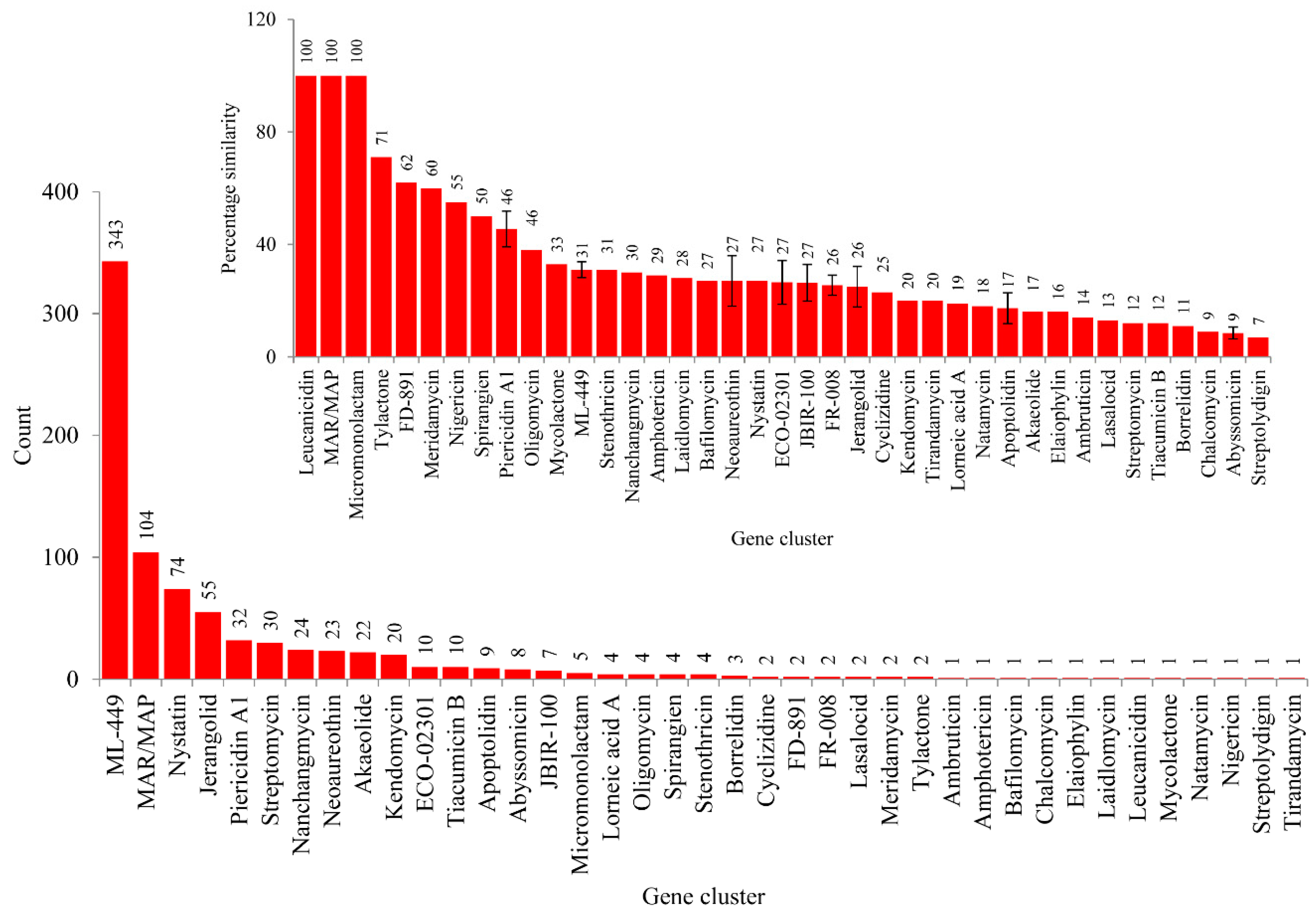
Analysis of secondary metabolite BGCs revealed that CYP139A P450s were part of only three different cluster types (Figure 4C and Table S2). Among three different cluster types, CYP139A P450s were found to be present dominantly as part of Type 3-Type 1 polyketide synthase (T3PKS-T1PKS) (97%) compared to T3 PKS (2%) and T1 PKS (1%) (Figure 4C and Table S2). There were 796 CYP139A P450s found to be part of T3PKS-T1PKS, followed by 17 and 11 CYP139 P450s found to be part of T3 PKS and T1 PKS, respectively (Figure 4C and Table S2). Analysis of gene clusters revealed that 824 CYP139A P450s were part of 39 different gene clusters (Figure 4). There were 34 CYP139A P450 gene clusters found in MTBC species, followed by seven gene clusters in NTM species and six gene clusters in MAC (Figure 4B). Among different gene clusters, ML-449 was dominant, with 349 CYP139A P450s followed by methylated alkyl-resorcinol/methylated acyl-phloroglucinol (MAR/MAP) with 104 CYP139A P450s, Nystatin with 74 CYP139A P450s and Jerangolid with 55 CYP139A P450s (Figure 5). Among 39 gene clusters only 11 gene clusters were found to have 10 or more CYP139A P450s (Figure 5). Analysis of DNA sequence percentage identity between CYP139A P450 gene clusters compared to known gene clusters revealed that some of the gene clusters have 100% identity, such as Leucanicidin, MAR/MAP and Micromonolactam (Figure 5), indicating CYP139A P450s are indeed involved in the synthesis of these secondary metabolites.
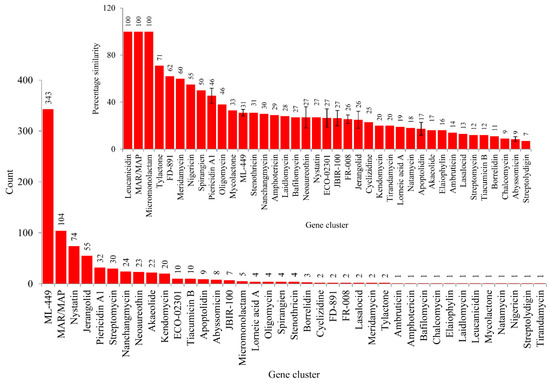
Figure 5.
CYP139A P450 gene cluster analysis in mycobacterial species. The 39 gene clusters were presented with their standard abbreviated names as per anti-SMASH. The number next to bars represents the number of CYP139A P450s that is part of that gene cluster. The inset figure shows the percentage identity of CYP139A P450 gene clusters to the known gene clusters available at anti-SMASH. The number next to the bars represents the percentage identity. For some gene clusters the percentage identity is represented with standard deviation (indicated with bars).
2.5. CYP139A P450s Involved in the Synthesis of Secondary Metabolites in Mycobacterial Species
Comprehensive comparative analysis of CYP139A P450s secondary BGCs in mycobacterial species revealed that CYP139A P450s are indeed involved in the synthesis of different secondary metabolites, as 92% of CYP139A P450s were found to be part of secondary metabolite BGCs (Figure 4 and Figure 5 and Table S2). To understand the role of CYP139A P450s in mycobacterial species’ physiology well, a functional comparison of CYP139A P450s gene clusters’ homolog secondary metabolites was carried out (Table 2). As shown in Table 2, it is clear that CYP139A P450s are involved in the production of chemicals that have antibacterial, antifungal, antiviral and antitumor properties. Interestingly, some of these metabolites in fact showed antimycobacterial activity (Table 2). This indicates that CYP139A P450s are possibly helping mycobacterial species to kill other bacteria, including other mycobacterial species, thus gaining the upper hand in the niche area for their survival. It is interesting to note that CYP139A P450s are present only in MTBC, NTM and MAC categories, but not present in SAP, MCAC or MCL. This necessitates understanding its role in mycobacterial species when they are surviving in hosts such as humans or other animals. In this direction, analysis of some secondary metabolite functions pointed out that some secondary metabolites are certainly helping mycobacterial species to survive in their hosts. For example, MAR/MAP BGC products are found to be part of the cell envelope in M. marinum, possibly complicating its access to host immune system or drug actions [30]; Akaeolide has cytotoxic activity against fibroblasts, suggesting it may play a role in tissue weakening in the host [31]; JBIR-100 exhibits cytotoxic activities and inhibition of proton pumps such as vacuolar-type ATPases (V-ATPases) activities and is thus linked with an increasing number of diseases such as osteopetrosis, male infertility and renal acidosis [32,33]. Lorneic acid A inhibits phosphodiesterase PDE5 blocking the degradation of cGMP [34] and thus it might be playing a role in pulmonary hypertension. Meridamycin has been found to bind FK506-binding proteins (FKBP12) [35]. FKBP12 proteins play a key role in regulating fundamental aspects of cell biology and have been found to be critical in mice survival [36]. Nigericin inhibits the Golgi functions in eukaryotic cells and is a well-known activator of the NLRP3 inflammasome [37,38,39], indicating bacterial infection. One secondary metabolite, namely mycolactone, a lipid-like toxin with cytotoxic, immunosuppressive and tissue necrosis activity, has been shown to be involved in the development of Buruli ulcer by M. ulcerans [40].

Table 2.
Functional analysis of homolog CYP139A P450 gene clusters.
3. Materials and Methods
3.1. Mycobacterial Species and Genome Databases
In total, 1111 mycobacterial species genomes that are available for public use (as of 12 June 2018) at Integrated Microbial Genomes & Microbiomes (IMG/M) [68] were used in the study (Table S1). Mycobacterial species used in the study, along with their name, genome ID and individual genome database links, were presented in Table S1.
3.2. Genome Data Mining and Annotation of CYP139 P450s
The M. tuberculosis H37Rv CYP139A1 (Rv1666c) P450 sequence has been blasted with the default settings against individual mycobacterial species genomes at IMG/M [68]. However, each time, only 20 mycobacterial species were selected for BLAST analysis. The hit proteins with more than 40% identity were selected and then subjected to BLAST analysis at the P450 BLAST server (https://ksyed.weebly.com/p450-blast.html) to identify the homolog P450. Hit proteins were then grouped into families and subfamilies based on the International Cytochrome P450 Nomenclature criteria, i.e., P450s showing >40% identity were assigned to the same P450 family and P450s that showed >55% identity were grouped under the same P450 subfamily [69,70,71]. Protein with more than 90% identity considered as ortholog and assigned the same subfamily number.
3.3. Phylogenetic Analysis of CYP139A P450s
The phylogenetic tree of CYP139 family members was built with M. tuberculosis CYP51B1 (Rv0764c) protein as outgroup. First, the protein sequences were aligned by MAFFT v6.864 [72], embedded on the Trex web serve [73]. Then, the alignments were automatically subjected to infer the best tree by the Trex web server with its embedded weighting procedure. Finally, the tree was visualised and colored by iTOL (http://itol.embl.de/about.cgi) [74].
3.4. Analysis of Homology and Amino Acid Conservation
Analysis of percentage identity among CYP139A P450s from species belonging to MAC and NTM categories was carried out as described elsewhere [23,29]. Briefly, the percentage identity between CYP139 P450s was determined using the Clustal Omega [75]. The Clustal Omega percentage identity matrix was downloaded and pasted into an Excel sheet by converting the text into a column option.
Amino acid conservation among CYP139A P450s was carried out following the method described elsewhere [23,25,29]. Briefly, CYP139 P450s were subjected to PROMALS3D [26] to identify invariantly conserved amino acids [27]. The conservation index follows numbers from 5–9, where 9 is the invariantly conserved amino acid across the sequences. The total number of conserved residues indicated by number 9 was recorded. The conserved nature of the CYP139 family was compared to other P450 families from different biological kingdoms, as reported elsewhere [23,25].
3.5. Generation of EXXR and CXG Sequence Logo
CYP139 P450 family EXXR and CXG sequence logos were generated following the method described elsewhere [25,28,29]. Briefly, CYP139 P450 sequences were aligned using ClustalW multiple alignments using MEGA7 [76]. After sequence alignment the EXXR and CXG region amino acids (4 and 10 amino acids, respectively), were selected and entered in the WebLogo program (http://weblogo.berkeley.edu/logo.cgi). As a selection parameter, the image format was selected as PNG (bitmap) at 300 dpi resolution. The percentage predominance of amino acids at particular positions is calculated considering the total number of amino acids as 100%. The generated EXXR and CXG logos were used for analysis and compared to the different P450 family EXXR and CXG logos that have been published and are available to the public [25,28,29].
3.6. Identification of CYP139 P450 Secondary Metabolite BGCs
BGCs listed on the IMG/M [68] website for each of the mycobacterial species were manually searched for the presence of CYP139 P450s using the protein ID. The BGCs that have CYP139 P450 were selected for further study. The listed BGCs at IMG/M are general [68] and in order to identify the specific type of BGCs, the selected BGCs genome sequences were subjected to secondary metabolite BGCs analysis, as described elsewhere [21]. Briefly, the individual BGC genome sequences downloaded from IMG/M [68] were submitted to anti-SMASH [77]. The type of BGC, percentage similarity to a known cluster and the cluster name were noted. Standard BGC abbreviation terminology developed by anti-SMASH [77] was used in the study.
4. Conclusions
The advancement of genome sequencing and bioinformatics tools helps significantly in understanding the role of orphan proteins in organisms. This study is an attempt to utilize the availability of quite a large number of mycobacterial species genome sequences and different bioinformatics tools to understand the role of the orphan CYP139 family in mycobacterial species. This study revealed that the CYP139 family indeed plays a role in the synthesis of secondary metabolites in mycobacterial species. Based on the functions of homolog CYP139 P450 gene clusters’ secondary metabolites, it can be assumed that these metabolites indeed help mycobacterial species to survive in the host, being part of the cell envelope and inhibiting fibroblast, thus causing tissue weakening and causing ulcers via tissue necrosis. The metabolites that exhibit antibacterial (including antimycobacterial), antifungal and antiviral properties certainly help mycobacterial species to gain the upper hand in the niche area compared to those agents. It would be interesting to determine the roles of CYP139A P450s that are not part of gene clusters. Predictions made in the study are based on the functions of homolog secondary metabolites. However, wet laboratory biosynthesis and functional analysis of secondary metabolites should be carried out to understand the role of these metabolites in mycobacterial physiology. Study results can be used as a reference for future experimental studies.
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/1422-0067/20/11/2690/s1.
Author Contributions
Conceptualization, R.K. and K.S.; data curation, P.R.S., W.C., D.R.N., A.P.K., J.-H.Y., R.K. and K.S.; formal analysis, P.R.S., W.C., D.R.N., A.P.K., J.-H.Y., R.K. and K.S.; funding acquisition, R.K., A.P.K. and K.S.; investigation, P.R.S., W.C., D.R.N., A.P.K., J.-H.Y., R.K. and K.S.; methodology, P.R.S, W.C., D.R.N., A.P.K., J.-H.Y., R.K. and K.S.; project administration, R.K. and K.S.; resources, R.K., A.P.K. and K.S.; supervision, R.K. and K.S.; validation, P.R.S., W.C., D.R.N., A.P.K., J.-H.Y., R.K. and K.S.; visualization, P.R.S., R.K. and K.S.; writing—original draft, P.R.S., W.C., D.R.N., A.P.K., J.-H.Y., R.K. and K.S.; writing—review and editing, P.R.S., R.K. and K.S.
Funding
Khajamohiddin Syed expresses sincere gratitude to the University of Zululand Research Committee for funding (Grant No. C686) and to the National Research Foundation (NRF), South Africa for a research grant (Grant No. 114159). Puleng Rosinah Syed thanks the NRF, South Africa for a DST-NRF Innovation Master’s Scholarship (Grant No. 114575). Rajshekhar Karpoormath (RK) and Puleng Rosinah Syed are grateful to the Discipline of Pharmaceutical Sciences, College of Health Sciences, University of KwaZulu-Natal, Durban, South Africa for providing access to necessary facilities. RK is also thankful to the NRF, South Africa for the research grants (Grant No. 103728 and 112079). Abidemi Paul Kappo is grateful to the South African Medical Research Council (SAMRC) for a research grant (Grant No. PC57009).
Acknowledgments
The authors want to thank Barbara Bradley, Pretoria, South Africa for English language editing.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- World Health Organization (WHO). Global Tuberculosis Report 2018. Available online: https://www.who.int/tb/publications/global_report/en/ (accessed on 22 March 2019).
- Mortality and Causes of Death in South Africa, 2016: Findings from Death Notification; Statistics South Africa: Pretoria, South Africa, 2018. Available online: http://www.statssa.gov.za/publications/P03093/P030932016.pdf (accessed on 22 March 2019).
- Cole, S.T.; Brosch, R.; Parkhill, J.; Garnier, T.; Churcher, C.; Harris, D.; Gordon, S.V.; Eiglmeier, K.; Gas, S.; Barry, C.E.; et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 1998, 393, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.R. Cytochrome P450 diversity in the tree of life. Biochim. Biophys. Acta Proteins Proteom. 2018, 1866, 141–154. [Google Scholar] [CrossRef]
- Ortiz de Montellano, P.R. Potential drug targets in the Mycobacterium tuberculosis cytochrome P450 system. J. Inorg. Biochem. 2018, 180, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Bellamine, A.; Mangla, A.T.; Dennis, A.L.; Nes, W.D.; Waterman, M.R. Structural requirements for substrate recognition of Mycobacterium tuberculosis 14 alpha-demethylase: Implications for sterol biosynthesis. J. Lipid Res. 2001, 42, 128–136. [Google Scholar] [PubMed]
- Bellamine, A.; Mangla, A.T.; Nes, W.D.; Waterman, M.R. Characterization and catalytic properties of the sterol 14alpha-demethylase from Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 1999, 96, 8937–8942. [Google Scholar] [CrossRef] [PubMed]
- McLean, K.J.; Warman, A.J.; Seward, H.E.; Marshall, K.R.; Girvan, H.M.; Cheesman, M.R.; Waterman, M.R.; Munro, A.W. Biophysical characterization of the sterol demethylase P450 from Mycobacterium tuberculosis, its cognate ferredoxin, and their interactions. Biochemistry 2006, 45, 8427–8443. [Google Scholar] [CrossRef]
- Belin, P.; Le Du, M.H.; Fielding, A.; Lequin, O.; Jacquet, M.; Charbonnier, J.B.; Lecoq, A.; Thai, R.; Courcon, M.; Masson, C.; et al. Identification and structural basis of the reaction catalyzed by CYP121, an essential cytochrome P450 in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 2009, 106, 7426–7431. [Google Scholar] [CrossRef]
- Johnston, J.B.; Ouellet, H.; Ortiz de Montellano, P.R. Functional redundancy of steroid C26-monooxygenase activity in Mycobacterium tuberculosis revealed by biochemical and genetic analyses. J. Biol. Chem. 2010, 285, 36352–36360. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, M.D.; McLean, K.J.; Levy, C.; Mast, N.; Pikuleva, I.A.; Lafite, P.; Rigby, S.E.; Leys, D.; Munro, A.W. Structural and biochemical characterization of Mycobacterium tuberculosis CYP142: Evidence for multiple cholesterol 27-hydroxylase activities in a human pathogen. J. Biol. Chem. 2010, 285, 38270–38282. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.B.; Kells, P.M.; Podust, L.M.; Ortiz de Montellano, P.R. Biochemical and structural characterization of CYP124: A methyl-branched lipid omega-hydroxylase from Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 2009, 106, 20687–20692. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.B.; Singh, A.A.; Clary, A.A.; Chen, C.K.; Hayes, P.Y.; Chow, S.; De Voss, J.J.; Ortiz de Montellano, P.R. Substrate analog studies of the omega-regiospecificity of Mycobacterium tuberculosis cholesterol metabolizing cytochrome P450 enzymes CYP124A1, CYP125A1 and CYP142A1. Bioorg. Med. Chem. 2012, 20, 4064–4081. [Google Scholar] [CrossRef] [PubMed]
- Sogi, K.M.; Holsclaw, C.M.; Fragiadakis, G.K.; Nomura, D.K.; Leary, J.A.; Bertozzi, C.R. Biosynthesis and regulation of sulfomenaquinone, a metabolite associated with virulence in Mycobacterium tuberculosis. ACS Infect. Dis. 2016, 2, 800–806. [Google Scholar] [CrossRef]
- McLean, K.J.; Munro, A.W. Structural biology and biochemistry of cytochrome P450 systems in Mycobacterium tuberculosis. Drug Metab. Rev. 2008, 40, 427–446. [Google Scholar] [CrossRef] [PubMed]
- Ouellet, H.; Johnston, J.B.; Ortiz de Montellano, P.R. The Mycobacterium tuberculosis cytochrome P450 system. Arch Biochem. Biophys. 2010, 493, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Griffin, J.E.; Gawronski, J.D.; Dejesus, M.A.; Ioerger, T.R.; Akerley, B.J.; Sassetti, C.M. High-resolution phenotypic profiling defines genes essential for mycobacterial growth and cholesterol catabolism. PLoS Pathog. 2011, 7, e1002251. [Google Scholar] [CrossRef]
- Sassetti, C.M.; Boyd, D.H.; Rubin, E.J. Genes required for mycobacterial growth defined by high density mutagenesis. Mol. Microbiol. 2003, 48, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Medema, M.H.; Kottmann, R.; Yilmaz, P.; Cummings, M.; Biggins, J.B.; Blin, K.; de Bruijn, I.; Chooi, Y.H.; Claesen, J.; Coates, R.C.; et al. Minimum information about a biosybnthetic gene cluster. Nat. Chem. Biol. 2015, 11, 625–631. [Google Scholar] [CrossRef]
- Greule, A.; Stok, J.E.; De Voss, J.J.; Cryle, M.J. Unrivalled diversity: The many roles and reactions of bacterial cytochromes P450 in secondary metabolism. Nat. Prod. Rep. 2018, 35, 757–791. [Google Scholar] [CrossRef] [PubMed]
- Mthethwa, B.C.; Chen, W.; Ngwenya, M.L.; Kappo, A.P.; Syed, P.R.; Karpoormath, R.; Yu, J.H.; Nelson, D.R.; Syed, K. Comparative analyses of cytochrome P450s and those associated with secondary metabolism in Bacillus species. Int. J. Mol. Sci. 2018, 19, 3623. [Google Scholar] [CrossRef]
- Senate, L.M.; Tjatji, M.P.; Pillay, K.; Chen, W.; Zondo, N.M.; Syed, P.R.; Mnguni, F.C.; Chiliza, Z.E.; Bamal, H.D.; Karpoormath, R.; et al. Similarities, variations, and evolution of cytochrome P450s in Streptomyces versus Mycobacterium. Sci. Rep. 2019, 9, 3962. [Google Scholar] [CrossRef]
- Parvez, M.; Qhanya, L.B.; Mthakathi, N.T.; Kgosiemang, I.K.; Bamal, H.D.; Pagadala, N.S.; Xie, T.; Yang, H.; Chen, H.; Theron, C.W.; et al. Molecular evolutionary dynamics of cytochrome P450 monooxygenases across kingdoms: Special focus on mycobacterial P450s. Sci. Rep. 2016, 6, 33099. [Google Scholar] [CrossRef] [PubMed]
- Jawallapersand, P.; Mashele, S.S.; Kovacic, L.; Stojan, J.; Komel, R.; Pakala, S.B.; Krasevec, N.; Syed, K. Cytochrome P450 monooxygenase CYP53 family in fungi: Comparative structural and evolutionary analysis and its role as a common alternative anti-fungal drug target. PLoS ONE 2014, 9, e107209. [Google Scholar] [CrossRef] [PubMed]
- Bamal, H.D.; Chen, W.; Mashele, S.S.; Nelson, D.R.; Kappo, A.P.; Mosa, R.A.; Yu, J.H.; Tuszynski, J.A.; Syed, K. Comparative analyses and structural insights of the novel cytochrome P450 fusion protein family CYP5619 in Oomycetes. Sci. Rep. 2018, 8, 6597. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Kim, B.H.; Grishin, N.V. PROMALS3D: A tool for multiple protein sequence and structure alignments. Nucleic Acids Res. 2008, 36, 2295–2300. [Google Scholar] [CrossRef]
- Pei, J.; Grishin, N.V. AL2CO: Calculation of positional conservation in a protein sequence alignment. Bioinformatics (Oxf. Engl.) 2001, 17, 700–712. [Google Scholar] [CrossRef]
- Syed, K.; Mashele, S.S. Comparative analysis of P450 signature motifs EXXR and CXG in the large and diverse kingdom of fungi: Identification of evolutionarily conserved amino acid patterns characteristic of P450 family. PLoS ONE 2014, 9, e95616. [Google Scholar] [CrossRef]
- Sello, M.M.; Jafta, N.; Nelson, D.R.; Chen, W.; Yu, J.H.; Parvez, M.; Kgosiemang, I.K.; Monyaki, R.; Raselemane, S.C.; Qhanya, L.B.; et al. Diversity and evolution of cytochrome P450 monooxygenases in Oomycetes. Sci. Rep. 2015, 5, 11572. [Google Scholar] [CrossRef]
- Parvez, A.; Giri, S.; Giri, G.R.; Kumari, M.; Bisht, R.; Saxena, P. Novel Type III polyketide synthases biosynthesize methylated polyketides in Mycobacterium marinum. Sci. Rep. 2018, 8, 6529. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Komaki, H.; Ichikawa, N.; Hosoyama, A.; Sato, S.; Igarashi, Y. Biosynthesis of akaeolide and lorneic acids and annotation of type I polyketide synthase gene clusters in the genome of Streptomyces sp. NPS554. Mar. Drugs 2015, 13, 581–596. [Google Scholar] [CrossRef]
- Ueda, J.Y.; Hashimoto, J.; Yamamura, H.; Hayakawa, M.; Takagi, M.; Shin-ya, K. A new 16-membered tetraene macrolide JBIR-100 from a newly identified Streptomyces species. J. Antibiot. 2010, 63, 627–629. [Google Scholar] [CrossRef]
- Huss, M.; Wieczorek, H. Inhibitors of V-ATPases: Old and new players. J. Exp. Biol. 2009, 212, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Iwata, F.; Sato, S.; Mukai, T.; Yamada, S.; Takeo, J.; Abe, A.; Okita, T.; Kawahara, H. Lorneic acids, trialkyl-substituted aromatic acids from a marine-derived actinomycete. J. Nat. Prod. 2009, 72, 2046–2048. [Google Scholar] [CrossRef] [PubMed]
- Salituro, G.M.; Zink, D.L.; Dahl, A.; Nielsen, J.; Wu, E.; Huang, L.; Kastner, C.; Dumont, F.J. Meridamycin: A novel nonimmunosuppressive FKBP12 ligand from Streptomyces hygroscopicus. Tetrahedron Lett. 1995, 36, 997–1000. [Google Scholar] [CrossRef]
- Aghdasi, B.; Ye, K.; Resnick, A.; Huang, A.; Ha, H.C.; Guo, X.; Dawson, T.M.; Dawson, V.L.; Snyder, S.H. FKBP12, the 12-kDa FK506-binding protein, is a physiologic regulator of the cell cycle. Proc. Natl. Acad. Sci. USA 2001, 98, 2425–2430. [Google Scholar] [CrossRef]
- Wawrocki, S.; Druszczynska, M. Inflammasomes in Mycobacterium tuberculosis-Driven Immunity. Can. J. Infect. Dis. Med Microbiol. J. Can. Mal. Infect. Microbiol. Med 2017, 2017, 2309478. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.P.; Alonso, S.; Rand, L.; Dick, T.; Pethe, K. The protonmotive force is required for maintaining ATP homeostasis and viability of hypoxic, nonreplicating Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 2008, 105, 11945–11950. [Google Scholar] [CrossRef]
- Katsnelson, M.A.; Rucker, L.G.; Russo, H.M.; Dubyak, G.R. K+ efflux agonists induce NLRP3 inflammasome activation independently of Ca2+ signaling. J. Immunol. (Baltim. Md. 1950) 2015, 194, 3937–3952. [Google Scholar] [CrossRef] [PubMed]
- Sarfo, F.S.; Phillips, R.; Wansbrough-Jones, M.; Simmonds, R.E. Recent advances: Role of mycolactone in the pathogenesis and monitoring of Mycobacterium ulcerans infection/Buruli ulcer disease. Cell. Microbiol. 2016, 18, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, H.; Degnes, K.F.; Dikiy, A.; Fjaervik, E.; Klinkenberg, G.; Zotchev, S.B. Insights into the evolution of macrolactam biosynthesis through cloning and comparative analysis of the biosynthetic gene cluster for a novel macrocyclic lactam, ML-449. Appl. Environ. Microbiol. 2010, 76, 283–293. [Google Scholar] [CrossRef]
- Brautaset, T.; Sekurova, O.N.; Sletta, H.; Ellingsen, T.E.; StrLm, A.R.; Valla, S.; Zotchev, S.B. Biosynthesis of the polyene antifungal antibiotic nystatin in Streptomyces noursei ATCC 11455: Analysis of the gene cluster and deduction of the biosynthetic pathway. Chem. Biol. 2000, 7, 395–403. [Google Scholar] [CrossRef]
- Julien, B.; Tian, Z.Q.; Reid, R.; Reeves, C.D. Analysis of the ambruticin and jerangolid gene clusters of Sorangium cellulosum reveals unusual mechanisms of polyketide biosynthesis. Chem. Biol. 2006, 13, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kong, L.; Shen, J.; Wang, Q.; Liu, Q.; Yang, W.; Deng, Z.; You, D. Characterization of the positive SARP family regulator PieR for improving piericidin A1 production in Streptomyces piomogeues var. Hangzhouwanensis. Synth. Syst. Biotechnol. 2019, 4, 16–24. [Google Scholar] [CrossRef]
- Distler, J.; Ebert, A.; Mansouri, K.; Pissowotzki, K.; Stockmann, M.; Piepersberg, W. Gene cluster for streptomycin biosynthesis in Streptomyces griseus: Nucleotide sequence of three genes and analysis of transcriptional activity. Nucleic Acids Res. 1987, 15, 8041–8056. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhou, X.; Dong, H.; Tu, G.; Wang, M.; Wang, B.; Deng, Z. A complete gene cluster from Streptomyces nanchangensis NS3226 encoding biosynthesis of the polyether ionophore nanchangmycin. Chem. Biol. 2003, 10, 431–441. [Google Scholar] [CrossRef]
- Rausch, K.; Hackett, B.A.; Weinbren, N.L.; Reeder, S.M.; Sadovsky, Y.; Hunter, C.A.; Schultz, D.C.; Coyne, C.B.; Cherry, S. Screening bioactives reveals nanchangmycin as a broad spectrum antiviral active against Zika virus. Cell Rep. 2017, 18, 804–815. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Lin, X.; Zhou, X.; Deng, Z.; Cane, D.E. Mechanism of thioesterase-catalyzed chain release in the biosynthesis of the polyether antibiotic nanchangmycin. Chem. Biol. 2008, 15, 449–458. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cassinelli, G.; Grein, A.; Orezzi, P.; Pennella, P.; Sanfilippo, A. New antibiotics produced by Streptoverticillium orinoci, n. sp. Arch. Fur Mikrobiol. 1967, 55, 358–368. [Google Scholar] [CrossRef]
- Elnakady, Y.A.; Chatterjee, I.; Bischoff, M.; Rohde, M.; Josten, M.; Sahl, H.G.; Herrmann, M.; Muller, R. Investigations to the antibacterial mechanism of action of kendomycin. PLoS ONE 2016, 11, e0146165. [Google Scholar] [CrossRef]
- McAlpine, J.B.; Bachmann, B.O.; Piraee, M.; Tremblay, S.; Alarco, A.M.; Zazopoulos, E.; Farnet, C.M. Microbial genomics as a guide to drug discovery and structural elucidation: ECO-02301, a novel antifungal agent, as an example. J. Nat. Prod. 2005, 68, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Li, S.; Niu, S.; Ma, L.; Zhang, G.; Zhang, H.; Zhang, G.; Ju, J.; Zhang, C. Characterization of tiacumicin B biosynthetic gene cluster affording diversified tiacumicin analogues and revealing a tailoring dihalogenase. J. Am. Chem. Soc. 2011, 133, 1092–1105. [Google Scholar] [CrossRef]
- Glaus, F.; Altmann, K.H. Total synthesis of the tiacumicin B (lipiarmycin A3/fidaxomicin) aglycone. Angew. Chem. (Int. Ed. Engl.) 2015, 54, 1937–1940. [Google Scholar] [CrossRef]
- Salomon, A.R.; Voehringer, D.W.; Herzenberg, L.A.; Khosla, C. Apoptolidin, a selective cytotoxic agent, is an inhibitor of F0F1-ATPase. Chem. Biol. 2001, 8, 71–80. [Google Scholar] [CrossRef]
- Wender, P.A.; Sukopp, M.; Longcore, K. Apoptolidins B and C: Isolation, structure determination, and biological activity. Org. Lett. 2005, 7, 3025–3028. [Google Scholar] [CrossRef]
- Kim, J.W.; Adachi, H.; Shin-ya, K.; Hayakawa, Y.; Seto, H. Apoptolidin, a new apoptosis inducer in transformed cells from Nocardiopsis sp. J. Antibiot. 1997, 50, 628–630. [Google Scholar] [CrossRef][Green Version]
- Sadaka, C.; Ellsworth, E.; Hansen, P.R.; Ewin, R.; Damborg, P.; Watts, J.L. Review on abyssomicins: Inhibitors of the chorismate pathway and folate biosynthesis. Molecules (Basel Switz.) 2018, 23, 1371. [Google Scholar] [CrossRef]
- Skellam, E.J.; Stewart, A.K.; Strangman, W.K.; Wright, J.L. Identification of micromonolactam, a new polyene macrocyclic lactam from two marine Micromonospora strains using chemical and molecular methods: Clarification of the biosynthetic pathway from a glutamate starter unit. J. Antibiot. 2013, 66, 431–441. [Google Scholar] [CrossRef]
- Isogai, A.; Sakuda, S.; Matsumoto, S.; Ogura, M.; Furihata, K.; Seto, H.; Suzuki, A. The structure of leucanicidin, a novel insecticidal macrolide produced by Streptomyces halstedii. Agric. Biol. Chem. 1984, 48, 1379–1381. [Google Scholar] [CrossRef]
- Symersky, J.; Osowski, D.; Walters, D.E.; Mueller, D.M. Oligomycin frames a common drug-binding site in the ATP synthase. Proc. Natl. Acad. Sci. USA 2012, 109, 13961–13965. [Google Scholar] [CrossRef] [PubMed]
- Niggemann, J.; Bedorf, N.; Flörke, U.; Steinmetz, H.; Gerth, K.; Reichenbach, H.; Höfle, G. Spirangien A and B, highly cytotoxic and antifungal spiroketals from the myxobacterium Sorangium cellulosum: Isolation, structure elucidation and chemical modifications. Eur. J. Org. Chem. 2005, 2005, 5013–5018. [Google Scholar] [CrossRef]
- Hasenbohler, A.; Kneifel, H.; Konig, W.A.; Zahner, H.; Zeiler, H.J. Metabolic products of microorganisms. 134. Stenothricin, a new inhibitor of the bacterial cell wall synthesis (author’s transl). Arch. Microbiol. 1974, 99, 307–321. [Google Scholar]
- Habibi, D.; Ogloff, N.; Jalili, R.B.; Yost, A.; Weng, A.P.; Ghahary, A.; Ong, C.J. Borrelidin, a small molecule nitrile-containing macrolide inhibitor of threonyl-tRNA synthetase, is a potent inducer of apoptosis in acute lymphoblastic leukemia. Investig. New Drugs 2012, 30, 1361–1370. [Google Scholar] [CrossRef]
- Olano, C.; Wilkinson, B.; Sanchez, C.; Moss, S.J.; Sheridan, R.; Math, V.; Weston, A.J.; Brana, A.F.; Martin, C.J.; Oliynyk, M.; et al. Biosynthesis of the angiogenesis inhibitor borrelidin by Streptomyces parvulus Tu4055: Cluster analysis and assignment of functions. Chem. Biol. 2004, 11, 87–97. [Google Scholar] [PubMed]
- Kataoka, T.; Yamada, A.; Bando, M.; Honma, T.; Mizoue, K.; Nagai, K. FD-891, a structural analogue of concanamycin A that does not affect vacuolar acidification or perforin activity, yet potently prevents cytotoxic T lymphocyte-mediated cytotoxicity through the blockage of conjugate formation. Immunology 2000, 100, 170–177. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, J.; Zhu, J.; Chen, S.; Bai, L.; Zhou, X.; Wu, H.; Deng, Z. Incomplete beta-ketone processing as a mechanism for polyene structural variation in the FR-008/candicidin complex. Chem. Biol. 2008, 15, 629–638. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vetcher, L.; Menzella, H.G.; Kudo, T.; Motoyama, T.; Katz, L. The antifungal polyketide ambruticin targets the HOG pathway. Antimicrob. Agents Chemother. 2007, 51, 3734–3736. [Google Scholar] [CrossRef]
- Chen, I.A.; Chu, K.; Palaniappan, K.; Pillay, M.; Ratner, A.; Huang, J.; Huntemann, M.; Varghese, N.; White, J.R.; Seshadri, R.; et al. IMG/M v.5.0: An integrated data management and comparative analysis system for microbial genomes and microbiomes. Nucleic Acids Res. 2019, 47, D666–D677. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.R. Cytochrome P450 nomenclature. Methods Mol. Biol. (Clifton N.J.) 1998, 107, 15–24. [Google Scholar]
- Nelson, D.R. Cytochrome P450 nomenclature, 2004. Methods Mol. Biol. (Clifton N.J.) 2006, 320, 1–10. [Google Scholar]
- Nelson, D.R.; Kamataki, T.; Waxman, D.J.; Guengerich, F.P.; Estabrook, R.W.; Feyereisen, R.; Gonzalez, F.J.; Coon, M.J.; Gunsalus, I.C.; Gotoh, O.; et al. The P450 superfamily: Update on new sequences, gene mapping, accession numbers, early trivial names of enzymes, and nomenclature. DNA Cell Biol. 1993, 12, 1–51. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Kuma, K.; Toh, H.; Miyata, T. MAFFT version 5: Improvement in accuracy of multiple sequence alignment. Nucleic Acids Res. 2005, 33, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Boc, A.; Diallo, A.B.; Makarenkov, V. T-REX: A web server for inferring, validating and visualizing phylogenetic trees and networks. Nucleic Acids Res. 2012, 40, W573–W579. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive tree of life (iTOL) v3: An online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 2016, 44, W242–W245. [Google Scholar] [CrossRef]
- McWilliam, H.; Li, W.; Uludag, M.; Squizzato, S.; Park, Y.M.; Buso, N.; Cowley, A.P.; Lopez, R. Analysis tool web services from the EMBL-EBI. Nucleic Acids Res. 2013, 41, W597–W600. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Blin, K.; Pascal Andreu, V.; de Los Santos, E.L.C.; Del Carratore, F.; Lee, S.Y.; Medema, M.H.; Weber, T. The antiSMASH database version 2: A comprehensive resource on secondary metabolite biosynthetic gene clusters. Nucleic Acids Res. 2019, 47, D625–D630. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).