Skullcapflavone II Inhibits Degradation of Type I Collagen by Suppressing MMP-1 Transcription in Human Skin Fibroblasts
Abstract
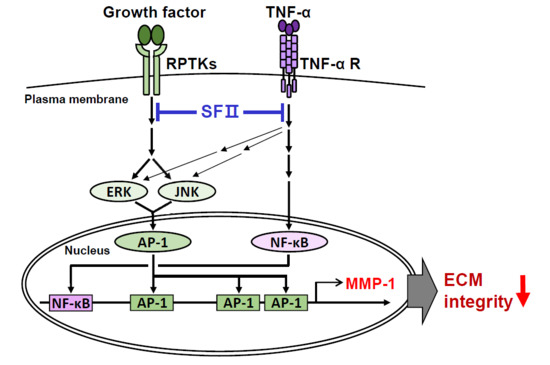
:1. Introduction
2. Results
2.1. Skullcapflavone II Decreased MMP-1 Expression in Foreskin Fibroblasts
2.2. Skullcapflavone II Decreased Transcription of the MMP-1 Gene in Foreskin Fibroblasts
2.3. Skullcapflavone II Inhibited MMP-1 Expression by Blocking the Activation of Activator Protein-1 (AP-1)
2.4. Skullcapflavone II Inhibited Tumor Necrosis Factor (TNF)-α-Induced MMP-1 Expression by Blocking Activation of NF-κB
2.5. Skullcapflavone II Decreased the Breakdown of Type I Collagen in 3D Culture of Foreskin Fibroblasts
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Cloning of the Human MMP-1 Promoter in a Reporter Plasmid
4.3. Cell Culture
4.4. RNA Isolation and Reverse Transcription (RT)-PCR Analysis
4.5. Preparation of Conditioned Media and Cell Lysates, and Western Blot Analysis
4.6. Cell Growth Assay
4.7. Flow Cytometry
4.8. Dual-Luciferase Reporter Assay
4.9. Collagenolysis in 3D Culture, Confocal Fluorescence Microscopy, and Image Acquisition
4.10. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 3D | three-dimensional |
| 7-AAD | 7-aminoactinomycin D |
| AP-1 | activator protein-1 |
| cAMP | cyclic cyclic adenosine monophosphate |
| DMEM | Dulbecco’s Modified Eagle’s medium |
| DMSO | dimethyl sulfoxide |
| EGF | epidermal growth factor |
| ERK | extracellular signal-regulated kinase |
| FBS | fetal bovine serum |
| FITC | fluorescein isothiocyanate |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase |
| JNK | c-Jun N-terminal kinase |
| LDLR | low-density-lipoprotein receptor |
| MAPK | mitogen-activated protein kinase |
| MMP | matrix metalloproteinase |
| MTT | 3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NF-κB | nuclear factor kappa light chain enhancer of activated B cells |
| pN-ColIα1 | pro-collagen α1(I) N-propeptide |
| RIPA | radio-immunoprecipitation assay |
| ROS | reactive oxygen species |
| TNF | tumor necrosis factor |
References
- Kimura, Y.; Okuda, H.; Ogita, Z. Effects of flavonoids isolated from Scutellariae radix on fibrinolytic system induced by trypsin in human umbilical vein endothelial cells. J. Nat. Prod. 1997, 60, 598–601. [Google Scholar] [CrossRef]
- Tayarani-Najarani, Z.; Asili, J.; Parsaee, H.; Mousavi, S.H.; Mashhadian, N.V.; Mirzaee, A.; Emami, S.A. Wogonin and neobaicalein from Scutellaria litwinowii roots are apoptotic for HeLa cells. Rev. Bras. Farmacogn. 2012, 22, 268–276. [Google Scholar] [CrossRef]
- Boozari, M.; Mohammadi, A.; Asili, J.; Emami, S.A.; Tayarani-Najaran, Z. Growth inhibition and apoptosis induction by Scutellaria pinnatifida A. Ham. on HL-60 and K562 leukemic cell lines. Environ. Toxicol. Pharmacol. 2015, 39, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.Y.; Ahn, K.S.; Park, M.J.; Kwon, O.K.; Lee, H.K.; Oh, S.R. Skullcapflavone II inhibits ovalbumin-induced airway inflammation in a mouse model of asthma. Int. Immunopharmacol. 2012, 12, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Son, H.S.; Lee, H.I.; Lee, G.R.; Jo, Y.J.; Hong, S.E.; Kim, N.; Kwon, M.; Kim, N.Y.; Kim, H.J.; et al. Skullcapflavone II inhibits osteoclastogenesis by regulating reactive oxygen species and attenuates the survival and resorption function of osteoclasts by modulating integrin signaling. FASEB J. 2019, 33, 2026–2036. [Google Scholar] [CrossRef] [PubMed]
- Bonham, M.; Posakony, J.; Coleman, I.; Montgomery, B.; Simon, J.; Nelson, P.S. Characterization of chemical constituents in Scutellaria baicalensis with antiandrogenic and growth-inhibitory activities toward prostate carcinoma. Clin. Cancer Res. 2005, 11, 3905–3914. [Google Scholar] [CrossRef] [PubMed]
- Nhoek, P.; Chae, H.S.; Masagalli, J.N.; Mailar, K.; Pel, P.; Kim, Y.M.; Choi, W.J.; Chin, Y.W. Discovery of flavonoids from Scutellaria baicalensis with inhibitory activity against PCSK 9 expression: Isolation, synthesis and their biological evaluation. Molecules 2018, 23, 504. [Google Scholar] [CrossRef]
- Kafienah, W.; Buttle, D.J.; Burnett, D.; Hollander, A.P. Cleavage of native type I collagen by human neutrophil elastase. Biochem. J. 1998, 330, 897–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gelse, K.; Poschl, E.; Aigner, T. Collagens-structure, function, and biosynthesis. Adv. Drug. Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.W.; Buehler, M.J. Molecular biomechanics of collagen molecules. Materials Today 2014, 17, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.A.; Quan, T.; Voorhees, J.J.; Fisher, G.J. Extracellular matrix regulation of fibroblast function: Redefining our perspective on skin aging. J. Cell Commun. Signal 2018, 12, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fanjul-Fernandez, M.; Folgueras, A.R.; Cabrera, S.; Lopez-Otin, C. Matrix metalloproteinases: Evolution, gene regulation and functional analysis in mouse models. Biochim. Biophys. Acta 2010, 1803, 3–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, F.; Wisithphrom, K.; Zhou, J.; Windsor, L.J. Matrix metalloproteinase dependent and independent collagen degradation. Front. Biosci. 2006, 11, 3100–3120. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Cho, S.; Chung, J.H.; Hammerberg, C.; Fisher, G.J.; Voorhees, J.J. Inflammation and extracellular matrix degradation mediated by activated transcription factors nuclear factor-kappaB and activator protein-1 in inflammatory acne lesions in vivo. Am. J. Pathol. 2005, 166, 1691–1699. [Google Scholar] [CrossRef]
- Ivarsson, M.; McWhirter, A.; Borg, T.K.; Rubin, K. Type I collagen synthesis in cultured human fibroblasts: Regulation by cell spreading, platelet-derived growth factor and interactions with collagen fibers. Matrix Biol. 1998, 16, 409–425. [Google Scholar] [CrossRef]
- Lindner, D.; Zietsch, C.; Becher, P.M.; Schulze, K.; Schultheiss, H.P.; Tschope, C.; Westermann, D. Differential expression of matrix metalloproteases in human fibroblasts with different origins. Biochem. Res. Int. 2012, 2012, 875742. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Liu, C.; Li, X.; Chen, W.; He, R.; Wang, X.; Feng, P.; Lan, W. Induction of matrix metalloproteinase-1 by tumor necrosis factor-alpha is mediated by interleukin-6 in cultured fibroblasts of keratoconus. Exp. Biol. Med. (Maywood) 2016, 241, 2033–2041. [Google Scholar] [CrossRef]
- Wilhelm, S.M.; Eisen, A.Z.; Teter, M.; Clark, S.D.; Kronberger, A.; Goldberg, G. Human fibroblast collagenase: Glycosylation and tissue-specific levels of enzyme synthesis. Proc. Natl. Acad. Sci. USA 1986, 83, 3756–3760. [Google Scholar] [CrossRef]
- Cortez, D.M.; Feldman, M.D.; Mummidi, S.; Valente, A.J.; Steffensen, B.; Vincenti, M.; Barnes, J.L.; Chandrasekar, B. IL-17 stimulates MMP-1 expression in primary human cardiac fibroblasts via p38 MAPK- and ERK1/2-dependent C/EBP-beta, NF-kappaB, and AP-1 activation. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H3356–H3365. [Google Scholar] [CrossRef] [PubMed]
- Vincenti, M.P.; Brinckerhoff, C.E. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: Integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. 2002, 4, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Hozawa, S.; Nakamura, T.; Nakano, M.; Adachi, M.; Tanaka, H.; Takahashi, Y.; Tetsuya, M.; Miyata, N.; Soma, H.; Hibi, T. Induction of matrix metalloproteinase-1 gene transcription by tumour necrosis factor alpha via the p50/p50 homodimer of nuclear factor-kappa B in activated human hepatic stellate cells. Liver Int. 2008, 28, 1418–1425. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.K.; Wang, C.C.; Huang, S.; Lee, J.J.; Chiang, C.P.; Lan, W.H.; Hong, C.Y. Induction of dental pulp fibroblast matrix metalloproteinase-1 and tissue inhibitor of metalloproteinase-1 gene expression by interleukin-1alpha and tumor necrosis factor-alpha through a prostaglandin-dependent pathway. J. Endod. 2001, 27, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Jung, Y.; Choi, Y.M.; Li, S. Effects of Er-Miao-San extracts on TNF-alpha-induced MMP-1 expression in human dermal fibroblasts. Biol. Res. 2015, 48, 8. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; May, M.J.; Kopp, E.B. NF-kappa B and Rel proteins: Evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 1998, 16, 225–260. [Google Scholar] [CrossRef] [PubMed]
- Delazar, A.; Nazemiyeh, H.; Afshar, F.H.; Barghi, N.; Esnaashari, S.; Asgharian, P. Chemical compositions and biological activities of Scutellaria pinnatifida A. Hamilt aerial parts. Res. Pharm. Sci. 2017, 12, 187–195. [Google Scholar] [PubMed]
- Shang, X.; He, X.; He, X.; Li, M.; Zhang, R.; Fan, P.; Zhang, Q.; Jia, Z. The genus Scutellaria an ethnopharmacological and phytochemical review. J. Ethnopharmacol. 2010, 128, 279–313. [Google Scholar] [CrossRef]
- Benbow, U.; Brinckerhoff, C.E. The AP-1 site and MMP gene regulation: What is all the fuss about? Matrix Biol. 1997, 15, 519–526. [Google Scholar] [CrossRef]
- Wang, Y.P.; Liu, I.J.; Chiang, C.P.; Wu, H.C. Astrocyte elevated gene-1 is associated with metastasis in head and neck squamous cell carcinoma through p65 phosphorylation and upregulation of MMP1. Mol. Cancer 2013, 12, 109. [Google Scholar] [CrossRef]
- Rowan, A.D.; Young, D.A. Collagenase gene regulation by pro-inflammatory cytokines in cartilage. Front. Biosci. 2007, 12, 536–550. [Google Scholar] [CrossRef] [PubMed]
- Shaulian, E.; Karin, M. AP-1 in cell proliferation and survival. Oncogene 2001, 20, 2390–2400. [Google Scholar] [CrossRef] [Green Version]
- Trop-Steinberg, S.; Azar, Y. AP-1 expression and its clinical relevance in immune eisorders and cancer. Am. J. Med. Sci. 2017, 353, 474–483. [Google Scholar] [CrossRef]
- Pardo, A.; Selman, M. MMP-1: The elder of the family. Int. J. Biochem. Cell Biol. 2005, 37, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Barchowsky, A.; Frleta, D.; Vincenti, M.P. Integration of the NF-kappaB and mitogen-activated protein kinase/AP-1 pathways at the collagenase-1 promoter: Divergence of IL-1 and TNF-dependent signal transduction in rabbit primary synovial fibroblasts. Cytokine 2000, 12, 1469–1479. [Google Scholar] [CrossRef] [PubMed]
- Gloire, G.; Legrand-Poels, S.; Piette, J. NF-kappaB activation by reactive oxygen species: Fifteen years later. Biochem. Pharmacol. 2006, 72, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Volanti, C.; Matroule, J.Y.; Piette, J. Involvement of oxidative stress in NF-kappaB activation in endothelial cells treated by photodynamic therapy. Photochem. Photobiol. 2002, 75, 36–45. [Google Scholar] [CrossRef]
- Schreck, R.; Rieber, P.; Baeuerle, P.A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991, 10, 2247–2258. [Google Scholar] [CrossRef]
- Kamata, H.; Manabe, T.; Oka, S.; Kamata, K.; Hirata, H. Hydrogen peroxide activates IkappaB kinases through phosphorylation of serine residues in the activation loops. FEBS Lett. 2002, 519, 231–237. [Google Scholar] [CrossRef]
- Takada, Y.; Mukhopadhyay, A.; Kundu, G.C.; Mahabeleshwar, G.H.; Singh, S.; Aggarwal, B.B. Hydrogen peroxide activates NF-kappa B through tyrosine phosphorylation of I kappa B alpha and serine phosphorylation of p65: Evidence for the involvement of I kappa B alpha kinase and Syk protein-tyrosine kinase. J. Biol. Chem. 2003, 278, 24233–24241. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef]
- Qin, Z.; Robichaud, P.; He, T.; Fisher, G.J.; Voorhees, J.J.; Quan, T. Oxidant exposure induces cysteine-rich protein 61 (CCN1) via c-Jun/AP-1 to reduce collagen expression in human dermal fibroblasts. PLoS ONE 2014, 9, e115402. [Google Scholar] [CrossRef]
- Knebel, A.; Rahmsdorf, H.J.; Ullrich, A.; Herrlich, P. Dephosphorylation of receptor tyrosine kinases as target of regulation by radiation, oxidants or alkylating agents. EMBO J. 1996, 15, 5314–5325. [Google Scholar] [CrossRef]
- Giannoni, E.; Buricchi, F.; Raugei, G.; Ramponi, G.; Chiarugi, P. Intracellular reactive oxygen species activate Src tyrosine kinase during cell adhesion and anchorage-dependent cell growth. Mol. Cell. Biol. 2005, 25, 6391–6403. [Google Scholar] [CrossRef]
- Natarajan, V.; Scribner, W.M.; al-Hassani, M.; Vepa, S. Reactive oxygen species signaling through regulation of protein tyrosine phosphorylation in endothelial cells. Environ. Health Perspect. 1998, 106, 1205–1212. [Google Scholar]
- Amar, S.; Smith, L.; Fields, G.B. Matrix metalloproteinase collagenolysis in health and disease. Biochim. Biophys. Acta. Mol. Cell Res. 2017, 1864, 1940–1951. [Google Scholar] [CrossRef]
- Orgaz, J.L.; Pandya, P.; Dalmeida, R.; Karagiannis, P.; Sanchez-Laorden, B.; Viros, A.; Albrengues, J.; Nestle, F.O.; Ridley, A.J.; Gaggioli, C.; et al. Diverse matrix metalloproteinase functions regulate cancer amoeboid migration. Nat. Commun. 2014, 5, 4255. [Google Scholar] [CrossRef]
- Lagoutte, E.; Villeneuve, C.; Lafanechere, L.; Wells, C.M.; Jones, G.E.; Chavrier, P.; Rosse, C. LIMK regulates tumor-cell invasion and matrix degradation through tyrosine phosphorylation of MT1-MMP. Sci. Rep. 2016, 6, 24925. [Google Scholar] [CrossRef]
- Lim, H.; Kim, H.P. Inhibition of mammalian collagenase, matrix metalloproteinase-1, by naturally-occurring flavonoids. Planta Med. 2007, 73, 1267–1274. [Google Scholar] [CrossRef]
- Hwang, Y.P.; Oh, K.N.; Yun, H.J.; Jeong, H.G. The flavonoids apigenin and luteolin suppress ultraviolet A-induced matrix metalloproteinase-1 expression via MAPKs and AP-1-dependent signaling in HaCaT cells. J. Dermatol. Sci. 2011, 61, 23–31. [Google Scholar] [CrossRef]
- Gan, L.S.; Hsyu, P.H.; Pritchard, J.F.; Thakker, D. Mechanism of intestinal absorption of ranitidine and ondansetron: Transport across Caco-2 cell monolayers. Pharm. Res. 1993, 10, 1722–1725. [Google Scholar] [CrossRef]
- Li, S.M.; Pan, M.H.; Lo, C.Y.; Tan, D.; Wang, Y.; Shahidi, F.; Ho, C.T. Chemistry and health effects of polymethoxyflavones and hydroxylated polymethoxyflavones. J. Funct. Foods 2009, 1, 2–12. [Google Scholar] [CrossRef]
- Kim, E.J.; Kim, Y.K.; Kim, M.K.; Kim, S.; Kim, J.Y.; Lee, D.H.; Chung, J.H. UV-induced inhibition of adipokine production in subcutaneous fat aggravates dermal matrix degradation in human skin. Sci. Rep. 2016, 6, 25616. [Google Scholar] [CrossRef] [Green Version]
- Shin, W.S.; Hong, Y.; Lee, H.W.; Lee, S.T. Catalytically defective receptor protein tyrosine kinase PTK7 enhances invasive phenotype by inducing MMP-9 through activation of AP-1 and NF-kappaB in esophageal squamous cell carcinoma cells. Oncotarget 2016, 7, 73242–73256. [Google Scholar] [CrossRef]
- Shin, W.S.; Na, H.W.; Lee, S.T. Biphasic effect of PTK7 on KDR activity in endothelial cells and angiogenesis. Biochim. Biophys. Acta 2015, 1853, 2251–2260. [Google Scholar] [CrossRef] [Green Version]
- Shin, W.S.; Gim, J.; Won, S.; Lee, S.T. Biphasic regulation of tumorigenesis by PTK7 expression level in esophageal squamous cell carcinoma. Sci. Rep. 2018, 8, 8519. [Google Scholar] [CrossRef]
- Lee, Y.H.; Park, J.H.; Cheon, D.H.; Kim, T.; Park, Y.E.; Oh, E.S.; Lee, J.E.; Lee, S.T. Processing of syndecan-2 by matrix metalloproteinase-14 and effect of its cleavage on VEGF-induced tube formation of HUVECs. Biochem. J. 2017, 474, 3719–3732. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.H.; Seo, E.K.; Lee, S.-T. Skullcapflavone II Inhibits Degradation of Type I Collagen by Suppressing MMP-1 Transcription in Human Skin Fibroblasts. Int. J. Mol. Sci. 2019, 20, 2734. https://doi.org/10.3390/ijms20112734
Lee YH, Seo EK, Lee S-T. Skullcapflavone II Inhibits Degradation of Type I Collagen by Suppressing MMP-1 Transcription in Human Skin Fibroblasts. International Journal of Molecular Sciences. 2019; 20(11):2734. https://doi.org/10.3390/ijms20112734
Chicago/Turabian StyleLee, Young Hun, Eun Kyoung Seo, and Seung-Taek Lee. 2019. "Skullcapflavone II Inhibits Degradation of Type I Collagen by Suppressing MMP-1 Transcription in Human Skin Fibroblasts" International Journal of Molecular Sciences 20, no. 11: 2734. https://doi.org/10.3390/ijms20112734
APA StyleLee, Y. H., Seo, E. K., & Lee, S.-T. (2019). Skullcapflavone II Inhibits Degradation of Type I Collagen by Suppressing MMP-1 Transcription in Human Skin Fibroblasts. International Journal of Molecular Sciences, 20(11), 2734. https://doi.org/10.3390/ijms20112734