Antimicrobial Polymers: The Potential Replacement of Existing Antibiotics?
Abstract
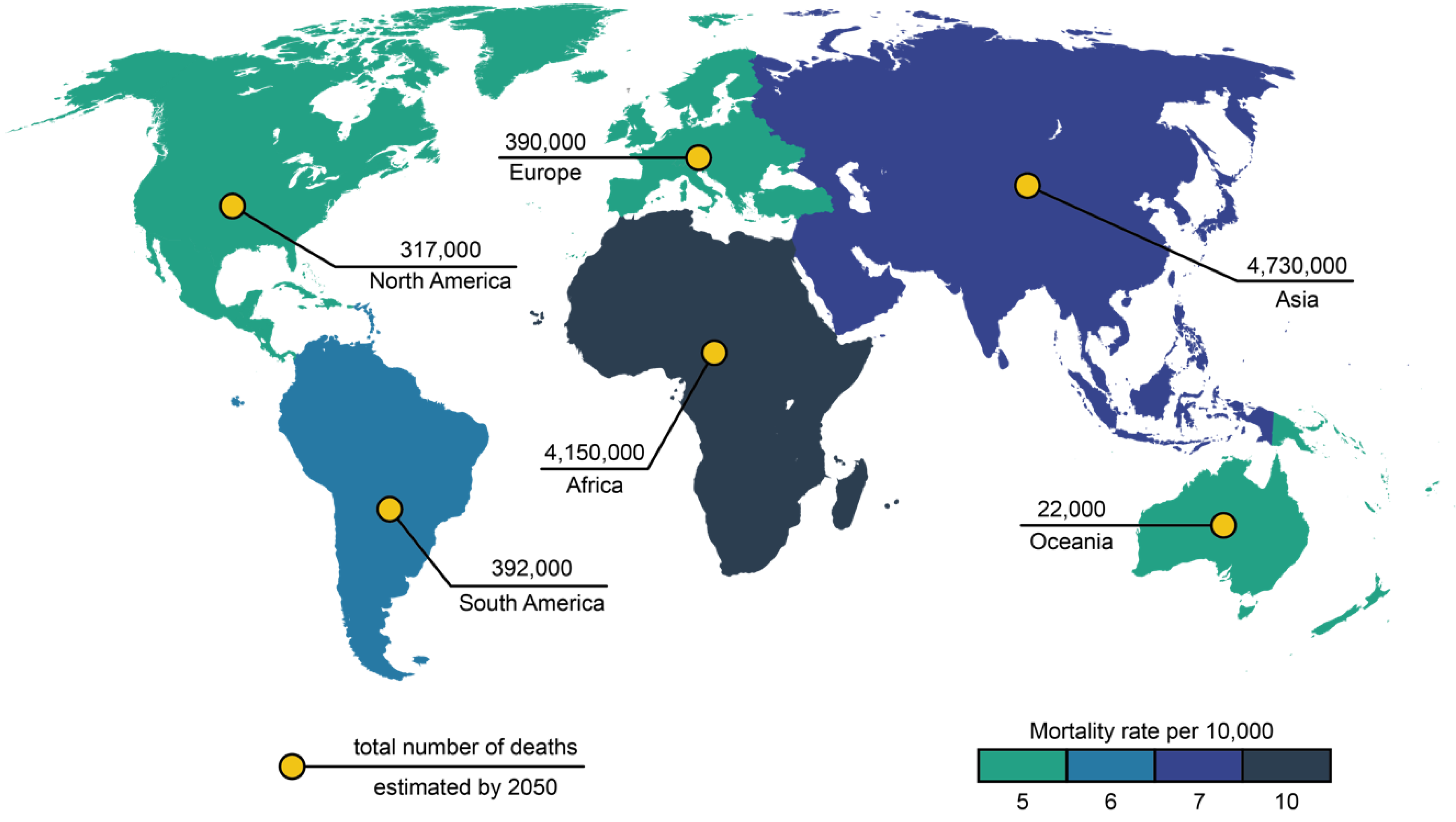
:1. Introduction
2. ESKAPE (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp.) pathogens
2.1. Enterococcus faecium
2.2. Staphylococcus aureus
2.3. Pseudomonas aeruginosa
2.4. Klebsiella pneumoniae
2.5. Acinetobacter baumnannii
2.6. Enterobacter spp.
3. The Progress in Antimicrobial Development
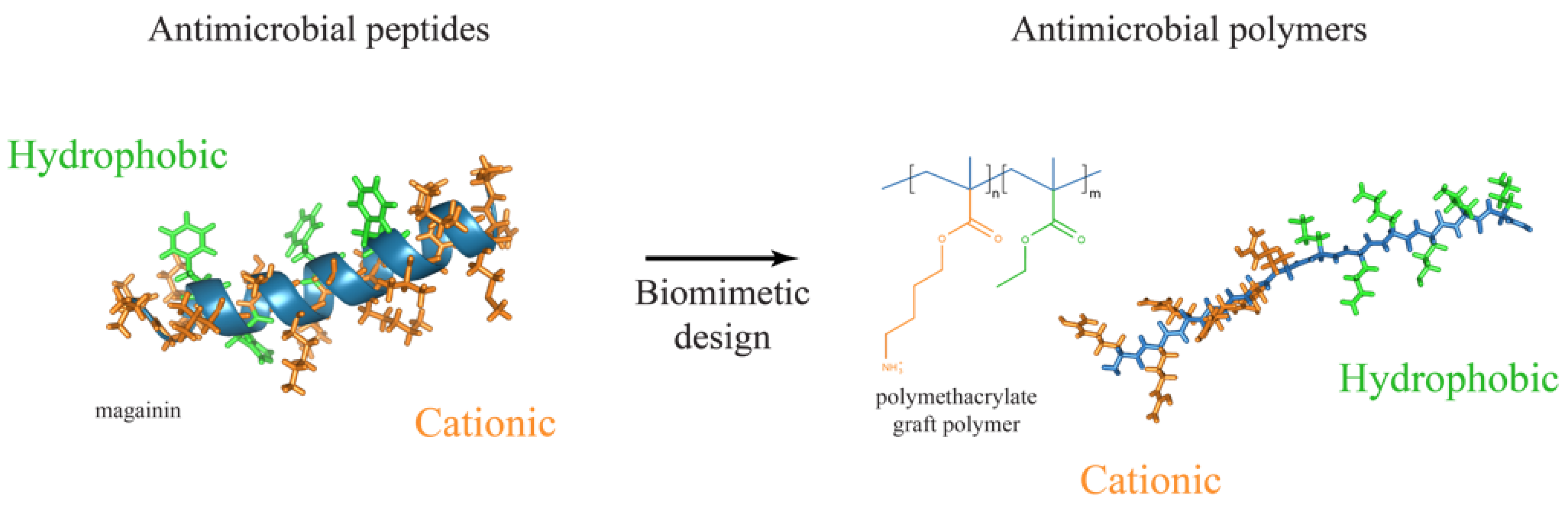
4. Antimicrobial Polymers Are the New Generation of Antimicrobials
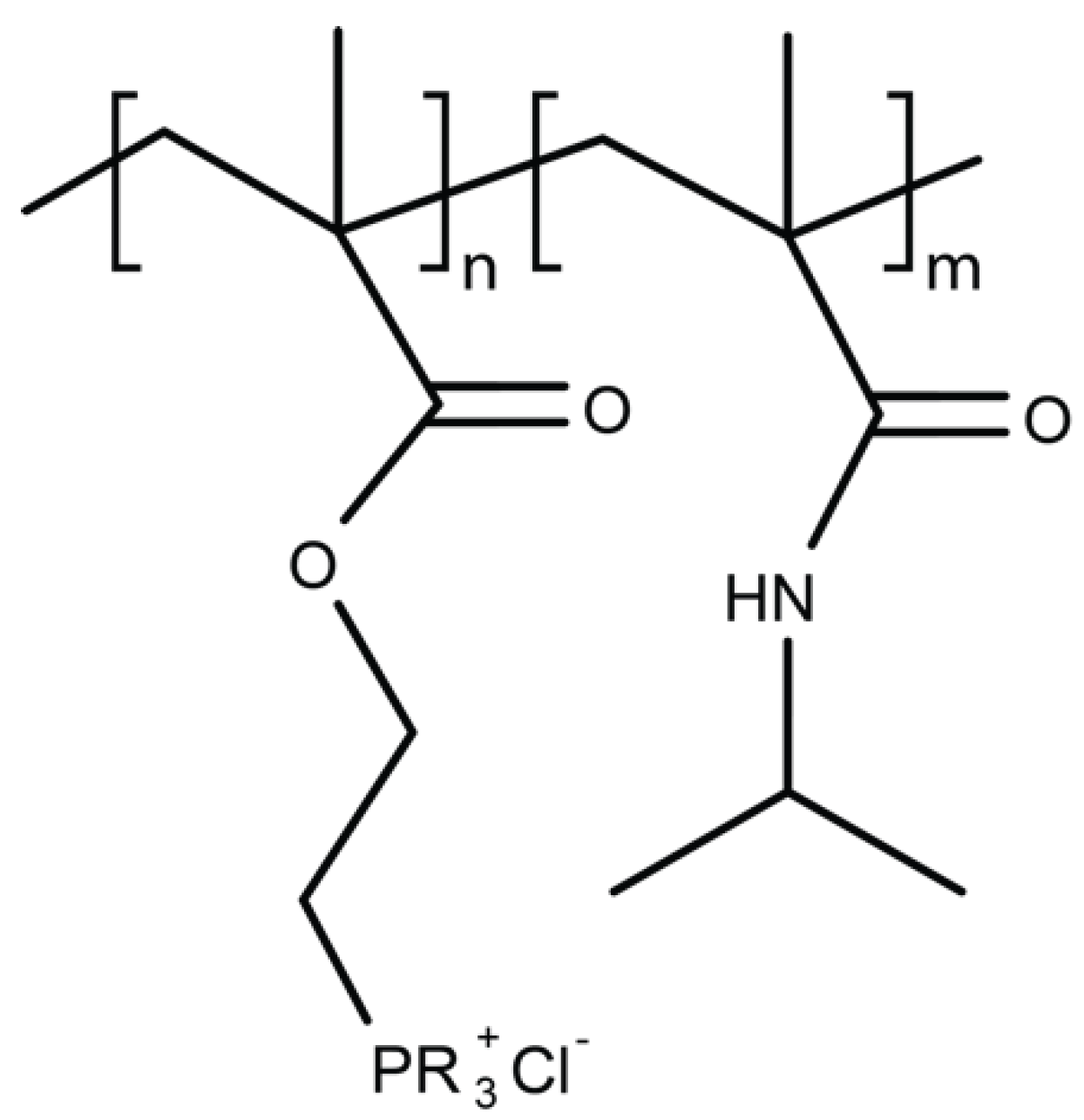
4.1. Amphiphilic Antibacterial Polymers
4.2. Polymers Containing Natural Peptides
4.3. Halogen-Containing Polymers
4.4. Polymers Containing Phosphor and Sulfo Derivatives
4.5. Phenol and Benzoic Derivative Polymers
4.6. Organometallic Polymers
4.7. Metal Nanoparticles Included in Polymeric Carriers
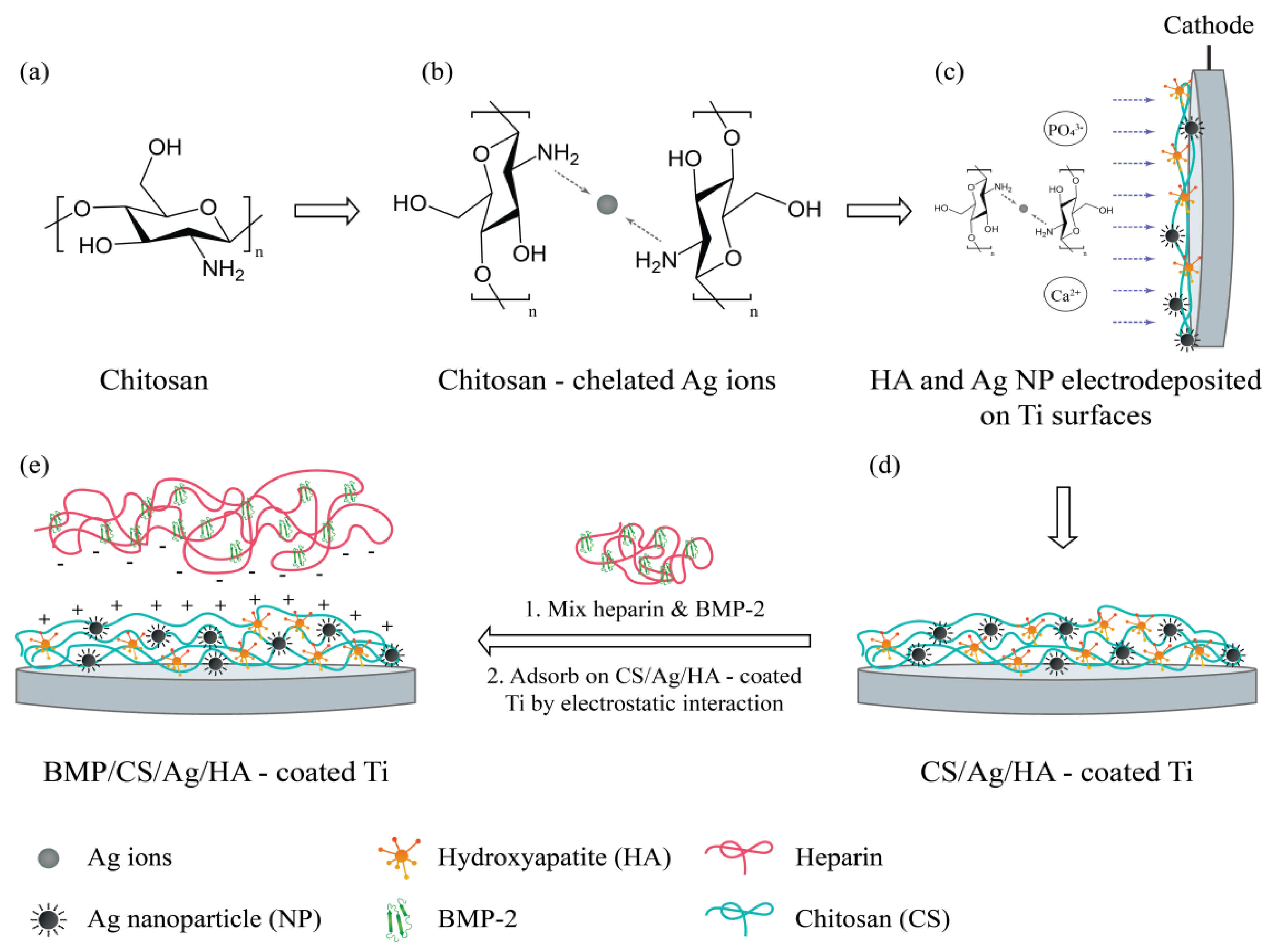
4.7.1. Polymeric Systems Containing Silver Nanoparticles
4.7.2. Nanofiber Systems Containing Silver Nanoparticles
4.7.3. Hydrogels Containing Silver Nanoparticles
4.7.4. Inclusion of Other Metal Nanoparticles
4.7.5. Inclusion of Titanium Dioxide and Zinc Oxide
4.8. Dendrimers
4.9. Polymer-Based Guanidine
5. Challenges in Bringing Antimicrobial Polymers into Clinics
6. Conclusions and Future Considerations
Author Contributions
Funding
Conflicts of Interest
References
- WHO High Levels of Antibiotic Resistance Found Worldwide, New Data Shows. WHO, News Release. Available online: https://www.who.int/mediacentre/news/releases/2018/antibiotic-resistance-found/en/ (accessed on 29 January 2018).
- O’Neill, J. Antimicrobial Resistance: Tackling a crisis for the health and wealth of nations. Rev. Antimicrob. Resist. 2014, 1, 1–16. [Google Scholar]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef]
- Bjarnsholt, T. The role of bacterial biofilms in chronic infections. APMIS 2013, 121, 1–58. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, S.V.; Dhanasekar, U. Trends in antibiotic resistance pattern against ESKAPE pathogens from tertiary care teaching institute – South India. Infect. Dis. Heal. 2016, 21, 142–143. [Google Scholar] [CrossRef]
- Mohamed, J.A.; Huang, D.B. Biofilm formation by enterococci. J. Med. Microbiol. 2007, 56, 1581–1588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, W.; Howden, B.P.; Stinear, T.P. Evolution of virulence in Enterococcus faecium, a hospital-adapted opportunistic pathogen. Curr. Opin. Microbiol. 2018, 41, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Al-Talib, H.; Yean, C.; Al-Jashamy, K.; Hasan, H. Methicillin-resistant Staphylococcus aureus nosocomial infection trends in Hospital Universiti Sains Malaysia during 2002–2007. Ann. Saudi Med. 2010, 30, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Harch, S.A.J.; MacMorran, E.; Tong, S.Y.C.; Holt, D.C.; Wilson, J.; Athan, E.; Hewagama, S. High burden of complicated skin and soft tissue infections in the Indigenous population of Central Australia due to dominant Panton Valentine leucocidin clones ST93-MRSA and CC121-MSSA. BMC Infect. Dis. 2017, 17, 405. [Google Scholar] [CrossRef] [PubMed]
- Enright, M.C.; Robinson, D.A.; Randle, G.; Feil, E.J.; Grundmann, H.; Spratt, B.G. The evolutionary history of methicillin-resistant Staphylococcus aureus (MRSA). Proc. Natl. Acad. Sci. USA 2002, 99, 7687–7692. [Google Scholar] [CrossRef] [PubMed]
- Macmorran, E.; Harch, S.; Athan, E.; Lane, S.; Tong, S.; Crawford, L.; Krishnaswamy, S.; Hewagama, S. The rise of methicillin resistant Staphylococcus aureus: Now the dominant cause of skin and soft tissue infection in Central Australia. Epidemiol. Infect. 2017, 145, 2817–2826. [Google Scholar] [CrossRef]
- Malani, P.N. National burden of invasive methicillin-resistant Staphylococcus aureus infection. Jama 2014, 311, 1438–1439. [Google Scholar] [CrossRef] [PubMed]
- Sisirak, M.; Zvizdic, A.; Hukic, M. Methicillin-resistant Staphylococcus aureus (MRSA) as a cause of nosocomial wound infections. Bosn J. Basic Med. Sci. 2010, 10, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Zhang, X.D.; Zhao, Q.; Peng, B.; Zheng, J. Analysis of global prevalence of antibiotic resistance in Acinetobacter baumannii infections disclosed a faster increase in OECD countries. Emerg. Microbes Infect. 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Olaitan, A.O.; Morand, S.; Rolain, J.M. Emergence of colistin-resistant bacteria in humans without colistin usage: A new worry and cause for vigilance. Int. J. Antimicrob. Agents 2016, 47, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Girardello, R.; Cury, A.P.; Di Gioia, T.S.R.; de Almeida, J.N.; Duarte, A.J.D.S. Emergence of colistin resistance in the largest university hospital complex of São Paulo, Brazil, over five years. Brazilian J. Infect. Dis. 2017, 21, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Klein, E.Y.; Laxminarayan, R. Seasonality and temporal correlation between community antibiotic use and resistance in the United States. Clin. Infect. Dis. 2012, 55, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Sundqvist, M. Reversibility of antibiotic resistance. Ups. J. Med. Sci. 2014, 119, 142–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biotech Connection. Trends and Opportunities in Antibiotic Development for Multidrug-Resistant Bacterial Infections. 2018. Available online: https://biotechconnectionbay.org/market-reports/antibioticdevelopment/ (accessed on 1 March 2019).
- Kamaruzzaman, N.; Pina, M.; Chivu, A.; Good, L. Polyhexamethylene biguanide and nadifloxacin self-assembled nanoparticles: Antimicrobial effects against intracellular methicillin-resistant Staphylococcus aureus. Polymers 2018, 10, 521. [Google Scholar] [CrossRef] [PubMed]
- Weiner, L.M.; Webb, A.K.; Limbago, B.; Dudeck, M.A.; Patel, J.; Kallen, A.J.; Edwards, J.R.; Sievert, D.M. Antimicrobial-Resistant Pathogens Associated With Healthcare-Associated Infections: Summary of Data Reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011-2014. Infect. Control. Hosp. Epidemiol. 2016, 37, 1288–1301. [Google Scholar] [CrossRef]
- Kamaruzzaman, N.F.; Firdessa, R.; Good, L. Bactericidal effects of polyhexamethylene biguanide against intracellualar Staphylococcus aureus EMRSA-15 and USA 300. J. Antimicrob. Chemother. 2016, 71, 1252–1259. [Google Scholar] [CrossRef]
- Ruparelia, J.P.; Chatterjee, A.K.; Duttagupta, S.P.; Mukherji, S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 2008, 4, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Golan, Y. Empiric therapy for hospital-acquired, Gram-negative complicated intra-abdominal infection and complicated urinary tract infections: A systematic literature review of current and emerging treatment options. BMC Infect. Dis. 2015, 15, 313. [Google Scholar] [CrossRef] [PubMed]
- Cornejo-Juárez, P.; Vilar-Compte, D.; Pérez-Jiménez, C.; Ñamendys-Silva, S.A.; Sandoval-Hernández, S.; Volkow-Fernández, P. The impact of hospital-acquired infections with multidrug-resistant bacteria in an oncology intensive care unit. Int. J. Infect. Dis. 2015, 31, e31–e34. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Chaftari, A.M.; El Zakhem, A.; Jamal, M.A.; Jiang, Y.; Hachem, R.; Raad, I. The use of minocycline-rifampin coated central venous catheters for exchange of catheters in the setting of staphylococcus aureus central line associated bloodstream infections. BMC Infect. Dis. 2014, 14, 518. [Google Scholar] [CrossRef] [PubMed]
- McGuffie, B.A.; Vallet-Gely, I.; Dove, S.L. sigma Factor and Anti-sigma Factor That Control Swarming Motility and Biofilm Formation in Pseudomonas aeruginosa. J. Bacteriol. 2015, 198, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Solomkin, J.S.; Mazuski, J.E.; Bradley, J.S.; Rodvold, K.A.; Goldstein, E.J.; Baron, E.J.; O’Neill, P.J.; Chow, A.W.; Dellinger, E.P.; Eachempati, S.R.; et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: Guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Surg. Infect. 2010, 11, 79–109. [Google Scholar] [CrossRef] [PubMed]
- Codjoe, F.; Donkor, E. Carbapenem Resistance: A Review. Med. Sci. 2017, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Johansen, H.K.; Moskowitz, S.M.; Ciofu, O.; Pressler, T.; Høiby, N. Spread of colistin resistant non-mucoid Pseudomonas aeruginosa among chronically infected Danish cystic fibrosis patients. J. Cyst. Fibros. 2008, 7, 391–397. [Google Scholar] [CrossRef]
- Fiaccadori, E.; Antonucci, E.; Morabito, S.; d’Avolio, A.; Maggiore, U.; Regolisti, G. Colistin Use in Patients With Reduced Kidney Function. Am. J. Kidney Dis. 2016, 68, 296–306. [Google Scholar] [CrossRef]
- Singla, S.; Harjai, K.; Chhibber, S. Artificial Klebsiella pneumoniae biofilm model mimicking in vivo system: Altered morphological characteristics and antibiotic resistance. J. Antibiot. 2014, 67, 305–309. [Google Scholar] [CrossRef] [PubMed]
- ECDC. Surveillance of Antimicrobial Resistance in Europe 2016; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2017; ISBN 9789294980991. [Google Scholar]
- Otter, J.A.; Doumith, M.; Davies, F.; Mookerjee, S.; Dyakova, E.; Gilchrist, M.; Brannigan, E.T.; Bamford, K.; Galletly, T.; Donaldson, H.; et al. Emergence and clonal spread of colistin resistance due to multiple mutational mechanisms in carbapenemase-producing Klebsiella pneumoniae in London. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Maragakis, L.L.; Perl, T.M. Acinetobacter baumannii: Epidemiology, antimicrobial resistance, and treatment options. Clin. Infect. Dis. 2008, 46, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Bonomo, R.A.; Szabo, D. Mechanisms of Multidrug Resistance in Acinetobacter Species and Pseudomonas aeruginosa. Clin. Infect. Dis. 2006, 44106, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Nation, R.L.; Li, J.; Cars, O.; Couet, W.; Dudley, M.N.; Kaye, K.S.; Mouton, J.W.; Paterson, D.L.; Tam, V.H.; Theuretzbacher, U.; et al. Framework for optimisation of the clinical use of colistin and polymyxin B: The Prato polymyxin consensus. Lancet Infect. Dis. 2015, 15, 225–234. [Google Scholar] [CrossRef]
- Potron, A.; Poirel, L.; Nordmann, P. Emerging broad-spectrum resistance in Pseudomonas aeruginosa and Acinetobacter baumannii: Mechanisms and epidemiology. Int. J. Antimicrob Agents 2015, 45, 568–585. [Google Scholar] [CrossRef]
- Qureshi, Z.A.; Hittle, L.E.; O’Hara, J.A.; Rivera, J.I.; Syed, A.; Shields, R.K.; Pasculle, A.W.; Ernst, R.K.; Doi, Y. Colistin-resistant Acinetobacter baumannii: Beyond carbapenem resistance. Clin. Infect. Dis. 2015, 60, 1295–1303. [Google Scholar] [CrossRef]
- Wilks, M.; Wilson, A.; Warwick, S.; Price, E.; Kennedy, D.; Ely, A.; Millar, M.R. Control of an outbreak of multidrug-resistant Acinetobacter baumannii-calcoaceticus colonization and infection in an intensive care unit (ICU) without closing the ICU or placing patients in isolation. Infect. Control. Hosp. Epidemiol. 2006, 27, 654–658. [Google Scholar] [CrossRef]
- Antunes, L.C.; Visca, P.; Towner, K.J. Acinetobacter baumannii: Evolution of a global pathogen. Pathog. Dis. 2014, 71, 292–301. [Google Scholar] [CrossRef]
- Davin-Regli, A.; Pagès, J.-M. Enterobacter aerogenes and Enterobacter cloacae; versatile bacterial pathogens confronting antibiotic treatment. Front. Microbiol. 2015, 6, 392. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wang, Y.; Zhou, Y.; Li, J.; Yin, W.; Wang, S.; Zhang, S.; Shen, J.; Shen, Z.; Wang, Y. Emergence of a novel mobile colistin resistance gene, mcr-8, in NDM-producing Klebsiella pneumoniae. Emerg. Microbes Infect. 2018, 7, 122. [Google Scholar] [CrossRef] [PubMed]
- Robinson, K.M.; Janes, M.S.; Pehar, M.; Monette, J.S.; Ross, M.F.; Hagen, T.M.; Murphy, M.P.; Beckman, J.S. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc. Natl. Acad. Sci. USA 2006, 103, 15038–15043. [Google Scholar] [CrossRef]
- Jackson, N.; Czaplewski, L.; Piddock, L.J.V. Discovery and development of new antibacterial drugs: Learning from experience? J. Antimicrob. Chemother. 2018, 73, 1452–1459. [Google Scholar] [CrossRef] [PubMed]
- Delplace, V.; Nicolas, J. Degradable vinyl polymers for biomedical applications. Nat. Chem. 2015, 7, 771–784. [Google Scholar] [CrossRef]
- Frade, S. German Government Funds GARDP’s Efforts to Discover, Develop, and Deliver Affordable Antibiotics: Funding Supports Delivery of R&D Strategy That Focuses on Treating Gram-Negative Infections. Available online: https://www.gardp.org/2018/news-resources/press-releases/german-government-funds-gardp-discover-develop-deliver-affordable-antibiotics/ (accessed on 15 March 2019).
- Jain, A.; Duvvuri, L.S.; Farah, S.; Beyth, N.; Domb, A.J.; Khan, W. Antimicrobial Polymers. Adv. Healthc. Mater. 2014, 3, 1969–1985. [Google Scholar] [CrossRef]
- Mowery, B.P.; Lee, S.E.; Kissounko, D.A.; Epand, R.F.; Epand, R.M.; Weisblum, B.; Stahl, S.S.; Gellman, S.H. Mimicry of Antimicrobial Host-Defense Peptides by Random Copolymers. J. Am. Chem. Soc. 2007, 129, 15474–15476. [Google Scholar] [CrossRef] [PubMed]
- Bechinger, B.; Gorr, S.U. Antimicrobial Peptides: Mechanisms of Action and Resistance. J. Dent. Res. 2017, 96, 254–260. [Google Scholar] [CrossRef]
- Park, A.J.; Okhovat, J.P.; Kim, J. Antimicrobial peptides. Clin. Basic Immunodermatol. Second Ed. 2017, 1548, 81–95. [Google Scholar] [CrossRef]
- Takahashi, H.; Palermo, E.F.; Yasuhara, K.; Caputo, G.A.; Kuroda, K. Molecular Design, Structures, and Activity of Antimicrobial Peptide-Mimetic Polymers. Macromol. Biosci. 2013, 13, 1285–1299. [Google Scholar] [CrossRef] [Green Version]
- Foster, L.L.; Mizutani, M.; Oda, Y.; Palermo, E.F.; Kuroda, K. Polymers for Biomedicine. John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Locock, K.E.S.; Michl, T.D.; Griesser, H.J.; Haeussler, M.; Meagher, L. Structure–activity relationships of guanylated antimicrobial polymethacrylates. Pure Appl. Chem. 2014, 86, 1281–1291. [Google Scholar] [CrossRef]
- Palermo, E.F.; Lienkamp, K.; Gillies, E.R.; Ragogna, P.J. Antibacterial Activity of Polymers: Discussions on the Nature of Amphiphilic Balance. Angew. Chem. Int. Ed. 2019, 58, 3690–3693. [Google Scholar] [CrossRef] [PubMed]
- Mankoci, S.; Kaiser, R.L.; Sahai, N.; Barton, H.A.; Joy, A. Bactericidal Peptidomimetic Polyurethanes with Remarkable Selectivity against Escherichia coli. ACS Biomater. Sci. Eng. 2017, 3, 2588–2597. [Google Scholar] [CrossRef]
- Mizutani, M.; Palermo, E.F.; Thoma, L.M.; Satoh, K.; Kamigaito, M.; Kuroda, K. Design and synthesis of self-degradable antibacterial polymers by simultaneous chain- and step-growth radical copolymerization. Biomacromolecules 2012, 13, 1554–1563. [Google Scholar] [CrossRef]
- Holden, M.T.G.; Hauser, H.; Sanders, M.; Ngo, T.H.; Cherevach, I.; Cronin, A.; Goodhead, I.; Mungall, K.; Quail, M.; Price, C.; et al. Rapid evolution of virulence and drug resistance in the emerging zoonotic pathogen Streptococcus suis. PLoS ONE 2009, 4, 6072. [Google Scholar] [CrossRef] [PubMed]
- Palermo, E.F.; Vemparala, S.; Kuroda, K. Cationic spacer arm design strategy for control of antimicrobial activity and conformation of amphiphilic methacrylate random copolymers. Biomacromolecules 2012, 13, 1632–1641. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Fei, J.; Deirmegian, C. Diagnosis of periprosthetic infection: Novel developments. J. Knee Surg. 2014, 27, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, K.; Caputo, G.A. Antimicrobial polymers as synthetic mimics of host-defense peptides. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2012, 5, 49–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Exley, S.E.; Paslay, L.C.; Sahukhal, G.S.; Abel, B.A.; Brown, T.D.; McCormick, C.L.; Heinhorst, S.; Koul, V.; Choudhary, V.; Elasri, M.O.; et al. Antimicrobial Peptide Mimicking Primary Amine and Guanidine Containing Methacrylamide Copolymers Prepared by Raft Polymerization. Biomacromolecules 2015, 16, 3845–3852. [Google Scholar] [CrossRef]
- Oda, Y.; Kanaoka, S.; Sato, T.; Aoshima, S.; Kuroda, K. Block versus random amphiphilic copolymers as antibacterial agents. Biomacromolecules 2011, 12, 3581–3591. [Google Scholar] [CrossRef]
- Burian, M.; Schittek, B. The secrets of dermcidin action. Int. J. Med. Microbiol. 2015, 305, 283–286. [Google Scholar] [CrossRef]
- McDermott, A.M. Antimicrobial compounds in tears. Exp. Eye Res. 2013, 117, 53–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz-Bonilla, A.; Fernández-García, M. Polymeric materials with antimicrobial activity. Prog. Polym. Sci. 2012, 37, 281–339. [Google Scholar] [CrossRef]
- Friedrich, C.L.; Moyles, D.; Beveridge, T.J.; Hancock, R.E.W. Antibacterial Action of Structurally Diverse Cationic Peptides on Gram-Positive Bacteria. Antimicrob. Agents Chemother. 2000, 44, 2086. [Google Scholar] [CrossRef] [PubMed]
- Le, C.-F.; Fang, C.-M.; Sekaran, S.D. Intracellular Targeting Mechanisms by Antimicrobial Peptides. Antimicrob. Agents Chemother. 2017, 61, e02340-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dartois, V.; Sanchez-Quesada, J.; Cabezas, E.; Chi, E.; Dubbelde, C.; Dunn, C.; Granja, J.; Gritzen, C.; Weinberger, D.; Ghadiri, M.R. Systemic antibacterial activity of novel synthetic cyclic peptides. Antimicrob. Agents Chemother. 2005, 49, 3302–3310. [Google Scholar] [CrossRef] [PubMed]
- Aoki, W.; Ueda, M. Characterization of Antimicrobial Peptides toward the Development of Novel Antibiotics. Pharmaceuticals 2013, 6, 1055–1081. [Google Scholar] [CrossRef] [Green Version]
- Peters, B.M.; Shirtliff, M.E.; Jabra-Rizk, M.A. Antimicrobial peptides: Primeval molecules or future drugs? PLoS Pathog. 2010, 6, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.S.; Blaskovich, M.A.T.; Cooper, M.A. Antibiotics in the clinical pipeline at the end of 2015. J. Antibiot. 2016, 70, 3. [Google Scholar] [CrossRef]
- Caillier, L.; Taffin de Givenchy, E.; Levy, R.; Vandenberghe, Y.; Geribaldi, S.; Guittard, F. Polymerizable semi-fluorinated gemini surfactants designed for antimicrobial materials. J. Colloid Interface Sci. 2009, 332, 201–207. [Google Scholar] [CrossRef]
- Lin, J.; Chen, X.; Chen, C.; Hu, J.; Zhou, C.; Cai, X.; Wang, W.; Zheng, C.; Zhang, P.; Cheng, J.; et al. Durably Antibacterial and Bacterially Antiadhesive Cotton Fabrics Coated by Cationic Fluorinated Polymers. ACS Appl. Mater. Interfaces 2018, 10, 6124–6136. [Google Scholar] [CrossRef]
- Mesallati, H.; Umerska, A.; Paluch, K.J.; Tajber, L. Amorphous Polymeric Drug Salts as Ionic Solid Dispersion Forms of Ciprofloxacin. Mol. Pharm. 2017, 14, 2209–2223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parwe, S.P.; Chaudhari, P.N.; Mohite, K.K.; Selukar, B.S.; Nande, S.S.; Garnaik, B. Synthesis of ciprofloxacin-conjugated poly (l-lactic acid) polymer for nanofiber fabrication and antibacterial evaluation. Int. J. Nanomed. 2014, 9, 1463–1477. [Google Scholar] [CrossRef]
- Kocer, H.B.; Worley, S.D.; Broughton, R.M.; Huang, T.S. A novel N-halamine acrylamide monomer and its copolymers for antimicrobial coatings. React. Funct. Polym. 2011, 71, 561–568. [Google Scholar] [CrossRef]
- Demir, B.; Broughton, R.M.; Qiao, M.; Huang, T.S.; Worley, S.D. N-halamine biocidal materials with superior antimicrobial efficacies for wound dressings. Molecules 2017, 22, 1582. [Google Scholar] [CrossRef] [PubMed]
- Cuthbert, T.J.; Hisey, B.; Harrison, T.D.; Trant, J.F.; Gillies, E.R.; Ragogna, P.J. Surprising Antibacterial Activity and Selectivity of Hydrophilic Polyphosphoniums Featuring Sugar and Hydroxy Substituents. Angew. Chem. Int. Ed. 2018, 57, 12707–12710. [Google Scholar] [CrossRef] [PubMed]
- Park, E.S.; Moon, W.S.; Song, M.J.; Kim, M.N.; Chung, K.H.; Yoon, J.S. Antimicrobial activity of phenol and benzoic acid derivatives. Int. Biodeterior. Biodegrad. 2001, 47, 209–214. [Google Scholar] [CrossRef]
- Alamri, A.; El-Newehy, M.H.; Al-Deyab, S.S. Biocidal polymers: Synthesis and antimicrobial properties of benzaldehyde derivatives immobilized onto amine-terminated polyacrylonitrile. Chem. Cent. J. 2012, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Awad, M.A.; Mekhamer, W.K.; Merghani, N.M.; Hendi, A.A.; Ortashi, K.M.O.; Al-Abbas, F.; Eisa, N.E. Green Synthesis, Characterization, and Antibacterial Activity of Silver/Polystyrene Nanocomposite. J. Nanomater. 2015, 2015. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, Y.; Vlahovic, B. A Review on Preparation and Applications of Silver-Containing Nanofibers. Nanoscale Res. Lett. 2016, 11, 80. [Google Scholar] [CrossRef]
- de Faria, A.F.; Perreault, F.; Shaulsky, E.; Arias Chavez, L.H.; Elimelech, M. Antimicrobial Electrospun Biopolymer Nanofiber Mats Functionalized with Graphene Oxide–Silver Nanocomposites. ACS Appl. Mater. Interfaces 2015, 7, 12751–12759. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Li, C.-W.; Tsai, C.-H.; Chou, C.-W.; Chen, D.-R.; Wang, G.-J. Synthesis of antibacterial TiO2/PLGA composite biofilms. Nanomed. Nanotechnol. Biol. Med. 2014, 10, e1097–e1107. [Google Scholar] [CrossRef]
- Liu, J.; Sonshine, D.A.; Shervani, S.; Hurt, R.H. Controlled release of biologically active silver from nanosilver surfaces. ACS Nano 2010, 4, 6903–6913. [Google Scholar] [CrossRef]
- Hussain, M.A.; Shah, A.; Jantan, I.; Shah, M.R.; Tahir, M.N.; Ahmad, R.; Bukhari, S.N.A. Hydroxypropylcellulose as a novel green reservoir for the synthesis, stabilization, and storage of silver nanoparticles. Int. J. Nanomed. 2015, 10, 2079–2088. [Google Scholar] [CrossRef]
- Sharma, V.K.; Yngard, R.A.; Lin, Y. Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv. Colloid Interface Sci. 2009, 145, 83–96. [Google Scholar] [CrossRef]
- Divya, K.P.; Miroshnikov, M.; Dutta, D.; Vemula, P.K.; Ajayan, P.M.; John, G. In Situ Synthesis of Metal Nanoparticle Embedded Hybrid Soft Nanomaterials. Acc. Chem. Res. 2016, 49, 1671–1680. [Google Scholar] [CrossRef]
- Francesko, A.; Cano Fossas, M.; Petkova, P.; Fernandes, M.M.; Mendoza, E.; Tzanov, T. Sonochemical synthesis and stabilization of concentrated antimicrobial silver-chitosan nanoparticle dispersions. J. Appl. Polym. Sci. 2017, 134, 45136. [Google Scholar] [CrossRef]
- Ayala Valencia, G.; Cristina de Oliveira Vercik, L.; Ferrari, R.; Vercik, A. Synthesis and characterization of silver nanoparticles using water-soluble starch and its antibacterial activity on Staphylococcus aureus. Starch Stärke 2013, 65, 931–937. [Google Scholar] [CrossRef]
- An, J.; Luo, Q.; Li, M.; Wang, D.; Li, X.; Yin, R. A facile synthesis of high antibacterial polymer nanocomposite containing uniformly dispersed silver nanoparticles. Colloid Polym. Sci. 2015, 293, 1997–2008. [Google Scholar] [CrossRef]
- Sánchez-Valdes, S.; Ortega-Ortiz, H.; Ramos-de Valle, L.F.; Medellín-Rodríguez, F.J.; Guedea-Miranda, R. Mechanical and antimicrobial properties of multilayer films with a polyethylene/silver nanocomposite layer. J. Appl. Polym. Sci. 2009, 111, 953–962. [Google Scholar] [CrossRef]
- Fortunati, E.; Mattioli, S.; Visai, L.; Imbriani, M.; Fierro, J.L.G.; Kenny, J.M.; Armentano, I. Combined Effects of Ag Nanoparticles and Oxygen Plasma Treatment on PLGA Morphological, Chemical, and Antibacterial Properties. Biomacromolecules 2013, 14, 626–636. [Google Scholar] [CrossRef] [Green Version]
- Xie, C.-M.; Lu, X.; Wang, K.-F.; Meng, F.-Z.; Jiang, O.; Zhang, H.-P.; Zhi, W.; Fang, L.-M. Silver Nanoparticles and Growth Factors Incorporated Hydroxyapatite Coatings on Metallic Implant Surfaces for Enhancement of Osteoinductivity and Antibacterial Properties. ACS Appl. Mater. Interfaces 2014, 6, 8580–8589. [Google Scholar] [CrossRef]
- Song, J.; Kang, H.; Lee, C.; Hwang, S.H.; Jang, J. Aqueous Synthesis of Silver Nanoparticle Embedded Cationic Polymer Nanofibers and Their Antibacterial Activity. ACS Appl. Mater. Interfaces 2012, 4, 460–465. [Google Scholar] [CrossRef]
- Wu, C.-N.; Fuh, S.-C.; Lin, S.-P.; Lin, Y.-Y.; Chen, H.-Y.; Liu, J.-M.; Cheng, K.-C. TEMPO-Oxidized Bacterial Cellulose Pellicle with Silver Nanoparticles for Wound Dressing. Biomacromolecules 2018, 19, 544–554. [Google Scholar] [CrossRef]
- Boonkaew, B.; Suwanpreuksa, P.; Cuttle, L.; Barber, P.M.; Supaphol, P. Hydrogels containing silver nanoparticles for burn wounds show antimicrobial activity without cytotoxicity. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- de Lima, G.G.; de Lima, D.W.F.; de Oliveira, M.J.A.; Lugão, A.B.; Alcântara, M.T.S.; Devine, D.M.; de Sá, M.J.C. Synthesis and in Vivo Behavior of PVP/CMC/Agar Hydrogel Membranes Impregnated with Silver Nanoparticles for Wound Healing Applications. ACS Appl. Bio Mater. 2018. [Google Scholar] [CrossRef]
- Baek, K.; Liang, J.; Lim, W.T.; Zhao, H.; Kim, D.H.; Kong, H. In Situ Assembly of Antifouling/Bacterial Silver Nanoparticle-Hydrogel Composites with Controlled Particle Release and Matrix Softening. ACS Appl. Mater. Interfaces 2015, 7, 15359–15367. [Google Scholar] [CrossRef]
- Ghosh, P.; Han, G.; De, M.; Kim, C.K.; Rotello, V.M. Gold nanoparticles in delivery applications. Adv. Drug Deliv. Rev. 2008, 60, 1307–1315. [Google Scholar] [CrossRef]
- Zhou, Y.; Kong, Y.; Kundu, S.; Cirillo, J.D.; Liang, H. Antibacterial activities of gold and silver nanoparticles against Escherichia coli and bacillus Calmette-Guérin. J. Nanobiotechnol. 2012, 10, 19. [Google Scholar] [CrossRef]
- Jokerst, J.V.; Lobovkina, T.; Zare, R.N.; Gambhir, S.S. Nanoparticle PEGylation for imaging and therapy. Nanomedicine 2011, 6, 715–728. [Google Scholar] [CrossRef] [Green Version]
- Niidome, T.; Yamagata, M.; Okamoto, Y.; Akiyama, Y.; Takahashi, H.; Kawano, T.; Katayama, Y.; Niidome, Y. PEG-modified gold nanorods with a stealth character for in vivo applications. J. Control. Release 2006, 114, 343–347. [Google Scholar] [CrossRef]
- Regiel-Futyra, A.; Kus-Liśkiewicz, M.; Sebastian, V.; Irusta, S.; Arruebo, M.; Stochel, G.; Kyzioł, A. Development of Noncytotoxic Chitosan–Gold Nanocomposites as Efficient Antibacterial Materials. ACS Appl. Mater. Interfaces 2015, 7, 1087–1099. [Google Scholar] [CrossRef]
- Mendoza, G.; Regiel-Futyra, A.; Andreu, V.; Sebastián, V.; Kyzioł, A.; Stochel, G.; Arruebo, M. Bactericidal Effect of Gold–Chitosan Nanocomposites in Coculture Models of Pathogenic Bacteria and Human Macrophages. ACS Appl. Mater. Interfaces 2017, 9, 17693–17701. [Google Scholar] [CrossRef]
- Politi, J.; Spadavecchia, J.; Fiorentino, G.; Antonucci, I.; De Stefano, L. Arsenate reductase from Thermus thermophilus conjugated to polyethylene glycol-stabilized gold nanospheres allow trace sensing and speciation of arsenic ions. J. R. Soc. Interface 2016, 13. [Google Scholar] [CrossRef]
- Politi, J.; Spadavecchia, J.; Iodice, M.; de Stefano, L. Oligopeptide–heavy metal interaction monitoring by hybrid gold nanoparticle based assay. Analyst 2015, 140, 149–155. [Google Scholar] [CrossRef]
- Palmieri, G.; Tatè, R.; Gogliettino, M.; Balestrieri, M.; Rea, I.; Terracciano, M.; Proroga, Y.T.; Capuano, F.; Anastasio, A.; De Stefano, L. Small Synthetic Peptides Bioconjugated to Hybrid Gold Nanoparticles Destroy Potentially Deadly Bacteria at Submicromolar Concentrations. Bioconjug. Chem. 2018, 29, 3877–3885. [Google Scholar] [CrossRef]
- Yang, X.; Yang, J.; Wang, L.; Ran, B.; Jia, Y.; Zhang, L.; Yang, G.; Shao, H.; Jiang, X. Pharmaceutical Intermediate-Modified Gold Nanoparticles: Against Multidrug-Resistant Bacteria and Wound-Healing Application via an Electrospun Scaffold. ACS Nano 2017, 11, 5737–5745. [Google Scholar] [CrossRef]
- Manzl, C.; Enrich, J.; Ebner, H.; Dallinger, R.; Krumschnabel, G. Copper-induced formation of reactive oxygen species causes cell death and disruption of calcium homeostasis in trout hepatocytes. Toxicology 2004, 196, 57–64. [Google Scholar] [CrossRef]
- Sehmi, S.K.; Noimark, S.; Weiner, J.; Allan, E.; MacRobert, A.J.; Parkin, I.P. Potent Antibacterial Activity of Copper Embedded into Silicone and Polyurethane. ACS Appl. Mater. Interfaces 2015, 7, 22807–22813. [Google Scholar] [CrossRef]
- Lu, Y.; Li, L.; Zhu, Y.; Wang, X.; Li, M.; Lin, Z.; Hu, X.; Zhang, Y.; Yin, Q.; Xia, H.; et al. Multifunctional Copper-Containing Carboxymethyl Chitosan/Alginate Scaffolds for Eradicating Clinical Bacterial Infection and Promoting Bone Formation. ACS Appl. Mater. Interfaces 2018, 10, 127–138. [Google Scholar] [CrossRef]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R Rep. 2004, 47, 49–121. [Google Scholar] [CrossRef] [Green Version]
- Liou, J.-W.; Chang, H.-H. Bactericidal Effects and Mechanisms of Visible Light-Responsive Titanium Dioxide Photocatalysts on Pathogenic Bacteria. Arch. Immunol. Ther. Exp. 2012, 60, 267–275. [Google Scholar] [CrossRef]
- Toniatto, T.V.; Rodrigues, B.V.M.; Marsi, T.C.O.; Ricci, R.; Marciano, F.R.; Webster, T.J.; Lobo, A.O. Nanostructured poly (lactic acid) electrospun fiber with high loadings of TiO2 nanoparticles: Insights into bactericidal activity and cell viability. Mater. Sci. Eng. C 2017, 71, 381–385. [Google Scholar] [CrossRef]
- Fan, X.; Chen, K.; He, X.; Li, N.; Huang, J.; Tang, K.; Li, Y.; Wang, F. Nano-TiO2/collagen-chitosan porous scaffold for wound repairing. Int. J. Biol. Macromol. 2016, 91, 15–22. [Google Scholar] [CrossRef]
- Archana, D.; Singh, B.K.; Dutta, J.; Dutta, P.K. In vivo evaluation of chitosan–PVP–titanium dioxide nanocomposite as wound dressing material. Carbohydr. Polym. 2013, 95, 530–539. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. Nano-TiO2 Reinforced PEEK/PEI Blends as Biomaterials for Load-Bearing Implant Applications. ACS Appl. Mater. Interfaces 2015, 7, 5561–5573. [Google Scholar] [CrossRef]
- Laurenti, M.; Cauda, V. ZnO Nanostructures for Tissue Engineering Applications. Nanomaterials 2017, 7, 374. [Google Scholar] [CrossRef]
- Grenho, L.; Salgado, C.L.; Fernandes, M.H.; Monteiro, F.J.; Ferraz, M.P. Antibacterial activity and biocompatibility of three-dimensional nanostructured porous granules of hydroxyapatite and zinc oxide nanoparticles—An in vitro and in vivo study. Nanotechnology 2015, 26, 315101. [Google Scholar] [CrossRef]
- Wahid, F.; Yin, J.-J.; Xue, D.-D.; Xue, H.; Lu, Y.-S.; Zhong, C.; Chu, L.-Q. Synthesis and characterization of antibacterial carboxymethyl Chitosan/ZnO nanocomposite hydrogels. Int. J. Biol. Macromol. 2016, 88, 273–279. [Google Scholar] [CrossRef]
- García-Gallego, S.; Franci, G.; Falanga, A.; Gómez, R.; Folliero, V.; Galdiero, S.; De La Mata, F.J.; Galdiero, M. Function oriented molecular design: Dendrimers as novel antimicrobials. Molecules 2017, 22, 1581. [Google Scholar] [CrossRef]
- Gupta, U.; Perumal, O. Dendrimers and Its Biomedical Applications, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands; ISBN 9780123969835.
- Winnicka, K.; Wroblewska, M.; Wieczorek, P.; Sacha, P.T.; Tryniszewska, E.A. The effect of PAMAM dendrimers on the antibacterial activity of antibiotics with different water solubility. Molecules 2013, 18, 8607–8617. [Google Scholar] [CrossRef]
- Mofrad, A.S.; Mohammadi, M.J.; Mansoorian, H.J.; Khaniabadid, Y.O.; Jebeli, M.A.; Khanjani, N.; Khoshgoftar, M.; Yari, A.R. The antibacterial effect of g3-poly-amidoamine dendrimer on gram negative and gram positive bacteria in aqueous solutions. Desalin. Water Treat. 2018, 124, 223–231. [Google Scholar] [CrossRef]
- Siriwardena, T.N.; Stach, M.; Tinguely, R.; Kasraian, S.; Luzzaro, F.; Leib, S.L.; Darbre, T.; Reymond, J.; Endimiani, A. In Vitro Activity of the Novel Antimicrobial Peptide Dendrimer G3KL against Multidrug-Resistant Acinetobacter baumannii and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2015, 59, 7915–7918. [Google Scholar] [CrossRef]
- Wong, P.T.; Tang, S.; Mukherjee, J.; Tang, K.; Gam, K.; Isham, D.; Murat, C.; Sun, R.; Baker, J.R.; Choi, S.K. Light-Controlled Active Release of Photocaged Ciprofloxacin for Lipopolysaccharide-Targeted Drug Delivery using Dendrimer Conjugates Pamela. Chem. Commun. 2016, 52, 10357–10360. [Google Scholar] [CrossRef]
- Serri, A.; Mahboubi, A.; Zarghi, A.; Moghimi, H.R. PAMAM-dendrimer Enhanced Antibacterial Effect Hydrochloride Against Gram-Negative Bacteria of Vancomycin. J. Pharm. Sci. 2019, 22, 10–21. [Google Scholar] [CrossRef]
- Choi, S.K.; Myc, A.; Silpe, J.E.; Sumit, M.; Wong, P.T.; McCarthy, K.; Desai, A.M.; Thomas, T.P.; Kotlyar, A.; Holl, M.M.B.; et al. Dendrimer-based multivalent vancomycin nanoplatform for targeting the drug-resistant bacterial surface. ACS Nano 2013, 7, 214–228. [Google Scholar] [CrossRef]
- Bosnjakovic, A.; Mishra, M.K.; Ren, W.; Kurtoglu, Y.E.; Shi, T.; Fan, D.; Kannan, R.M. Poly(amidoamine) dendrimer-erythromycin conjugates for drug delivery to macrophages involved in periprosthetic inflammation. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 284–294. [Google Scholar] [CrossRef]
- Wrońska, N.; Felczak, A.; Zawadzka, K.; Poszepczy Nska, M.; Rózalska, S.; Bryszewska, M.; Appelhans, D.; Lisowska, K. Poly(propylene imine) dendrimers and amoxicillin as dual-action antibacterial agents. Molecules 2015, 20, 19330–19342. [Google Scholar] [CrossRef]
- Aghayari, M.; Salouti, M.; Kazemizadeh, A.R.; Zabihian, A.; Hamidi, M.; Shajari, N.; Moghtader, F. Enhanced antibacterial activity of ceftazidime against pseudomonas aeruginosa using poly (propyleneimine) dendrimer as a nanocarrier. Sci. Iran. F 2015, 22, 1330–1336. [Google Scholar]
- Sikwal, D.R.; Kalhapure, R.S.; Jadhav, M.; Rambharose, S.; Mocktar, C.; Govender, T. Non-ionic self-assembling amphiphilic polyester dendrimers as new drug delivery excipients. RSC Adv. 2017, 7, 14233–14246. [Google Scholar] [CrossRef] [Green Version]
- Michaud, G.; Visini, R.; Bergmann, M.; Salerno, G.; Bosco, R.; Gillon, E.; Richichi, B.; Nativi, C.; Imberty, A.; Stocker, A.; et al. Overcoming antibiotic resistance in Pseudomonas aeruginosa biofilms using glycopeptide dendrimers. Chem. Sci. 2016, 7, 166–182. [Google Scholar] [CrossRef]
- Saczewski, F.; Balewski, Ł. Biological activities of guanidine compounds. Expert Opin. Therap. Patents 2009, 19, 1417–1448. [Google Scholar] [CrossRef]
- Mignani, S.; Rodrigues, J.; Tomas, H.; Roy, R.; Shi, X.; Majoral, J. Bench-to-bedside translation of dendrimers: Reality or utopia? A concise analysis. Adv. Drug Deliv. Rev. 2017, 136–137, 73–81. [Google Scholar] [CrossRef]
- Berlinck, R.G.S.; Burtoloso, A.C.B.; Kossuga, M.H. The chemistry and biology of organic guanidine derivatives. Nat. Prod. Rep. 2005, 22, 919–954. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, J.; Chen, Y. Synthesis and antimicrobial activity of polymeric guanidine and biguanidine salts. Polymer 1999, 40, 6189–6198. [Google Scholar] [CrossRef]
- Moore, K.; Gray, D. Using PHMB antimicrobial to prevent wound infection. Wounds 2007, 3, 96. [Google Scholar]
- Kusnetsov, J.M.; Tulkki, I.; Ahonen, H.E.; Martikainen, P.J. Efficacy of three prevention strategies against legionella in cooling water systems. J. Appl. Microbiol. 1997, 82, 763–768. [Google Scholar] [CrossRef]
- Hiti, K. Viability of Acanthamoeba after exposure to a multipurpose disinfecting contact lens solution and two hydrogen peroxide systems. Br. J. Ophthalmol. 2002, 86, 144–146. [Google Scholar] [CrossRef]
- Gilbert, P.; Moore, L.E. Cationic antiseptics: Diversity of action under a common epithet. J. Appl. Microbiol. 2005, 99, 703–715. [Google Scholar] [CrossRef]
- Messick, C.R.; Pendland, S.L.; Moshirfar, M.; Fiscellac, R.G.; Losnedah, K.J.; Schriever, C.A.; Schreckenb, P.C. In-vitro activity of polyhexamethylene biguanide (PHMB) against fungal isolates associated with infective keratitits. J. Antimicrob. Chemother. 1999, 44, 291–302. [Google Scholar] [CrossRef]
- Romanowski, E.G.; Yates, K.A.; Connor, K.E.O.; Francis, S.; Shanks, R.M.Q.; Kowalski, R.P.; Ascp, M.S.M. The Evaluation of Polyhexamethylene Biguanide (PHMB) as a Disinfectant for Adenovirus. JAMA Opthalmol. 2013, 131, 495–498. [Google Scholar] [CrossRef]
- Chindera, K.; Mahato, M.; Kumar Sharma, A.; Horsley, H.; Kloc-Muniak, K.; Kamaruzzaman, N.F.; Kumar, S.; McFarlane, A.; Stach, J.; Bentin, T.; et al. The antimicrobial polymer PHMB enters cells and selectively condenses bacterial chromosomes. Sci. Rep. 2016, 6, 23121. [Google Scholar] [CrossRef]
- Firdessa, R.; Good, L.; Amstalden, M.C.; Chindera, K.; Kamaruzzaman, N.F.; Schultheis, M.; Röger, B.; Hecht, N.; Oelschlaeger, T.A.; Meinel, L.; et al. Pathogen- and Host-Directed Antileishmanial Effects Mediated by Polyhexanide (PHMB). PLoS Negl. Trop. Dis. 2015, 9. [Google Scholar] [CrossRef]
- Müller, G.; Kramer, A. Biocompatibility index of antiseptic agents by parallel assessment of antimicrobial activity and cellular cytotoxicity. J. Antimicrob. Chemother. 2008, 61, 1281–1287. [Google Scholar] [CrossRef] [Green Version]
- Lewis, K. Platforms for antibiotic discovery. Nat. Publ. Gr. 2013, 12, 371–387. [Google Scholar] [CrossRef]
- Shea, R.O.; Moser, H.E. Physicochemical Properties of Antibacterial Compounds: Implications for Drug Discovery. J. Med. Chem. 2008, 51. [Google Scholar] [CrossRef]
- Chang, R.-K.; Raw, A.; Lionberger, R.; Yu, L.X. Generic development of topical dermatologic products: Formulation development, process development, and testing of topical dermatologic products. AAPS J. 2013, 15, 41–52. [Google Scholar] [CrossRef]
- Nellums, L.B.; Thompson, H.; Holmes, A.; Castro-Sánchez, E.; Otter, J.A.; Norredam, M.; Friedland, J.S.; Hargreaves, S. Antimicrobial resistance among migrants in Europe: A systematic review and meta-analysis. Lancet Infect. Dis. 2018, 18, 796–811. [Google Scholar] [CrossRef]
- Kamaruzzaman, N.F.; Chong, S.Q.Y.; Edmondson-Brown, K.M.; Ntow-Boahene, W.; Bardiau, M.; Good, L. Bactericidal and anti-biofilm effects of polyhexamethylene Biguanide in models of intracellular and biofilm of Staphylococcus aureus isolated from bovine mastitis. Front. Microbiol. 2017, 8, 1–10. [Google Scholar] [CrossRef]







| Pathogens | Enterococcus faecium | Staphylococcus aureus | Klebsiella pneumoniae | Acinetobacter baumannii | Pseudomonas aeruginosa | Enterobacter spp. | References | |
|---|---|---|---|---|---|---|---|---|
| Country | ||||||||
| Thailand | - | - | ER | ER | - | - | [4] | |
| South India | - | - | - | - | S | - | [5] | |
| India | ER | ER | ER | ER | ER | ER | [6] | |
| India (Veterinary Cases) | ER | ER | ER | ER | ER | ER | [7] | |
| Iran | ER | HR | R | ER | ER | HR | [14] | |
| Asia-Pacific | - | - | R | ER | S | S | [8] | |
| Southern Italy | S | R | S | ER | R | - | [9] | |
| Romania | HR | R | HR | HR | HR | - | [10] | |
| Romania | H | ER | ER | ER | S | - | [11] | |
| South Africa | R | S | ER | H | S | - | [12] | |
| Brazil | ER | ER | HR | HR | ER | ER | [13] | |
| Latin-America | - | - | R | ER | S | S | [8] | |
| Polymers | Class | Description | Susceptibility | Haemolytic Activity | References | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| E | S | K | A | P | E | |||||
| 4-aminobutylene side chain coupled with hydrophobic ethylmethacrylate in a roughly 70/30 ratio | Amphiphilic Methacrylate Copolymers | Cationic amphiphilic random copolymers with ethyl methacrylate (EMA) comonomer were prepared with a range of comonomer fractions, and the library of copolymers was screened for antimicrobial and hemolytic activities. | BC | BS | - | BS | BS | BS | Low | [60] |
| PDMAEMA-g-rosin | Cationic polymers | Quaternary ammonium-containing poly(N,N-dimethylaminoethyl methacrylate) with natural rosin as the pendant group. | BS | BS | - | - | - | - | NA | [61] |
| Methacrylate Copolymer (E429) | Methacrylate Copolymer | Amphiphilic random copolymers with modulated cationic side chain spacer arms structure which include 2-aminoethylene, 4-aminobutylene, and 6-aminohexylene groups. | BS | BS | - | BS | BS | BS | NA | [62] |
| PAPMA | Amphiphilic Methacrylamide Copolymers | A series of copolymers containing lysine mimicking aminopropyl methacrylamide (APMA) and arginine mimicking guanadinopropyl methacrylamide (GPMA). | BS | BS | - | - | BS | - | NA | [63] |
| Cationic polyester-based copolymer | Self-Degradable Antibacterial Polymers | Auto-degradable antimicrobial copolymers bearing cationic side chains and main-chain ester linkages synthesized using the simultaneous chain- and step-growth radical polymerization of t-butyl acrylate and 3-butenyl 2-chloropropionate, followed by the transformation of t-butyl groups into primary ammonium salts. | BS | - | - | - | - | - | Low-Moderate | [58] |
| AMP-mimicking polyurethanes | Peptidomimetic Polyurethanes | Peptidomimetic polyurethanes with pendant functional groups that mimic lysine and valine amino acid residues | BC | - | - | - | - | - | Low | [57] |
| Block Amphiphilic Copolymers | Amphiphilic copolymers of Poly(Vinyl Ether)s | A series of amphiphilic block copolymers of poly(vinyl ether) derivatives prepared by base-assisting living cationic polymerization. | BS | - | - | - | - | - | Low | [64] |
| Random Amphiphilic Copolymers | Amphiphilic copolymers of Poly(Vinyl Ether)s | A series of amphiphilic random copolymers of poly(vinyl ether) derivatives prepared by base-assisting living cationic polymerization. | BS | - | - | - | - | - | High | [64] |
| Polymers | Class | Description | Susceptibility | Haemolytic Activity | References | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| E | S | K | A | P | E | |||||
| Idolidicin variants | Peptide | A 13-residue cationic antimicrobial peptide (sequence carboxy-terminal amidated ILPWKWPWWPWRR-NH2) | BC | High | [66,73] | |||||
| Gratisin analogues | Peptide | cyclo(-Val1-Orn2-Leu3-d-Phe4-Pro5-d-Tyr6-)2 | BS | BS | Low | [69] | ||||
| LL-37 | Peptide | A cathelin-associated antimicrobial peptide | BS | BS | None | [74] | ||||
| α/β-Peptides | Peptide | Helix-forming α/β-peptides, i.e., oligomers containing a 1:1 pattern of α- and β- amino acid residues in the backbone | BS | BS | None | [75] | ||||
| cecropin/melittin | Peptide | Hybrid peptide produced by recombinant DNA technology in S. aureus | BC | NA | [76] | |||||
| Maleic anhydride copolymers | Peptide mimics | Peptides Mimicking Copolymers of Maleic Anhydride and 4-Methyl-1-pentene | None | None | High | [77] | ||||
| Brilacidin | Peptide mimics | also known as PMX-30063, a defensin-mimetic and a membrane-targeting arylamide oligomer | BC | NA | [71] | |||||
| cecropin/melittin | Synthetic peptide | Recombinant hybrid peptide | BC | NA | [76] | |||||
| LL-37LTX 109 | Peptide mimics | a synthetic antimicrobial peptidomimetic containing a modified tryptophan derivate as lipophilic bulk, displayed a combination of high antibacterial activity against MRSA and Staphylococcus spp. biofilm | BC | BS | Low | [78] | ||||
| poly(m-phenylene ethynylene)s | Peptide mimics | Nonhemolytic abiogenic polymers | BS | BS | BS | BS | BS | BS | Low | [79] |
| Pandinin 2 | Peptide Variants | A scorpion venom AMP contains a central proline residue | BC | High | [80] | |||||
| Pyridinium Functionalized Polynorbornenes | Synthetic peptide | Amphiphilic polyoxanorbornene with different quaternary alkyl pyridinium side-chains | BS | NA | [81] | |||||
| Amino-Functionalized Poly(norbornene) | Synthetic peptide | Homopolymers of the amine bearing monomers and random copolymers of amine- and alkyl-substituted monomers of high average molar mass was produced by ring-opening metathesis polymerization. | BS | BS | None | [82] | ||||
| Dendrimers | Antibiotics Conjugates | Pathogens Tested | Mechanism of Antibiotic Release | References |
|---|---|---|---|---|
| Polyamidoamines (PAMAM) | Ciprofloxacin | E. coli | Light-active release | [130] |
| PAMAM | Vancomycin | S. aureus | Temperature-active release | [131] |
| PAMAM | Vancomycin | S. aureus | NA | [132] |
| PAMAM | Erythromycin | S. aureus | Hydrolysis of the ester linkage | [133] |
| Polypropylene imine (PPI)-modified maltose | Amoxicillin | E. coli and P. aeruginosa | NA | [134] |
| PPI | Ceftazidime | P. aeruginosa | pH-active release | [135] |
| Polyesters | Fusidic acid | S. aureus | Water-active release | [136] |
| Carbohydrate-glycopeptide | Tobramycin | P. aeruginosa | Temperature-active release | [137] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamaruzzaman, N.F.; Tan, L.P.; Hamdan, R.H.; Choong, S.S.; Wong, W.K.; Gibson, A.J.; Chivu, A.; Pina, M.d.F. Antimicrobial Polymers: The Potential Replacement of Existing Antibiotics? Int. J. Mol. Sci. 2019, 20, 2747. https://doi.org/10.3390/ijms20112747
Kamaruzzaman NF, Tan LP, Hamdan RH, Choong SS, Wong WK, Gibson AJ, Chivu A, Pina MdF. Antimicrobial Polymers: The Potential Replacement of Existing Antibiotics? International Journal of Molecular Sciences. 2019; 20(11):2747. https://doi.org/10.3390/ijms20112747
Chicago/Turabian StyleKamaruzzaman, Nor Fadhilah, Li Peng Tan, Ruhil Hayati Hamdan, Siew Shean Choong, Weng Kin Wong, Amanda Jane Gibson, Alexandru Chivu, and Maria de Fatima Pina. 2019. "Antimicrobial Polymers: The Potential Replacement of Existing Antibiotics?" International Journal of Molecular Sciences 20, no. 11: 2747. https://doi.org/10.3390/ijms20112747
APA StyleKamaruzzaman, N. F., Tan, L. P., Hamdan, R. H., Choong, S. S., Wong, W. K., Gibson, A. J., Chivu, A., & Pina, M. d. F. (2019). Antimicrobial Polymers: The Potential Replacement of Existing Antibiotics? International Journal of Molecular Sciences, 20(11), 2747. https://doi.org/10.3390/ijms20112747







