A Critical Review of Electroporation as A Plasmid Delivery System in Mouse Skeletal Muscle
Abstract
1. Introduction
2. Electroporation
3. The Usefulness and Safety of Plasmid Vectors for Electroporation
4. Markers of Electroporation: Luciferase, A Green Fluorescent Protein (GFP), Beta-Galactosidase
5. Skeletal Muscle as A Target for Electroporation
6. Hyaluronidase Pre-Treatment Improves Vectors Distribution within Muscles
7. Procedure of Electroporation
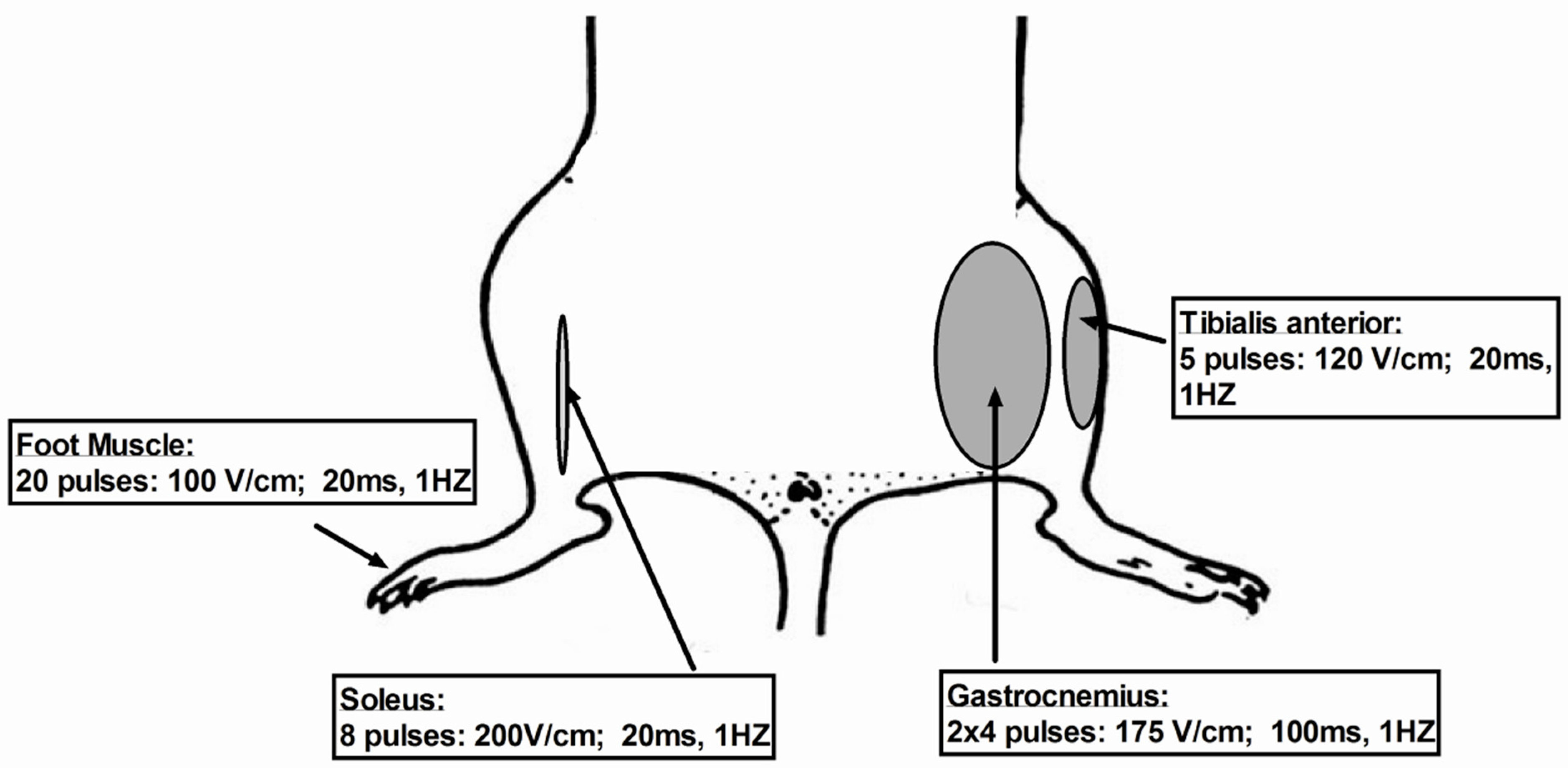
7.1. Critical Parameters of Electroporation Depending Muscles Used
7.1.1. Gastrocnemious Muscle
7.1.2. Tibialis
7.1.3. Soleus
7.1.4. Foot Muscles: Flexor Digitorum Brevis (FDB) and Interosseus (IO) Muscles
8. Toxicity
9. Effectiveness of Electroporation
10. Limitations of Electroporation
11. Discussion
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AAV | Adeno-associated viruses |
| CCD | charge coupled device |
| ECM | extracellular matrix layer |
| FDB | flexor digitorum brevis |
| GFP | Green Fluorescent protein |
| HV | high voltage |
| IO | interosseus |
| mRNA | messenger Ribonucleic Acid |
| LV | low voltage |
| PBS | phosphate buffered saline |
| pDNA | plasmid DNA |
References
- Gothelf, A.; Gehl, J. Gene electrotransfer to skin; review of existing literature and clinical perspectives. Curr. Gene Ther. 2010, 10, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Golzio, M.; Rols, M.P.; Teissie, J. In vitro and in vivo electric field-mediated permeabilization, gene transfer and expression. Methods 2004, 32, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Abdulhaqq, S.A.; Weiner, D.B. DNA vaccines: Developing new strategies to enhance immune responses. Immunol. Res. 2008, 42, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Ragot, T.; Vincent, N.; Chafey, P.; Vigne, E.; Gilgenkrantz, H.; Couton, D.; Cartaud, J.; Briand, P.; Kaplan, J.C.; Perricaudet, M. Efficient adenovirus-mediated transfer of a human minidystrophin gene to skeletal muscle of mdx mice. Nature 1993, 361, 647–650. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.Y.; Barron, L.G.; Meyer, K.B.; Szoka, F.C., Jr. Characterization of plasmid DNA transfer into mouse skeletal muscle: Evaluation of uptake mechanism, expression and secretion of gene products into blood. Gene Ther. 1996, 3, 201–211. [Google Scholar] [PubMed]
- Cappelletti, M.; Zampaglione, I.; Rizzuto, G.; Ciliberto, G.; La Monica, N.; Fattori, E. Gene electro-transfer improves transduction by modifying the fate of intramuscular DNA. J. Gene Med. 2003, 5, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Mir, L.M.; Bureau, F.B.; Gehl, J.; Rangara, R.; Rouy, D.; Caillaud, J.M.; Branellec, D.; Schwartz, B.; Scherman, D. High-efficiency gene transfer into skeletal muscle mediated by electric pulses. Proc. Natl. Acad. Sci. USA 1999, 96, 4262–4267. [Google Scholar] [CrossRef]
- Aihara, H.; Miyazaki, J.I. Gene transfer into muscle by electroporation in vivo. Nat. Biotechnol. 1998, 16, 867–870. [Google Scholar] [CrossRef]
- Cemazar, M.; Golzio, M.; Sersa, G.; Rols, M.P.; Teissié, J. Electrically-assisted nucleic acids delivery to tissues in vivo: Where do we stand? Curr. Pharm. Des. 2006, 12, 3817–3825. [Google Scholar] [CrossRef]
- Heller, L.C.; Heller, R. In vivo electroporation for gene therapy. Hum. Gene Ther. 2006, 17, 890–897. [Google Scholar] [CrossRef]
- Faria, M.; Spiller, D.G.; Dubertret, C.; Nelson, J.S.; White, M.R.; Scherman, D.; Hélène, C.; Giovannangeli, C. Phosphoramidate oligonucleotides as potent antisense molecules in cells and in vivo. Nat. Biotechnol. 2001, 19, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Kessler, P.D.; Podsakoff, G.M.; Chen, X.; McQuiston, S.; Colosi, P.C.; Matelis, L.A.; Kurtzman, G.J.; Byrne, B.J. Gene delivery to skeletal muscle results in sustained expression and systemic delivery of a therapeutic protein. Proc. Natl. Acad. Sci. USA 1996, 93, 14082–14087. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Steinbrecher, R.A.; Murdock, P.J.; Tuddenham, E.G.D.; Lee, C.A.; Pasi, K.J.; Goldspink, G. Expression of factor VII by muscle cells in vitro and in vivo following direct gene transfer: Modelling gene therapy for haemophilia. Gene Ther. 1995, 2, 736–742. [Google Scholar] [PubMed]
- Naffakh, N.; Pinset, C.; Montarras, D.; Li, Z.; Paulin, D.; Danos, O.; Heard, J.M. Long-Term secretion of therapeutic proteins from genetically modified skeletal muscles. Hum. Gene Ther. 1996, 7, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, S.K.; Goldwasser, E.; Min-Min, L.; Barr, E.; Leiden, J.F. Stable delivery of physiologic levels of recombinant erythropoietin to the systemic circulation by intramuscular injection of replication-defective adenovirus. Proc. Natl. Acad. Sci. USA 1994, 91, 11557–11561. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, T.; Mizutani, Y.; Ohmori, Y.; Okumura, J. Comparison of three nonviral transfection methods for foreign gene expression in early chicken embryos in ovo. Biochem. Biophys. Res. Commun. 1997, 230, 376–380. [Google Scholar] [CrossRef]
- Heller, R.; Jaroszeski, M.; Atkin, A.; Moradpour, D.; Gilbert, R.; Wands, J.; Nicolau, C. In vivo gene electroinjection and expression in rat liver. FEBS Lett. 1996, 389, 225–228. [Google Scholar] [CrossRef]
- Isaka, Y.; Yamada, K.; Takabatake, Y.; Mizui, M.; Miura-Tsujie, M.; Ichimaru, N.; Yazawa, K.; Utsugi, R.; Okuyama, A.; Hori, M.; et al. Electroporationmediated HGF gene transfection protected the kidney against graft injury. Gene Ther. 2005, 12, 815–820. [Google Scholar] [CrossRef]
- Khoury, M.; Bigey, P.; Louis-Plence, P.; Noel, D.; Rhinn, H.; Scherman, D.; Jorgensen, C.; Apparailly, F. A comparative study on intra-articular versus systemic gene electrotransfer in experimental arthritis. Gene Ther. 2006, 8, 1027–1036. [Google Scholar] [CrossRef]
- Wang, H.; Ko, C.H.; Koletar, M.M.; Ralph, M.R.; Yeomans, J. Casein kinase I epsilon gene transfer into the suprachiasmatic nucleus via electroporation lengthens circadian periods of tau mutant hamsters. Eur. J. Neurosci. 2007, 25, 3359–3366. [Google Scholar] [CrossRef]
- Blair-Parks, K.; Weston, B.C.; Dean, D.A. High-level gene transfer to the cornea using electroporation. J. Gene Med. 2002, 4, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Pringle, I.A.; McLachlan, G.; Collie, D.D.; SumnerJones, S.G.; Lawton, A.E.; Tennant, P.; Baker, A.; Gordon, C.; Blundell, R.; Varathalingam, A.; et al. Electroporation enhances reporter gene expression following delivery of naked plasmid DNA to the lung. J. Gene Med. 2007, 9, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.L.; Byrne, B.J.; Tung, L. Electroporation-mediated gene transfer in cardiac tissue. FEBS Lett. 1998, 435, 1–5. [Google Scholar] [CrossRef]
- Matsumoto, T.; Komori, K.; Shoji, T.; Kuma, S.; Kume, M.; Yamaoka, T.; Mori, E.; Furuyama, T.; Yonemitsu, Y.; Sugimachi, K. Successful and optimized in vivo gene transfer to rabbit carotid artery mediated by electronic pulse. Gene Ther. 2001, 8, 1174–1179. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Matsuda, T.; Cepko, C.L. Electroporation and RNA interference in the rodent retina in vivo and in vitro. Proc. Natl. Acad. Sci. USA 2004, 101, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Pedron-Mazoyer, S.; Plouët, J.; Hellaudais, L.; Teissie, J.; Golzio, M. New anti-angiogenesis developments through electro-immunization: Optimization by in vivo optical imaging of intradermal electrogenetransfer. Biochim. Biophys. Acta 2007, 1770, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Horan, R.; Powell, R.; Bird, J.M.; Gannon, F.; Houghton, J.A. Effects of electropermeabilization on the association of foreign DNA with pig sperm. Arch. Androl. 1992, 28, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Neumann, E.; Schaefer-Ridder, M.; Wang, Y.; Hofschneider, P.H. Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J. 1982, 1, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.L.; Whalen, R.G.; Demeneix, B.A. Direct gene transfer into skeletal muscle in vivo: Factors affecting efficiency of transfer and stability of expression. Hum. Gene Ther. 1993, 4, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Potter, H. Electroporation in biology: Methods, applications and instrumentation. Anal. Biochem. 1988, 174, 361–373. [Google Scholar] [CrossRef]
- Gowrishankar, T.R.; Pliquett, U.; Lee, R.C. Dynamics of membrane sealing in transient electropermeabilization of skeletal muscle membranes. Ann. N. Y. Acad. Sci. 1999, 888, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Bodine-Fowler, S. Skeletal muscle regeneration after injury: An overview. J. Voice 1994, 8, 53–62. [Google Scholar] [CrossRef]
- McMahon, J.M.; Signori, E.; Wells, K.E.; Fazio, V.M.; Wells, D.J. Optimisation of electrotransfer of plasmid into skeletal muscle by pre-treatment with hyaluronidase—Increased expression with reduced muscle damage. Gene Ther. 2001, 8, 1264–1270. [Google Scholar] [CrossRef] [PubMed]
- Mennuni, C.; Calvaruso, F.; Zampaglione, I.; Rizzuto, G.; Rinaudo, D.; Dammassa, E.; Ciliberto, G.; Fattori, E.; La Monica, N. Hyaluronidase increases electrogene transfer efficiency in skeletal muscle. Hum. Gene Ther. 2002, 13, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Vilquin, J.T.; Kennel, P.F.; Paturneau-Jouas, M.; Chapdelaine, P.; Boissel, N.; Delaère, P.; Tremblay, J.P.; Scherman, D.; Fiszman, M.Y.; Schwartz, K. Electrotransfer of naked DNA in the skeletal muscles of animal models of muscular dystrophies. Gene Ther. 2001, 8, 1097–1107. [Google Scholar] [CrossRef]
- Gollins, H.; McMahon, J.; Wells, K.E.; Wells, D.J. High-efficiency plasmid gene transfer into dystrophic muscle. Gene Ther. 2003, 10, 504–512. [Google Scholar] [CrossRef]
- Vicat, J.M.; Boisseau, S.; Jourdes, P.; Lainé, M.; Wion, D.; Bouali-Benazzouz, R.; Benabid, A.L.; Berger, F. Muscle transfection by electroporation with high-voltage and short-pulse currents provides high-level and long-lasting gene expression. Hum. Gene Ther. 2000, 11, 909–916. [Google Scholar] [CrossRef]
- Golzio, M.; Teissie, J.; Rols, M.P. Direct visualization at the single-cell level of electrically mediated gene delivery. Proc. Natl. Acad. Sci. USA 2002, 99, 1292–1297. [Google Scholar] [CrossRef]
- Rosazza, C.; Buntz, A.; Ries, T.; Woll, D.; Zumbusch, A.; Rols, M.P. Intracellular tracking of single-plasmid DNA particles after delivery by electroporation. Mol. Ther. 2013, 21, 2217–2226. [Google Scholar] [CrossRef]
- Lechardeur, D.; Sohn, K.J.; Haardt, M.; Joshi, P.B.; Monck, M.; Graham, R.W.; Beatty, B.; Squire, J.; O’Brodovich, H.; Lukacs, G.L. Metabolic instability of plasmid DNA in the cytosol: A potential barrier to gene transfer. Gene Ther. 1999, 6, 482–497. [Google Scholar] [CrossRef]
- Faurez, F.; Dory, D.; Le Moigne, V.; Gravier, R.; Jestin, A. Biosafety of DNA vaccines: New generation of DNA vectors and current knowledge on the fate of plasmid after injection. Vaccine 2010, 28, 3888–3895. [Google Scholar] [CrossRef] [PubMed]
- Gillies, A.R.; Lieber, R.L. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve 2011, 44, 318–331. [Google Scholar] [CrossRef] [PubMed]
- Dauty, E.; Verkman, A.S. Actin cytoskeleton as the principal determinant of size-dependent DNA mobility in cytoplasm: A new barrier for non-viral gene delivery. J. Biol. Chem. 2008, 280, 7823–7828. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, E.E.; Geiger, R.C.; Miller, A.M.; Loh-Marley, P.L.; Suzuki, T.; Miyata, N.; Dean, D.A. Microtubule acetylation through HDAC6 inhibition results in increased transfection efficiency. Mol. Ther. 2008, 16, 1841–1847. [Google Scholar] [CrossRef]
- Badding, M.A.; Lapek, J.D.; Friedman, A.E.; Dean, D.A. Proteomic and functional analyses of protein-DNA complexes during gene transfer. Mol. Ther. 2013, 21, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Rols, M.P. Gene Delivery by Electroporation In Vitro: Mechanisms. In Handbook of Electroporation; Miklavcic, D., Ed.; Springer: Cham, Switzerland, 2016; pp. 1–16. [Google Scholar]
- Wong, T.K.; Neumann, E. Electric field mediated gene transfer. Biochem. Biophys. Res. Commun. 1982, 107, 584–587. [Google Scholar] [CrossRef]
- Rols, M.P.; Delteil, C.; Golzio, M.; Dumond, P.; Cros, S.; Teissié, J. In vivo electrically mediated protein and gene transfer in murine melanoma. Nat. Biotechnol. 1998, 16, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Shin, B.C.; Fujikura, K.; Matsuzaki, T.; Takata, K. Direct gene transfer into rat liver cells by in vivo electroporation. FEBS Lett. 1998, 425, 436–440. [Google Scholar] [CrossRef]
- Yin, D.; Tang, J.G. Gene therapy for streptozotocin-induced diabetic mice by electroporational transfer of naked human insulin precursor DNA into skeletal muscle in vivo. FEBS Lett. 2001, 495, 16–20. [Google Scholar] [CrossRef]
- Watanabe, K.; Nakazawa, M.; Fuse, K.; Hanawa, H.; Kodama, M.; Aizawa, Y.; Ohnuki, T.; Gejyo, F.; Maruyama, H.; Miyazaki, J. Protection against autoimmune myocarditis by gene transfer of interleukin-10 by electroporation. Circulation 2001, 104, 1098–1100. [Google Scholar] [CrossRef]
- Maruyama, H.; Ataka, K.; Gejyo, F.; Higuchi, N.; Ito, Y.; Hirahara, H.; Imazeki, I.; Hirata, M.; Ichikawa, F.; Neichi, T.; et al. Long-term production of erythropoietin after electroporation-mediated transfer of plasmid DNA into the muscles of normal and uremic rats. Gene Ther. 2001, 8, 461–468. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kishida, T.; Asada, H.; Satoh, E.; Tanaka, S.; Shinya, M.; Hirai, H.; Iwai, M.; Tahara, H.; Imanishi, J.; Mazda, O. In vivo electroporation-mediated transfer of interleukin-12 and interleukin-18 genes induces significant antitumor effects against melanoma in mice. Gene Ther. 2001, 8, 1234–1240. [Google Scholar] [CrossRef] [PubMed]
- Bachy, M.; Boudet, F.; Bureau, M.; Girerd-Chambaz, Y.; Wils, P.; Scherman, D.; Meric, C. Electric pulses increase the immunogenicity of an influenza DNA vaccine injected intramuscularly in the mouse. Vaccine 2001, 19, 1688–1693. [Google Scholar] [CrossRef]
- Babiuk, S.; Baca-Estrada, M.E.; Foldvari, M.; Middleton, D.M.; Rabussay, D.; Widera, G.; Babiuk, L.A. Increased gene expression and inflammatory cell infiltration caused by electroporation are both important for improving the efficacy of DNA vaccines. J. Biotechnol. 2004, 110, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chiarella, P.; Massi, E.; De Robertis, M.; Sibilio, A.; Parrella, P.; Fazio, V.M.; Signori, E. Electroporation of skeletal muscle induces danger signal release and antigen-presenting cell recruitment independently of DNA vaccine administration. Expert Opin. Biol. Ther. 2008, 8, 1645–1657. [Google Scholar] [CrossRef]
- Mathiesen, I. Electropermeabilization of skeletal muscle enhances gene transfer in vivo. Gene Ther. 1999, 6, 508–514. [Google Scholar] [CrossRef]
- Satkauskas, S.; Andre, F.; Bureau, M.F.; Scherman, D.; Miklavcic, D.; Mir, L.M. Electrophoretic component of electric pulses determines the efficacy of in vivo DNA electrotransfer. Hum. Gene Ther. 2005, 16, 1194–1201. [Google Scholar] [CrossRef]
- Satkauskas, S.; Bureau, M.F.; Puc, M.; Mahfoudi, A.; Scherman, D.; Miklavcic, D.; Mir, L.M. Mechanisms of in vivo DNA electrotransfer: Respective contributions of cell electropermeabilization and DNA electrophoresis. Mol. Ther. 2002, 5, 133–140. [Google Scholar] [CrossRef]
- Bureau, M.F.; Gehl, J.; Deleuze, V.; Mir, L.M.; Scherman, D. Importance of association between permeabilization and electrophoretic forces for intramuscular DNA electrotransfer. Biochim. Biophys. Acta 2000, 1474, 353–359. [Google Scholar] [CrossRef]
- Von Groll, A.; Levin, Y.; Barbosa, M.C.; Ravazzolo, A.P. Linear DNA low efficiency transfection by liposome can be improved by the use of cationic lipid as charge neutralizer. Biotechnol. Prog. 2006, 22, 1220–1224. [Google Scholar] [CrossRef]
- Karpati, G.; Pari, G.; Molnar, M.J. Molecular therapy for genetic muscle diseases—Status 1999. Clin. Genet. 1999, 55, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.L.; Bou-Gharios, G.; Partridge, T.A. Non-viral gene delivery in skeletal muscle: A protein factory. Gene Ther. 2003, 10, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Herweijer, H.; Wolff, J.A. Progress and prospects: Naked DNA gene transfer and therapy. Gene Ther. 2003, 10, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Wolff, J.A.; Malone, R.W.; Williams, P.; Chong, W.; Acsadi, G.; Jani, A.; Felgner, P.L. Direct gene transfer into mouse muscle in vivo. Science 1990, 247, 1465–1468. [Google Scholar] [CrossRef]
- Acsadi, G.; Dickson, G.; Love, D.R.; Jani, A.; Walsh, F.S.; Gurusinghe, A.; Wolff, J.A.; Davies, K.E. Human dystrophin expression in mdx mice after intramuscular injection of DNA constructs. Nature 1991, 352, 815–818. [Google Scholar] [CrossRef] [PubMed]
- Schratzberger, P.; Krainin, J.G.; Schratzberger, G.; Silver, M.; Ma, H.; Kearney, M.; Zuk, R.F.; Brisken, A.F.; Losordo, D.W.; Isner, J.M. Transcutaneous ultrasound augments naked DNA transfection of skeletal muscle. Mol. Ther. 2002, 6, 576–583. [Google Scholar] [CrossRef]
- Yamashita, Y.; Shimada, M.; Tachibana, K.; Harimoto, N.; Tsujita, E.; Shirabe, K.; Miyazaki, J.; Sugimachi, K. In vivo gene transfer into muscle via electro-sonoporation. Hum. Gene Ther. 2002, 13, 2079–2084. [Google Scholar] [CrossRef]
- Danialou, G.; Comtois, A.S.; Dudley, R.W.; Nalbantoglu, J.; Gilbert, R.; Karpati, G.; Jones, D.H.; Petrof, B.J. Ultrasound increases plasmid-mediated gene transfer to dystrophic muscles without collateral damage. Mol. Ther. 2002, 6, 687–693. [Google Scholar] [CrossRef]
- Hacein-Bey-Abina, S.; Von Kalle, C.; Schmidt, M.; McCormack, M.P.; Wulffraat, N.; Leboulch, P.; Lim, A.; Osborne, C.S.; Pawliuk, R.; Morillon, E.; et al. LMO2—Associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science 2003, 302, 415–419. [Google Scholar] [CrossRef]
- Broeke, A.V.; Burny, A. Retroviral vector biosafety: Lessons from sheep. J. Biomed. Biotechnol. 2003, 1, 9–12. [Google Scholar] [CrossRef]
- Reyes-Sandoval, A.; Ertl, H.C. CpG methylation of a plasmid vector results in extended transgene product expression by circumventing induction of immune responses. Mol. Ther. 2004, 9, 249–261. [Google Scholar] [CrossRef] [PubMed]
- McMahon, J.M.; Wells, K.E.; Bamfo, J.E.; Cartwright, M.A.; Wells, D.J. Inflammatory responses following direct injection of plasmid DNA into skeletal muscle. Gene Ther. 1998, 5, 1283–1290. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wooddell, C.I.; Subbotin, V.M.; Sebestyén, M.G.; Griffin, J.B.; Zhang, G.; Schleef, M.; Braun, S.; Huss, T.; Wolff, J.A. Muscle damage after delivery of naked plasmid DNA into skeletal muscles is batch dependent. Hum. Gene Ther. 2011, 22, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Lara, A.R.; Ramirez, O.T. Plasmid DNA production for therapeutic applications. Methods Mol. Biol. 2012, 824, 271–303. [Google Scholar] [PubMed]
- Roda, A.; Pasini, P.; Mirasoli, M.; Michelini, E.; Guardigli, M. Biotechnological applications of bioluminescence and chemiluminescence. Trends Biotechnol. 2004, 22, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Close, D.M.; Xu, T.; Sayler, G.S.; Ripp, S. In vivo bioluminescent imaging (BLI): Noninvasive visualization and interrogation of biological processes in living animals. Sensors 2011, 11, 180–206. [Google Scholar] [CrossRef]
- Prescher, J.A.; Contag, C.H. Guided by the light: Visualizing biomolecular processes in living animals with bioluminescence. Curr. Opin. Chem. Biol. 2010, 14, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Pavselj, N.; Preat, V. DNA electrotransfer into the skin using a combination of one high- and one low-voltage pulse. J. Control. Release 2005, 106, 407–415. [Google Scholar] [CrossRef]
- Gothelf, A.; Mahmood, F.; Dagnaes-Hansen, F.; Gehl, J. Importance of electrodes in gene electrotransfer to porcine skin; evaluated by efficacy of transgene expression and electric field calculation. Mol. Ther. 2011, 19, S174. [Google Scholar]
- Andre, F.M.; Gehl, J.; Sersa, G.; Préat, V.; Hojman, P.; Eriksen, J.; Golzio, M.; Cemazar, M.; Pavselj, N.; Rols, M.P.; et al. Efficiency of high- and low voltage pulse combinations for gene electrotransfer in muscle, liver, tumor and skin. Hum. Gene Ther. 2008, 19, 1261–1272. [Google Scholar] [CrossRef]
- Inouye, S.; Tsuji, F.I. Aequorea green fluorescent protein. Expression of the gene and fluorescence characteristics of the recombinant protein. FEBS Lett. 1994, 341, 277–280. [Google Scholar] [CrossRef]
- Hojman, P.; Gissel, H.; Gehl, J. Sensitive and precise regulation of haemoglobin after gene transfer of erythropoietin to muscle tissue using electroporation. Gene Ther. 2007, 14, 950–959. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Couffinhal, T.; Kearney, M.; Sullivan, A.; Silver, M.; Tsurumi, Y.; Isner, J.M. Histochemical staining following LacZ gene transfer underestimates transfection efficiency. Hum. Gene Ther. 1997, 8, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Molnar, M.J.; Gilbert, R.; Lu, Y.; Liu, A.B.; Guo, A.; Larochelle, N.; Orlopp, K.; Lochmuller, H.; Petrof, B.J.; Nalbantoglu, J.; et al. Factors influencing the efficacy, longevity and safety of electroporation-assisted plasmid-based gene transfer into mouse muscles. Mol. Ther. 2004, 10, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.A.; Khan, A.S.; Pope, M.A.; Draghia-Akli, I.R. Muscle characteristics affect plasmid expression following electroporation in both large and small animals. Mol. Ther. 2007, 15, S349. [Google Scholar]
- Blaveri, K.; Heslop, L.; Yu, D.S.; Rosenblatt, J.D.; Gross, J.G.; Partridge, T.A.; Morgan, J.E. Patterns of repair of dystrophic mouse muscle: Studies on isolated fibers. Dev. Dyn. 1999, 216, 244–256. [Google Scholar] [CrossRef]
- Taylor, J.; Babbs, C.F.; Alzghoul, M.B.; Olsen, A.; Latour, M.; Pond, A.L.; Hannon, K. Optimization of ectopic gene expression in skeletal muscle through DNA transfer by electroporation. BMC Biotechnol. 2004, 4, 11. [Google Scholar] [CrossRef]
- Relaix, F.; Zammit, P.S. Satellite cells are essential for skeletal muscle regeneration: The cell on the edge returns centre stage. Development 2012, 139, 2845–2856. [Google Scholar] [CrossRef]
- Zammit, P.S.; Heslop, L.; Hudon, V.; Rosenblatt, J.D.; Tajbakhsh, S.; Buckingham, M.E.; Beauchamp, J.R.; Partridge, T.A. Kinetics of myoblast proliferation show that resident satellite cells are competent to fully regenerate skeletal muscle fibers. Exp. Cell Res. 2002, 281, 39–49. [Google Scholar] [CrossRef]
- Peng, B.W.; Zhao, Y.G.; Lu, H.L.; Pang, W.K.; Xu, Y.H. In vivo plasmid DNA electroporation resulted in transfection of satellite cells and lasting transgene expression in regenerated muscle fibers. Biochem. Biophys. Res. Commun. 2005, 338, 1490–1498. [Google Scholar] [CrossRef]
- Pruchnic, R.; Cao, B.; Peterson, Z.Q.; Xiao, X.; Li, J.; Samulski, R.J.; Epperly, M.; Huard, J. The use of adeno-associated virus to circumvent the maturation-dependent viral transduction of muscle fibers. Hum. Gene Ther. 2000, 11, 521–536. [Google Scholar] [CrossRef] [PubMed]
- Hartikka, J.; Sukhu, L.; Buchner, C.; Hazard, D.; Bozoukova, V.; Margalith, M.; Nishioka, W.K.; Wheeler, C.J.; Manthorp, M.; Sawdey, M. Electroporation-facilitated delivery of plasmid DNA in skeletal muscle: Plasmid dependence of muscle damage and effect of poloxamer 188. Mol. Ther. 2001, 4, 407–415. [Google Scholar] [CrossRef]
- Favre, D.; Cherel, Y.; Provost, N.; Blouin, V.; Ferry, N.; Moullier, P.; Salvetti, A. Hyaluronidase enhances recombinant adenoassociated virus (rAAV)- mediated gene transfer in the rat skeletal muscle. Gene Ther. 2000, 7, 1417–1420. [Google Scholar] [CrossRef] [PubMed]
- Hayes, K.E.; Gades, N.M.; Toth, L.A.; Raucci, J.A., Jr. An evaluation of analgesic regimens for abdominal surgery in mice. Contemp. Top. Lab. Anim. Sci. 2000, 39, 18–23. [Google Scholar] [PubMed]
- Salama, R.A.M.; El Gayar, N.H.; Georgy, S.S.; Hamza, M. Equivalent intraperitoneal doses of ibuprofen supplemented in drinking water or in diet: A behavioral and biochemical assay using antinociceptive and thromboxane inhibitory dose-response curves in mice. PeerJ 2016, 4, e2239. [Google Scholar] [CrossRef] [PubMed]
- André, F.M.; Cournil-Henrionnet, C.; Vernerey, D.; Opolon, P.; Mir, L.M. Variability of naked DNA expression after direct local injection: The influence of the injection speed. Gene Ther. 2006, 13, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Tevz, G.; Pavlin, D.; Kamensek, U.; Kranjc, S.; Mesojednik, S.; Coer, A.; Sersa, G.; Cemazar, M. Gene electrotransfer into murine skeletal muscle: A systematic analysis of parameters for long-term gene expression. Technol. Cancer Res. Treat. 2008, 7, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Bureau, M.F.; Naimi, S.; Ibad, R.T.; Seguin, J.; Georger, C.; Arnould, E.; Maton, L.; Blanche, F.; Delaere, P.; Schennan, D. Intramuscular plasmid DNA electrotransfer: Biodistribution and degradation. Biochim. Biophys. Acta 2004, 676, 138–148. [Google Scholar] [CrossRef]
- Faurie, C.; Golzio, M.; Moller, P.; Teissié, J.; Rols, M.P. Cell and animal imaging of electrically mediated gene transfer. DNA Cell Biol. 2003, 22, 777–783. [Google Scholar] [CrossRef]
- Lefesvre, P.; Attema, J.; Van Bekkum, D. A comparison of efficacy and toxicity between electroporation and adenoviral gene transfer. BMC Mol. Biol. 2002, 3, 12. [Google Scholar] [CrossRef]
- Bettan, M.; Emmanuel, F.; Darteil, R.; Caillaud, J.M.; Soubrier, F.; Delaere, P.; Branelec, D.; Mahfoudi, A.; Duverger, N.; Scherman, D. High-level protein secretion into blood circulation after electric pulse-mediated gene transfer into skeletal muscle. Mol. Ther. 2000, 2, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Schertzer, J.D.; Plant, D.R.; Lynch, G.S. Optimizing plasmid-based gene transfer for investigating skeletal muscle structure and function. Mol. Ther. 2006, 13, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Golzio, M.; Escoffre, J.M.; Teissié, J. shRNA-mediated gene knockdown in skeletal muscle. Methods Mol. Biol. 2012, 798, 491–501. [Google Scholar] [PubMed]
- Donà, M.; Sandri, M.; Rossini, K.; Dell’Aica, I.; Podhorska-Okolow, M.; Carraro, U. Functional in vivo gene transfer into the myofibers of adult skeletal muscle. Biochem. Biophys. Res. Commun. 2003, 312, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Hoover, F.; Kalhovde, J.M. A double-injection DNA electroporation protocol to enhance in vivo gene delivery in skeletal muscle. Anal. Biochem. 2000, 285, 175–178. [Google Scholar] [CrossRef]
- DiFranco, M.; Quinonez, M.; Capote, J.; Vergara, J. DNA transfection of mammalian skeletal muscles using in vivo electroporation. J. Vis. Exp. 2009, 19, 1520. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.V.; Faulkner, J.A. Contractile properties of skeletal muscles from young, adult and aged mice. J. Physiol. 1988, 404, 71–82. [Google Scholar] [CrossRef]
- Bloemberg, D.; Quadrilatero, J. Rapid determination of myosin heavy chain expression in rat, mouse and human skeletal muscle using multicolor immunofluorescence analysis. PLoS ONE 2012, 7, e35273. [Google Scholar] [CrossRef]
- Denies, M.S.; Johnson, J.; Maliphol, A.B.; Bruno, M.; Kim, A.; Rizvi, A.; Rustici, K.; Medler, S. Diet-induced obesity alters skeletal muscle fiber types of male but not female mice. Physiol. Rep. 2014, 2, e00204. [Google Scholar] [CrossRef]
- Kilikevicius, A.; Venckunas, T.; Zelniene, R.; Carroll, A.M.; Lionikaite, S.; Ratkevicius, A.; Lionikas, A. Divergent physiological characteristics and responses to endurance training among inbred mouse strains. Scand. J. Med. Sci. Sports. 2013, 23, 657–668. [Google Scholar] [CrossRef]
- Augusto, V.; Padovani, C.R.; Campos, R.; Eduardo, G. Skeletal muscle fiber types in C57Bl6J mice. Braz. J. Morphol. Sci. 2004, 21, 89–94. [Google Scholar]
- Sher, J.; Cardasis, C. Skeletal muscle fiber types in the adult mouse. Acta Neurol. Scand. 1976, 54, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Leem, M.J.; Cho, S.S.; Jang, H.S.; Lim, Y.S.; You, J.R.; Park, J.; Suhm, H.; Kim, J.A.; Park, J.S.; Kim, D.K. Optimal salt concentration of vehicle for plasmid DNA enhances gene transfer mediated by electroporation. Exp. Mol. Med. 2002, 34, 265–272. [Google Scholar]
- Gehl, J.; Sorensen, T.H.; Nielsen, K.; Raskmark, P.; Nielsen, S.L.; Skovsgaard, T.; Mir, L.M. In vivo electroporation of skeletal muscle: Threshold, efficacy and relation to electric field distribution. Biochim. Biophys. Acta 1999, 1428, 233–240. [Google Scholar] [CrossRef]
- Gehl, J.; Mir, L.M. Determination of optimal parameters for in vivo gene transfer by electroporation, using a rapid in vivo test for cell permeabilization. Biochem. Biophys. Res. Commun. 1999, 261, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Golzio, M.; Rols, M.P.; Gabriel, B.; Teissie, J. Optical imaging of in vivo gene expression: A critical assessment of the morphology and associated technologies. Gene Ther. 2004, 11, S85–S91. [Google Scholar] [CrossRef] [PubMed]
- Gravier, R.; Dory, D.; Laurentie, M.; Bougeard, S.; Cariolet, R.; Jestin, A. In vivo tissue distribution and kinetics of a pseudorabies virus plasmid DNA vaccine after intramuscular injection in swine. Vaccine 2007, 25, 6930–6938. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Sun, S.H.; Guo, Y.J.; Chen, Z.H.; Huang, L.; Gao, Y.J.; Wan, B.; Zhu, W.J.; Xu, G.X.; Wang, J.J. Tissue distribution of a plasmid DNA containing epitopes of foot-and-mouth disease virus in mice. Vaccine 2005, 23, 5632–5640. [Google Scholar] [CrossRef]
- Durieux, A.C.; Bonnefoy, R.; Busso, T.; Freyssenet, D. In vivo gene electrotransfer into skeletal muscle: Effects of plasmid DNA on the occurrence and extent of muscle damage. J. Gene Med. 2004, 6, 809–816. [Google Scholar] [CrossRef]
- Zhang, G.; Budker, V.; Williams, P.; Subbotin, V.; Wolff, J.A. Efficient expression of naked DNA delivered intraarterially to limb muscles of nonhuman primates. Hum. Gene Ther. 2001, 12, 427–438. [Google Scholar] [CrossRef]
- Roche, J.A.; Ford-Speelman, D.L.; Ru, L.W.; Densmore, A.L.; Roche, R.; Reed, P.W.; Bloch, R.J. Physiological and histological changes in skeletal muscle following in vivo gene transfer by electroporation. Am. J. Physiol. Cell Physiol. 2011, 301, C1239–C1250. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Nishikawa, M.; Takakura, Y. Development of safe and effective nonviral gene therapy by eliminating CpG motifs from plasmid DNA vector. Front. Biosci. 2012, 4, 133–141. [Google Scholar] [CrossRef]
- Tamura, T.; Nishi, T.; Goto, T.; Takeshima, H.; Dev, S.B.; Ushio, Y.; Sakata, T. Intratumoral delivery of interleukin 12 expression plasmids with in vivo electroporation is effective for colon and renal cancer. Hum. Gene Ther. 2001, 12, 1265–1276. [Google Scholar] [CrossRef] [PubMed]
- Rizzuto, G.; Cappelletti, M.; Maione, D.; Savino, R.; Lazzaro, D.; Costa, P.; Mathiesen, I.; Cortese, R.; Ciliberto, G.; Laufer, R.; et al. Efficient and regulated erythropoietin production by naked DNA injection and muscle electroporation. Proc. Natl. Acad. Sci. USA 1999, 96, 6417–6422. [Google Scholar] [CrossRef] [PubMed]
- Kreiss, P.; Bettan, M.; Crouzet, J.; Scherman, D. Erythropoietin secretion and physiological effect in mouse after intramuscular plasmid DNA electrotransfer. J. Gene Med. 1999, 1, 245–250. [Google Scholar] [CrossRef]
- Li, S.; Zhang, X.; Xia, X.; Zhou, L.; Breau, R.; Suen, J.; Hanna, E. Intramuscular electroporation delivery of IFN-a gene therapy for inhibition of tumor growth located at a distant site. Gene Ther. 2001, 8, 400–407. [Google Scholar] [CrossRef]
- Fewell, J.G.; MacLaughlin, F.; Mehta, V.; Gondo, M.; Nicol, F.; Wilson, E.; Smith, L.C. Gene therapy for the treatment of hemophilia B using PINC-formulated plasmid delivered to muscle with electroporation. Mol. Ther. 2001, 3, 574–583. [Google Scholar] [CrossRef]
- Bodles-Brakhop, A.M.; Heller, R.; Draghia-Akli, R. Electroporation for the delivery of DNA-based vaccines and immunotherapeutics. Mol. Ther. 2009, 17, 585–592. [Google Scholar] [CrossRef]
- Yu, Z.; Yan, B.; Gao, L.; Dong, C.; Zhong, J.; D’Ortenzio, M.; Nguyen, B.; Lee, S.S.; Hu, X.; Liang, F. Targeted delivery of bleomycin: A comprehensive anticancer review. Curr. Cancer Drug Targets 2016, 16, 509–521. [Google Scholar] [CrossRef]
- Andre, F.; Mir, L.M. DNA electrotransfer: Its principles and an updated review of its therapeutic applications. Gene Ther. 2004, 11, S33–S42. [Google Scholar] [CrossRef]
- Egeland, C.; Baeksgaard, L.; Johannesen, H.H.; Löfgren, J.; Plaschke, C.C.; Svendsen, L.B.; Gehl, J.; Achiam, M.P. Endoscopic electrochemotherapy for esophageal cancer: A phase I clinical study. Endosc. Int. Open 2018, 6, E727–E734. [Google Scholar] [CrossRef] [PubMed]
- Mir, L.M.; Orlowski, S.; Paoletti, C.; Belehradek, J., Jr. Electrochemotherapy potentiation of antitumour effect of bleomycin by local electric pulses. Eur. J. Cancer. 1991, 27, 68–72. [Google Scholar] [CrossRef]
- Miklavcic, D. Handbook of Electroporation, 1st ed.; Springer: Cham, Switzerland, 2017; pp. 1–2998. [Google Scholar]



| Used Vectors | The Main Advantages | Limitations |
|---|---|---|
| Adenoviruses | High efficiency of in vivo and ex vivo transduction; High level of transgene expression; Possibility of obtaining high titre virus preparations. | Cytotoxicity; Strong immune response to viral proteins restricting repeated administration; Short-term transgene expression (no integration with the genome). |
| Retroviruses | Long-term transgene expression (integration with the genome). | Introduction of the transgene possible only to dividing cells; Possible insertion mutations (integration into the genome); Application limited mainly to transducing cells ex vivo. |
| Lentiviruses | Introduction of the transgene possible also to non-dividing cells; Long-term transgene expression; | Possible insertion mutations. |
| AAV (Adeno-associated viruses) | Low immunogenicity; Introduction of the transgene possible also to non-dividing cells; Long-term transgene expression. | Possible insertion mutations; Difficult quality control. |
| Herpes virus | Introduction of the transgene possible also to non-dividing cells; Transgenes up to 15 kbp. | Short-term expression of the transgene. |
| Method for Genetic Material Introduction | Method of Application | Advantages | Limitations |
|---|---|---|---|
| Electroporation | Uses an electric current pulse to form a transient branches in the cell membrane | Highly effective, reproducible, directed gene transfer, the possibility of transferring the DNA macromolecule | Impossible to use on a large area; requires surgical intervention when transfer to internal organs; the use of high voltage can disturb the DNA |
| Sonoporation | Uses ultrasounds to induce a transient state of cell membrane permeability | Harmless, non-invasive, DNA transfer into internal organs without the need for surgical procedure | Low efficiency |
| Muscle | ||||
|---|---|---|---|---|
| Gastrocnemious | Tibialis | Soleus | Foot Muscle: (Flexor Digitorum Brevis (FDB) and Interosseus (IO) Muscle) | |
| Hyaluronidase in sterile Tyrode | 0.4 U/μL (30 μL) | 8 U/20 μL | - | 6 U/10 μL |
| Plasmid volume (μL) | 50 (4 × 12.5) (1–2 μg/1 μL) | 30 (2 μg/1 μL) | 20 (2 μg/1 μL) | 2–5 μg plasmid/μL volume (10–20 μL) |
| Syringe needle | 29 G | 26 G | 34 G | 33 G |
| Pulses | 2 × 4 | 5 | 8 | 20 |
| Voltage (V/cm) | 175 | 120 | 200 | 100 |
| Pulse width (ms) | 100 | 20 | 20 | 20 |
| Frequency (Hz) | 1 | 1 | 1 | 1 |
| Type of electrodes | plate electrodes 10 × 10 mm | plate electrodes 7 × 7 mm | plate electrodes 10 × 10 mm | plated acupuncture needle |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokołowska, E.; Błachnio-Zabielska, A.U. A Critical Review of Electroporation as A Plasmid Delivery System in Mouse Skeletal Muscle. Int. J. Mol. Sci. 2019, 20, 2776. https://doi.org/10.3390/ijms20112776
Sokołowska E, Błachnio-Zabielska AU. A Critical Review of Electroporation as A Plasmid Delivery System in Mouse Skeletal Muscle. International Journal of Molecular Sciences. 2019; 20(11):2776. https://doi.org/10.3390/ijms20112776
Chicago/Turabian StyleSokołowska, Emilia, and Agnieszka Urszula Błachnio-Zabielska. 2019. "A Critical Review of Electroporation as A Plasmid Delivery System in Mouse Skeletal Muscle" International Journal of Molecular Sciences 20, no. 11: 2776. https://doi.org/10.3390/ijms20112776
APA StyleSokołowska, E., & Błachnio-Zabielska, A. U. (2019). A Critical Review of Electroporation as A Plasmid Delivery System in Mouse Skeletal Muscle. International Journal of Molecular Sciences, 20(11), 2776. https://doi.org/10.3390/ijms20112776





