Emerging Biomarkers in Vascular Cognitive Impairment and Dementia: From Pathophysiological Pathways to Clinical Application
Abstract
1. Background
Vascular Cognitive Impairment and Dementia: An Umbrella Term
2. Methods
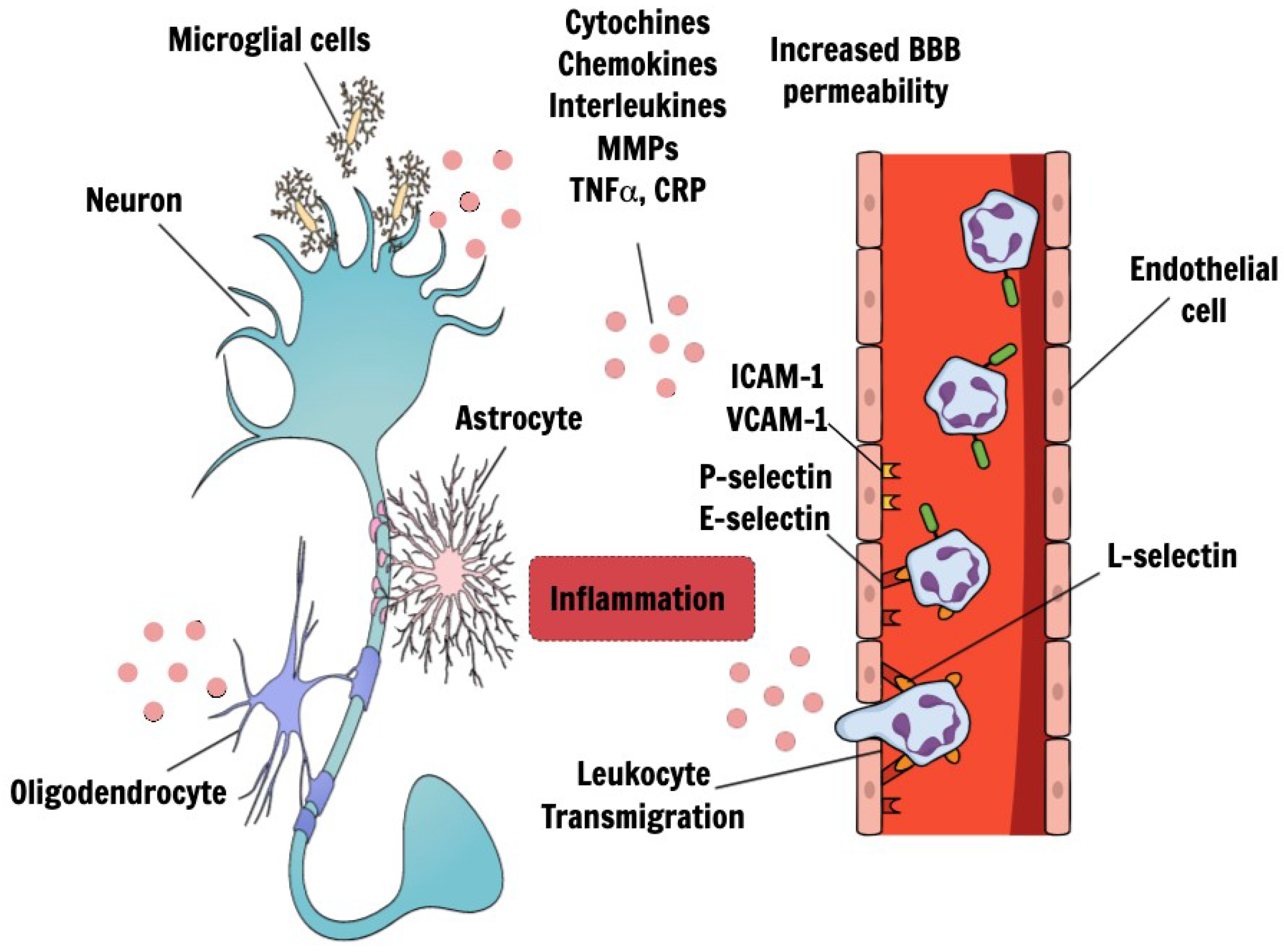
3. Pathophysiological Pathways of VCID
4. Endothelial Dysfunction
5. Blood-Brain Barrier and Choroid Lexus Breakdown
6. The Role of Inflammation
7. Biomarkers
- (1)
- Biomarkers of Inflammatory Response;
- (2)
- Biomarkers of Central Nervous System Tissue Injuries;
- (3)
- Biomarkers of Coagulation and Thrombosis;
- (4)
- Circulatory miRNAs.
7.1. Biomarkers of Inflammatory Response
7.2. Biomarkers of Central Nervous System Tissue Injuries
7.3. Biomarkers of Coagulation and Thrombosis
7.4. Circulating microRNA
8. Discussion
9. Conclusions and Future Perspectives
Conflicts of Interest
Abbreviations
| VCID | vascular cognitive impairment and dementia |
| AD | Alzheimer’s disease |
| CVD | cerebrovascular disease |
| VaD | vascular dementia |
| VASCOG | Society for Vascular Behavioral and Cognitive Disorders |
| BBB | blood–brain barrier |
| NVU | neurovascular unit |
| MID | multi-infarct dementia |
| SVD | small vessels disease |
| WMHs | white matter hyperintensities |
| CSF | cerebrospinal fluid |
| miRNA | MicroRNA |
| CBF | cerebral blood flow |
| CAA | cerebral amyloid angiopathy |
| WMLs | white matter lesions |
| EPVS | Enlarged Perivascular spaces |
| MRI | Magnetic Resonance Imaging |
| ICAM 1 | intercellular adhesion molecule-1 |
| VCAM-1 | vascular cell adhesion molecule-1 |
| MMP | matrix metalloproteinase |
| CNS | central nervous system |
| IL | interleukin |
| TNFα | Tumor necrosis factor α |
| TLR4 | toll-like receptor 4 |
| CRP | C-reactive protein |
| ACT | alpha 1-antichymotrypsin |
| NSE | neuron specific enolase |
| NMDAR | N-methyl-D-aspartate receptor |
| MBP | myelin basic protein |
| NfL | Neurofilament light chain |
| CADASIL | Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy |
| Lp PLA2 | lipoprotein-associated phospholipase A2 |
References
- Skrobot, O.A.; O’Brien, J.; Black, S.; Chen, C.; DeCarli, C.; Erkinjuntti, T.; Ford, G.A.; Kalaria, R.N.; Pantoni, L.; Pasquier, F.; et al. The Vascular Impairment of Cognition Classification Consensus Study. Alzheimer’s Dement. 2016, 13, 624–633. [Google Scholar] [CrossRef]
- Murphy, M.P.; Corriveau, R.A.; Wilcock, D.M. Vascular contributions to cognitive impairment and dementia (VCID). Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 2016, 1862, 857–859. [Google Scholar] [CrossRef] [PubMed]
- Lang, B.; Kindy, M.S.; Kozel, F.A.; Schultz, S.K.; Taheri, S. Multi-Parametric Classification of Vascular Cognitive Impairment and Dementia: The Impact of Diverse CerebrovRefascular Injury Biomarkers. J. Alzheimer’s Dis. 2018, 62, 39–60. [Google Scholar] [CrossRef] [PubMed]
- Paradise, M.B.; Sachdev, P.S. Vascular Cognitive Disorder. Semin. Neurol. 2019, 392, 241–250. [Google Scholar] [CrossRef]
- Sachdev, P.; Kalaria, R.; O’Brien, J.; Skoog, I.; Alladi, S.; Black, S.E.; Blacker, D.; Blazer, D.G.; Chen, C.; Chui, H.; et al. Diagnostic criteria for vascular cognitive disorders: A VASCOG statement. Alzheimer Dis. Assoc. Disord. 2014, 28, 206–218. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-V, 5th ed.; American Psychiatric Publishing: Arlington, VA, USA, 2014. [Google Scholar]
- Van der Flier, W.M.; Skoog, I.; Schneider, J.A.; Pantoni, L.; Mok, V.; Chen, C.L.H.; Scheltens, P. Vascular cognitive impairment. Nat. Rev. Dis. Primers 2018, 15, 18003. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, P.B.; Counts, S.E.; Nyenhuis, D. Vascular cognitive impairment and dementia. Biochim. Biophys. Acta (BBA)—Bioenerg. 2015, 1862, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Hachinski, V.C.; Lassen, N.A.; Marshall, J. Multi-infarct dementia. A cause of mental deterioration in the elderly. Lancet 1974, 2, 207–210. [Google Scholar] [CrossRef]
- Rosenberg, G.A. Extracellular matrix inflammation in vascular cognitive impairment and dementia. Clin. Sci. 2017, 131, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Hachinski, V.; World Stroke, O. Stroke and Potentially Preventable Dementias Proclamation: Updated World Stroke Day Proclamation. Stroke 2015, 46, 3039–3040. [Google Scholar] [CrossRef]
- Rosenberg, G.A.; Bjerke, M.; Wallin, A. Multimodal markers of inflammation in the subcortical ischemic vascular disease type of vascular cognitive impairment. Stroke 2014, 45, 1531–1538. [Google Scholar] [CrossRef] [PubMed]
- Vilar-Bergua, A.; Riba-Llena, I.; Nafría, C.; Bustamante, A.; Llombart, V.; Delgado, P.; Montaner, J. Blood and CSF biomarkers in brain subcortical ischemic vascular disease: Involved pathways and clinical applicability. J. Cereb. Blood Flow Metab. 2016, 36, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Kalaria, R.N. Neuropathological diagnosis of vascular cognitive impairment and vascular dementia with implications for Alzheimer’s disease. Acta Neuropathol. 2016, 131, 659–685. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Sun, Y.; Lu, Z.; Leak, R.K.; Zhang, F. The impact of cerebrovascular aging on vascular cognitive impairment and dementia. Ageing Res. Rev. 2017, 34, 15–29. [Google Scholar] [CrossRef] [PubMed]
- McAleese, K.E.; Alafuzoff, I.; Charidimou, A.; de Reuck, J.; Grinberg, L.T.; Hainsworth, A.H.; Hortobagyi, T.; Ince, P.; Jellinger, K.; Gao, J.; et al. Post-mortem assessment in vascular dementia: Advances and aspirations. BMC Med. 2016, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Murr, J.; Carmichael, P.-H.; Julien, P.; Laurin, D. Plasma oxidized low-density lipoprotein levels and risk of Alzheimer’s disease. Neurobiol. Aging 2014, 35, 1833–1838. [Google Scholar] [CrossRef]
- Dong, H.; Li, J.; Huang, L.; Chen, X.; Li, D.; Wang, T.; Hu, C.; Xu, J.; Zhang, C.; Zen, K. Serum MicroRNA profiles serve as novel biomarkers for the diagnosis of Alzheimer’s disease. Dis. Markers 2015, 2015, 625–659. [Google Scholar] [CrossRef] [PubMed]
- Duits, F.H.; Hernandez-Guillamon, M.; Montaner, J.; Goos, J.D.C.; Montañola, A.; Wattjes, M.P.; Barkhof, F.; Scheltens, P.; Teunissen, C.E.; Van Der Flier, W.M. Matrix Metalloproteinases in Alzheimer’s Disease and Concurrent Cerebral Microbleeds. J. Alzheimer’s Dis. 2015, 48, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, H.; Hanyu, H.; Fukasawa, R.; Hirao, K.; Shimizu, S.; Kanetaka, H.; Iwamoto, T. Differences in peripheral oxidative stress markers in Alzheimer’s disease, vascular dementia and mixed dementia patients. Geriatr. Gerontol. Int. 2015, 15, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Hilal, S.; Chai, Y.L.; Ikram, M.K.; Elangovan, S.; Yeow, T.B.; Xin, X.; Chong, J.Y.; Venketasubramanian, N.; Richards, A.M.; Chong, J.P. Markers of cardiac dysfunction in cognitive impairment and dementia. Medicine (Baltimore) 2015, 94, e297. [Google Scholar] [CrossRef] [PubMed]
- Teunissen, C.E.; van der Flier, W.M.; Scheltens, P.; Duits, A.; Wijnstok, N.; Nijpels, G.; Dekker, J.M.; Blankenstein, R.M.A.; Heijboer, A.C. Serum leptin is not altered nor related to cognitive decline in Alzheimer’s disease. J. Alzheimer’s Dis. 2015, 44, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhao, J.; Xu, R.; Zhao, J.; Duan, S. Identification of Pivotal Markers in Vascular Dementia Based on Proteomics Data. Dement. Geriatr. Cogn. Disord. 2015, 39, 312–320. [Google Scholar] [CrossRef]
- Castellazzi, M.; Trentini, A.; Romani, A.; Valacchi, G.; Bellini, T.; Bonaccorsi, G.; Fainardi, E.; Cavicchio, C.; Passaro, A.; Zuliani, G. Decreased arylesterase activity of paraoxonase-1 (PON-1) might be a common denominator of neuroinflammatory and neurodegenerative diseases. Int. J. Biochem. Cell Biol. 2016, 81, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liang, X.; Zhang, C.; Wang, J.; Chen, G.; Zhang, H.; Sun, Z. Correlation of thyroid dysfunction and cognitive impairments induced by subcortical ischemic vascular disease. Brain Behav. 2016, 6, e00452. [Google Scholar] [CrossRef] [PubMed]
- Dukic, L.; Simundic, A.-M.; Martinic-Popovic, I.; Kackov, S.; Diamandis, A.; Begcevic, I.; Diamandis, E.P. The role of human kallikrein 6, clusterin and adiponectin as potential blood biomarkers of dementia. Clin. Biochem. 2016, 49, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Horvath, I.; Jia, X.; Johansson, P.; Wang, C.; Moskalenko, R.; Steinau, A.; Forsgren, L.; Wågberg, T.; Svensson, J.; Zetterberg, H.; et al. Pro-inflammatory S100A9 Protein as a Robust Biomarker Differentiating Early Stages of Cognitive Impairment in Alzheimer’s Disease. ACS Chem. Neurosci. 2016, 20, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, K.; Miwa, K.; Okazaki, S.; Sakaguchi, M.; Mochizuki, H. Serum high-molecular-weight adiponectin level and incident dementia in patients with vascular risk factors. Eur. J. Neurol. 2016, 23, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Levada, O.A.; Cherednichenko, N.V.; Trailin, A.V.; Troyan, A.S. Plasma Brain-Derived Neurotrophic Factor as a Biomarker for the Main Types of Mild Neurocognitive Disorders and Treatment Efficacy: A Preliminary Study. Dis. Markers 2016, 2016, 1–7. [Google Scholar] [CrossRef]
- Mirza, S.S.; de Bruijn, R.F.; Koudstaal, P.J.; van den Meiracker, A.H.; Franco, O.H.; Hofman, A.; Tiemeier, H.; Ikram, M.A. The N-terminal pro B-type natriuretic peptide, and risk of dementia and cognitive decline: A 10-year follow-up study in the general population. J. Neurol. Neurosurg. Psychiatry 2016, 87, 356–362. [Google Scholar] [CrossRef]
- Nilsson, E.D.; Melander, O.; Elmståhl, S.; Lethagen, E.; Minthon, L.; Pihlsgård, M.; Nägga, K. Copeptin, a Marker of Vasopressin, Predicts Vascular Dementia but not Alzheimer’s Disease. J. Alzheimer’s Dis. 2016, 52, 1047–1053. [Google Scholar] [CrossRef]
- Pan, X.; Fei, G.; Lu, J.; Jin, L.; Pan, S.; Chen, Z.; Wang, C.; Sang, S.; Liu, H.; Hu, W. Measurement of Blood Thiamine Metabolites for Alzheimer’s Disease Diagnosis. EBio Med. 2015, 3, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Bednarska-Makaruk, M.; Graban, A.; Wiśniewska, A.; Łojkowska, W.; Bochyńska, A.; Gugała-Iwaniuk, M.; Sławińska, K.; Ługowska, A.; Ryglewicz, D.; Wehr, H. Association of adiponectin, leptin and resistin with inflammatory markers and obesity in dementia. Biogerontology 2017, 18, 561–580. [Google Scholar] [CrossRef] [PubMed]
- Busse, M.; Michler, E.; Von Hoff, F.; Dobrowolny, H.; Hartig, R.; Frodl, T. Alterations in the Peripheral Immune System in Dementia. J. Alzheimer’s Dis. 2017, 58, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Holm, H.; Nägga, K.; Nilsson, E.D.; Ricci, F.; Cinosi, E.; Melander, O.; Hansson, O.; Bachus, E.; Magnusson, M.; Fedorowski, A. N-Terminal Prosomatostatin and Risk of Vascular Dementia. Cerebrovasc. Dis. 2017, 44, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Holm, H.; Nägga, K.; Nilsson, E.D.; Ricci, F.; Melander, O.; Hansson, O.; Bachus, E.; Magnusson, M.; Fedorowski, A. Biomarkers of microvascular endothelial dysfunction predict incident dementia: A population-based prospective study. J. Intern. Med. 2017, 282, 94–101. [Google Scholar] [CrossRef]
- Hsu, P.-F.; Pan, W.-H.; Yip, B.-S.; Chen, R.C.-Y.; Cheng, H.-M.; Chuang, S.-Y. C-Reactive Protein Predicts Incidence of Dementia in an Elderly Asian Community Cohort. J. Am. Med Dir. Assoc. 2017, 18, 277.e7–277.e11. [Google Scholar] [CrossRef]
- Moretti, R.; Caruso, P.; Dal Ben, M.; Conti, C.; Gazzin, S.; Tiribelli, C. Vitamin D, Homocysteine, and Folate in Subcortical Vascular Dementia and Alzheimer Dementia. Front. Aging Neurosci. 2017, 30, 169. [Google Scholar] [CrossRef]
- Prabhakar, P.; Chandra, S.R.; Christopher, R. Circulating microRNAs as potential biomarkers for the identification of vascular dementia due to cerebral small vessel disease. Age Ageing 2017, 46, 861–864. [Google Scholar] [CrossRef]
- Quinlan, P.; Horvath, A.; Nordlund, A.; Wallin, A.; Svensson, J. Low serum insulin-like growth factor-I (IGF-I) level is associated with increased risk of vascular dementia. Psychoneuroendocrinology 2017, 86, 169–175. [Google Scholar] [CrossRef]
- Suridjan, I.; Herrmann, N.; Adibfar, A.; Saleem, M.; Andreazza, A.; Oh, P.I.; Lanctôt, K.L.; Naranjo, I.C. Lipid Peroxidation Markers in Coronary Artery Disease Patients with Possible Vascular Mild Cognitive Impairment. J. Alzheimer’s Dis. 2017, 58, 885–896. [Google Scholar] [CrossRef]
- Tang, S.-C.; Yang, K.-C.; Hu, C.-J.; Chiou, H.-Y.; Wu, C.C.; Jeng, J.-S. Elevated Plasma Level of Soluble Form of RAGE in Ischemic Stroke Patients with Dementia. Neuromol. Med. 2017, 19, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Vishnu, V.Y.; Modi, M.; Garg, V.K.; Mohanty, M.; Goyal, M.K.; Lal, V.; Mittal, B.R.; Prabhakar, S. Role of inflammatory and hemostatic biomarkers in Alzheimer’s and vascular dementia—A pilot study from a tertiary centre in Northern India. Asian J. Psychiatry 2017, 29, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Chen, Z.; Fu, Y.; Wei, X.; Liao, J.; Liu, X.; He, B.; Xu, Y.; Zou, J.; Yang, X.; et al. Plasma Cystatin C and High-Density Lipoprotein Are Important Biomarkers of Alzheimer’s Disease and Vascular Dementia: A Cross-Sectional Study. Front. Aging Neurosci. 2017, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Fu, S.; Zhao, L.; Zhen, B.; Ye, L.; Niu, X.; Li, X.; Zhang, P.; Bai, J. Quantitation of circulating GDF-11 and β2-MG in aged patients with age-related impairment in cognitive function. Clin. Sci. (Lond.) 2017, 131, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- Brombo, G.; Bonetti, F.; Ortolani, B.; Morieri, M.L.; Bosi, C.; Passaro, A.; Vigna, G.B.; Borgna, C.; Arcidicono, M.V.; Tisato, V.; et al. Lower Plasma Klotho Concentrations Are Associated with Vascular Dementia but Not Late-Onset Alzheimer’s Disease. Gerontology 2018, 64, 414–421. [Google Scholar] [CrossRef]
- Latourte, A.; Soumaré, A.; Bardin, T.; Perez-Ruiz, F.; Debette, S.; Richette, P. Uric acid and incident dementia over 12 years of follow-up: A population-based cohort study. Ann. Rheum. Dis. 2018, 77, 328–335. [Google Scholar] [CrossRef]
- Lauriola, M.; Paroni, G.; Ciccone, F.; Onofrio, G.D.; Cascavilla, L.; Paris, F.; Gravina, C.; Urbano, M.; Seripa, D.; Greco, A. Erythrocyte Associated Amyloid-β as Potential Biomarker to Diagnose Dementia. Curr. Alzheimer Res. 2018, 15, 381–385. [Google Scholar] [CrossRef]
- Shang, J.; Yamashita, T.; Fukui, Y.; Song, D.; Li, X.; Zhai, Y.; Nakano, Y.; Morihara, R.; Hishikawa, N.; Ohta, Y.; et al. Different Associations of Plasma Biomarkers in Alzheimer’s Disease, Mild Cognitive Impairment, Vascular Dementia, and Ischemic Stroke. J. Clin. Neurol. 2018, 14, 29–34. [Google Scholar] [CrossRef]
- Staszewski, J.; Piusińska-Macoch, R.; Brodacki, B.; Skrobowska, E.; Stępień, A. IL-6, PF-4, sCD40 L, and homocysteine are associated with the radiological progression of cerebral small-vessel disease: A 2-year follow-up study. Clin. Interv. Aging 2018, 13, 1135–1141. [Google Scholar] [CrossRef]
- Yang, T.T.; Liu, C.G.; Gao, S.C.; Zhang, Y.; Wang, P.C. The Serum Exosome Derived MicroRNA-135a, -193b, and -384 Were Potential Alzheimer’s Disease Biomarkers. Biomed. Environ. Sci. 2018, 31, 87–96. [Google Scholar]
- Staszewski, J.; Skrobowska, E.; Piusińska-Macoch, R.; Brodacki, B.; Stępień, A. IL-1α and IL-6 predict vascular events or death in patients with cerebral small vessel disease-Data from the SHEF-CSVD study. Adv. Med. Sci. 2019, 64, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Busse, S.; Busse, M.; Brix, B.; Probst, C.; Genz, A.; Bogerts, B.; Stoecker, W.; Steiner, J. Seroprevalence of n-methyl-d-aspartate glutamate receptor (NMDA-R) autoantibodies in aging subjects without neuropsychiatric disorders and in dementia patients. Eur. Arch. Psychiatry Clin. Neurosci. 2014, 264, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Herbert, M.K.; Aerts, M.B.; Kuiperij, H.B.; Claassen, J.A.H.R.; Spies, P.E.; Esselink, R.A.J.; Bloem, B.R.; Verbeek, M.M. Addition of MHPG to Alzheimer’s disease biomarkers improves differentiation of dementia with Lewy bodies from Alzheimer’s disease but not other dementias. Alzheimer’s Dement. 2014, 10, 448–455.e2. [Google Scholar] [CrossRef] [PubMed]
- Hermann, P.; Romero, C.; Schmidt, C.; Reis, C.; Zerr, I. CSF biomarkers and neuropsychological profiles in patients with cerebral small-vessel disease. PLoS ONE 2014, 9, e105000. [Google Scholar] [CrossRef] [PubMed]
- Skillbäck, T.; Farahmand, B.; Bartlett, J.W.; Rosén, C.; Mattsson, N.; Nägga, K.; Kilander, L.; Religa, D.; Wimo, A.; Winblad, B.; et al. CSF neurofilament light differs in neurodegenerative diseases and predicts severity and survival. Neurology 2014, 83, 1945–1953. [Google Scholar]
- Ewers, M.; Mattsson, N.; Minthon, L.; Molinuevo, J.L.; Antonell, A.; Popp, J.; Jessen, F.; Herukka, S.K.; Soininen, H.; Maetzler, W. CSF biomarkers for the differential diagnosis of Alzheimer’s disease: A large-scale international multicenter study. Alzheimer’s Dement. 2015, 11, 1306–1315. [Google Scholar] [CrossRef]
- Liguori, C.; Stefani, A.; Sancesario, G.; Sancesario, G.M.; Marciani, M.G.; Pierantozzi, M. CSF lactate levels, τ proteins, cognitive decline: A dynamic relationship in Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2015, 86, 655–659. [Google Scholar] [CrossRef]
- Rosenberg, G.A.; Prestopnik, J.; Adair, J.C.; Huisa, B.N.; Knoefel, J.; Caprihan, A.; Gasparovic, C.; Thompson, J.; Erhardt, E.B.; Schrader, R. Validation of biomarkers in subcortical ischaemic vascular disease of the Binswanger type: Approach to targeted treatment trials. J. Neurol. Neurosurg. Psychiatry 2015, 86, 1324–1330. [Google Scholar] [CrossRef]
- Skillbäck, T.; Farahmand, B.Y.; Rosén, C.; Mattsson, N.; Nägga, K.; Kilander, L.; Religa, D.; Wimo, A.; Winblad, B.; Schott, J.M.; et al. Cerebrospinal fluid tau and amyloid-β1-42 in patients with dementia. Brain 2015, 138, 2716–2731. [Google Scholar] [CrossRef]
- Struyfs, H.; Van Broeck, B.; Timmers, M.; Fransen, E.; Sleegers, K.; Van Broeckhoven, C.; de Deyn, P.P.; Streffer, J.R.; Mercken, M.; Engelborghs, S. Diagnostic Accuracy of Cerebrospinal Fluid Amyloid-β Isoforms for Early and Differential Dementia Diagnosis. J. Alzheimer’s Dis. 2015, 45, 813–822. [Google Scholar] [CrossRef]
- Skillbäck, T.; Delsing, L.; Synnergren, J.; Mattsson, N.; Janelidze, S.; Nägga, K.; Kilander, L.; Hicks, R.; Wimo, A.; Winblad, B.; et al. CSF/serum albumin ratio in dementias: A cross-sectional study on 1861 patients. Neurobiol. Aging 2017, 59, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kiđemet-Piskač, S.; Leko, M.B.; Blažeković, A.; Horvat, L.L.; Klepac, N.; Sonicki, Z.; Kolenc, D.; Hof, P.R.; Boban, M.; Mimica, N.; et al. Evaluation of cerebrospinal fluid phosphorylated tau231 as a biomarker in the differential diagnosis of Alzheimer’s disease and vascular dementia. CNS Neurosci. Ther. 2018, 24, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Barry Erhardt, E.; Pesko, J.C.; Prestopnik, J.; Thompson, J.; Caprihan, A.; Rosenberg, G.A. Biomarkers identify the Binswanger type of vascular cognitive impairment. J. Cereb. Blood Flow Metab. 2018, 7, 271678X18762655. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Chatterjee, M.; Twaalfhoven, H.; Del Campo Milan, M.; Teunissen, C.E.; Scheltens, P.; Fontijn, R.D.; van Der Flier, W.M.; de Vries, H.E. Vascular Endothelial Growth Factor remains unchanged in cerebrospinal fluid of patients with Alzheimer’s disease and vascular dementia. Alzheimer’s Res. Ther. 2018, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Skrobot, O.A.; Attems, J.; Esiri, M.; Hortobágyi, T.; Ironside, J.W.; Kalaria, R.N.; Lammie, G.A.; Mann, D.; Neal, J.; Ben-Shlomo, Y.; et al. Vascular cognitive impairment neuropathology guidelines (VCING): The contribution of cerebrovascular pathology to cognitive impairment. Brain 2016, 139, 2957–2969. [Google Scholar] [CrossRef] [PubMed]
- Wallin, A.; Román, G.C.; Esiri, M.; Kettunen, P.; Svensson, J.; Paraskevas, G.P.; Kapaki, E. Update on Vascular Cognitive Impairment Associated with Subcortical Small-Vessel Disease. J. Alzheimer’s Dis. 2018, 62, 1417–1441. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, J.M.; Smith, C.; Dichgans, M. Mechanisms of sporadic cerebral small vessel disease: Insights from neuroimaging. Lancet Neurol. 2013, 12, 483–497. [Google Scholar] [CrossRef]
- Ramirez, J.; Berezuk, C.; McNeely, A.A.; Gao, F.; McLaurin, J.; Black, S.E. Imaging the Perivascular Space as a Potential Biomarker of Neurovascular and Neurodegenerative Diseases. Cell. Mol. Neurobiol. 2016, 36, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Pantoni, L. Cerebral small vessel disease: From pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010, 9, 689–701. [Google Scholar] [CrossRef]
- Joutel, A.; Corpechot, C.; Ducros, A.; Vahedi, K.; Chabriat, H.; Mouton, P.; Alamowitch, S.; Domenga, V.; Cécillion, M.; Maréchal, E.; et al. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature 1996, 383, 707–710. [Google Scholar] [CrossRef]
- Corlobé, A.; Verdura, E.; Hervé, D.; Scharrer, E.; Amador, M.D.M.; Guyant-Maréchal, L.; Philippi, A.; Bergametti, F.; Gazal, S.; Prieto-Morin, C.; et al. Heterozygous HTRA1 mutations are associated with autosomal dominant cerebral small vessel disease. Brain 2015, 138, 2347–2358. [Google Scholar]
- Bugiani, M.; Waisfisz, Q.; Kevelam, S.H.; Bakels, H.S.; Groote, C.C.-D.; Niessen, H.W.M.; Abbink, T.E.M.; Oberstein, S.A.M.J.L.; Van Der Knaap, M.S. Cathepsin A–related arteriopathy with strokes and leukoencephalopathy (CARASAL). Neurology 2016, 87, 1777–1786. [Google Scholar] [CrossRef] [PubMed]
- Lynch, D.S.; Jaunmuktane, Z.; Sheerin, U.-M.; Phadke, R.; Brandner, S.; Milonas, I.; Dean, A.; Bajaj, N.; McNicholas, N.; Costello, D.; et al. Hereditary leukoencephalopathy with axonal spheroids: A spectrum of phenotypes from CNS vasculitis to parkinsonism in an adult onset leukodystrophy series. J. Neurol. Neurosurg. Psychiatry 2015, 87, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Poggesi, A.; Pasi, M.; Pescini, F.; Pantoni, L.; Inzitari, D. Circulating biologic markers of endothelial dysfunction in cerebral small vessel disease: A review. J. Cereb. Blood Flow Metab. 2016, 36, 72–94. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.; Niizuma, K.; Rashad, S.; Sumiyoshi, A.; Ryoke, R.; Endo, H.; Sato, K.; Kawashima, R.; Tominaga, T. A refined model of chronic cerebral hypoperfusion resulting in cognitive impairment and a low mortality rate in rats. J. Neurosurg. 2018, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Cao, Y.; Ma, L.; Pei, H.; Rausch, W.D.; Li, H. Dysfunction of Cerebrovascular Endothelial Cells: Prelude to Vascular Dementia. Front. Aging Neurosci. 2018, 10. [Google Scholar] [CrossRef]
- Young, V.G.; Halliday, G.; Kril, J. Neuropathologic correlates of white matter hyperintensities. Neurology 2008, 71, 804–811. [Google Scholar] [CrossRef]
- Fernando, M.S.; Simpson, J.E.; Matthews, F.; Brayne, C.; Lewis, C.E.; Barber, R.; Kalaria, R.N.; Forster, G.; Esteves, F.; Wharton, S.B.; et al. White matter lesions in an unselected cohort of the elderly: Molecular pathology suggests origin from chronic hypoperfusion injury. Stroke 2006, 37, 1391–1398. [Google Scholar] [CrossRef]
- Duncombe, J.; Kitamura, A.; Hase, Y.; Ihara, M.; Kalaria, R.N.; Horsburgh, K. Chronic cerebral hypoperfusion: A key mechanism leading to vascular cognitive impairment and dementia. Closing the translational gap between rodent models and human vascular cognitive impairment and dementia. Clin. Sci. 2017, 131, 2451–2468. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood–brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Ueno, M.; Chiba, Y.; Matsumoto, K.; Murakami, R.; Fujihara, R.; Kawauchi, M.; Miyanaka, H.; Nakagawa, T. Blood–brain barrier damage in vascular dementia. Neuropathology 2016, 36, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Farrall, A.J.; Wardlaw, J.M. Blood–brain barrier: Ageing and microvascular disease–systematic review and meta-analysis. Neurobiol. Aging 2009, 30, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Amor, S.; Peferoen, L.A.N.; Vogel, D.Y.S.; Breur, M.; Valk, P.; Baker, D.; Noort, J.M.; Van Der Valk, P.; van Noort, J.M. Inflammation in neurodegenerative diseases—An update. Immunology 2014, 142, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Rouhl, R.P.; Damoiseaux, J.G.; Lodder, J.; Theunissen, R.O.; Knottnerus, I.L.; Staals, J.; Henskens, L.H.; Kroon, A.A.; De Leeuw, P.W.; Tervaert, J.W.C.; et al. Vascular inflammation in cerebral small vessel disease. Neurobiol. Aging 2012, 33, 1800–1806. [Google Scholar] [CrossRef] [PubMed]
- Venkat, P.; Chopp, M.; Chen, J. Models and mechanisms of vascular dementia. Exp. Neurol. 2015, 272, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, M.; Kumar, S.; Bhatti, J.S.; Reddy, P.H. Molecular Links and Biomarkers of Stroke, Vascular Dementia, and Alzheimer’s Disease. Prog. Mol. Biol. Transl. Sci. 2017, 146, 95–126. [Google Scholar]
- Sullivan, G.W.; Sarembock, I.J.; Linden, J. The role of inflammation in vascular diseases. J. Leukoc. Biol. 2000, 67, 591–602. [Google Scholar] [CrossRef]
- Gu, Y.; Gutierrez, J.; Meier, I.B.; Guzman, V.A.; Manly, J.J.; Schupf, N.; Brickman, A.M.; Mayeux, R. Circulating inflammatory biomarkers are related to cerebrovascular disease in older adults. Neurol. - Neuroimmunol. Neuroinflamm. 2018, 6, e521. [Google Scholar] [CrossRef]
- Belkhelfa, M.; Beder, N.; Mouhoub, D.; Amri, M.; Hayet, R.; Tighilt, N.; Bakheti, S.; Laimouche, S.; Azzouz, D.; Belhadj, R.; et al. The involvement of neuroinflammation and necroptosis in the hippocampus during vascular dementia. J. Neuroimmunol. 2018, 15, 48–57. [Google Scholar] [CrossRef]
- Nagai, K.; Kozaki, K.; Sonohara, K.; Akishita, M.; Toba, K. Relationship between interleukin-6 and cerebral deep white matter and periventricular hyperintensity in elderly women. Geriatr. Gerontol. Int. 2011, 11, 328–332. [Google Scholar] [CrossRef]
- Hilal, S.; Ikram, M.A.; Verbeek, M.M.; Franco, O.H.; Stoops, E.; Vanderstichele, H.; Niessen, W.J.; Vernooij, M.W. C-Reactive Protein, Plasma Amyloid-β Levels, and Their Interaction with Magnetic Resonance Imaging Markers. Stroke 2018, 49, 2692–2698. [Google Scholar] [CrossRef] [PubMed]
- Walker, K.A.; Power, M.C.; Hoogeveen, R.C.; Folsom, A.R.; Ballantyne, C.M.; Knopman, D.S.; Windham, B.G.; Selvin, E.; Jack, C.R., Jr.; Gottesman, R.F. Midlife systemic inflammation, late-life white matter integrity, and cerebral small vessel disease: The atherosclerosis risk in communities study. Stroke 2017, 48, 3196–3202. [Google Scholar] [CrossRef] [PubMed]
- Satizabal, C.; Zhu, Y.C.; Mazoyer, B.; Dufouil, C.; Tzourio, C. Circulating IL-6 and CRP are associated with MRI findings in the elderly: The 3C-Dijon Study. Neurology 2012, 78, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Bettcher, B.M.; Yaffe, K.; Boudreau, R.M.; Neuhaus, J.; Aizenstein, H.; Ding, J.; Kritchevsky, S.B.; Launer, L.J.; Liu, Y.; Satterfield, S.; et al. Declines in inflammation predict greater white matter microstructure in older adults. Neurobiol. Aging 2015, 36, 948–954. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mitaki, S.; Nagai, A.; Oguro, H.; Yamaguchi, S. C-Reactive Protein Levels are Associated with Cerebral Small Vessel-Related Lesions. Acta Neurol. Scand. 2016, 133, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Wong, K.S.; Wang, Y.Y.; Fu, J.H.; Ding, D.; Hong, Z.; Wong, K.S.L. Plasma level of sICAM-1 is associated with the extent of white matter lesion among asymptomatic elderly subjects. Clin. Neurol. Neurosurg. 2009, 111, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Markus, H.S.; Hunt, B.; Palmer, K.; Enzinger, C.; Schmidt, H.; Schmidt, R. Markers of endothelial and hemostatic activation and progression of cerebral white matter hyperintensities: Longitudinal results of the Austrian Stroke Prevention Study. Stroke 2005, 36, 1410–1414. [Google Scholar] [CrossRef] [PubMed]
- Jalal, F.Y.; Yang, Y.; Thompson, J.; Lopez, A.C.; Rosenberg, G.A. Myelin loss associated with neuroinflammation in hypertensive rats. Stroke 2012, 43, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Weekman, E.M.; Wilcock, D.M. Matrix metalloproteinase in blood–brain barrier breakdown in dementia. J. Alzheimer’s Dis. 2016, 49, 893–903. [Google Scholar] [CrossRef]
- Bjerke, M.; Zetterberg, H.; Edman, Å.; Blennow, K.; Wallin, A.; Andreasson, U. Cerebrospinal Fluid Matrix Metalloproteinases and Tissue Inhibitor of Metalloproteinases in Combination with Subcortical and Cortical Biomarkers in Vascular Dementia and Alzheimer’s Disease. J. Alzheimer’s Dis. 2011, 27, 665–676. [Google Scholar] [CrossRef]
- Candelario-Jalil, E.; Thompson, J.; Taheri, S.; Grossetete, M.; Adair, J.C.; Edmonds, E.; Prestopnik, J.; Wills, J.; Rosenberg, G.A.; Adair, J. Matrix Metalloproteinases are Associated with Increased Blood-Brain Barrier Opening in Vascular Cognitive Impairment. Stroke 2011, 42, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Lüscher, C.; Malenka, R.C. NMDA Receptor-Dependent Long-Term Potentiation and Long-Term Depression (LTP/LTD). Cold Spring Harb. Perspect. Biol. 2012, 4, a005710. [Google Scholar]
- Ihara, M.; Polvikoski, T.M.; Hall, R.; Slade, J.Y.; Perry, R.H.; Oakley, A.E.; Englund, E.; O’Brien, J.T.; Ince, P.G.; Kalaria, R.N. Quantification of myelin loss in frontal lobe white matter in vascular dementia, Alzheimer’s disease, and dementia with Lewy bodies. Acta Neuropathol. 2010, 119, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Barker, R.; Wellington, D.; Esiri, M.M.; Love, S. Assessing white matter ischemic damage in dementia patients by measurement of myelin proteins. J. Cereb. Blood Flow Metab. 2013, 33, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Llorens, F.; Schmitz, M.; Knipper, T.; Schmidt, C.; Lange, P.; Fischer, A.; Hermann, P.; Zerr, I. Cerebrospinal Fluid Biomarkers of Alzheimer’s Disease Show Different but Partially Overlapping Profile Compared to Vascular Dementia. Front. Aging Neurosci. 2017, 9, 289. [Google Scholar] [CrossRef] [PubMed]
- Duering, M.; Konieczny, M.J.; Tiedt, S.; Baykara, E.; Tuladhar, A.M.; Van Leijsen, E.; Lyrer, P.; Engelter, S.T.; Gesierich, B.; Achmüller, M.; et al. Serum Neurofilament Light Chain Levels Are Related to Small Vessel Disease Burden. J. Stroke 2018, 20, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Gravesteijn, G.; Rutten, J.W.; Verberk, I.M.W.; Böhringer, S.; Liem, M.K.; van der Grond, J.; Aartsma-Rus, A.; Teunissen, C.E.; Oberstein, S.A.J.L. Serum Neurofilament light correlates with CADASIL disease severity and survival. Ann. Clin. Transl. Neurol. 2018, 6, 46–56. [Google Scholar] [CrossRef]
- Rothwell, P.M.; Howard, S.C.; Power, D.A.; Gutnikov, S.A.; Algra, A.; Van Gijn, J.; Clark, T.G.; Murphy, M.F.; Warlow, C.P. Fibrinogen Concentration and Risk of Ischemic Stroke and Acute Coronary Events in 5113 Patients with Transient Ischemic Attack and Minor Ischemic Stroke. Stroke 2004, 35, 2300–2305. [Google Scholar] [CrossRef]
- Di Napoli, M.; Papa, F.; Bocola, V. Prognostic influence of increased C-reactive protein and fibrinogen levels in ischemic stroke. Stroke 2001, 32, 133–138. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, H.; Zhang, S.; Fan, X.; Liu, X. Plasma fibrinogen is associated with cognitive decline and risk for dementia in patients with mild cognitive impairment. Int. J. Clin. Pract. 2008, 62, 1070–1075. [Google Scholar] [CrossRef]
- Fitzpatrick, A.L.; Irizarry, M.C.; Cushman, M.; Jenny, N.S.; Chi, G.C.; Koro, C. Lipoprotein-associated phospholipase A2 and Risk of Dementia in the Cardiovascular Health Study. Atherosclerosis 2014, 235, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Hunt, B.J.; Parmar, K.; Bamford, J.M.; Briley, D.; Brown, M.M.; Thomas, D.J.; Markus, H.S.; Hassan, A.; O’Sullivan, M. Markers of endothelial dysfunction in lacunar infarction and ischaemic leukoaraiosis. Brain 2003, 126, 424–432. [Google Scholar]
- Kearney-Schwartz, A.; Rossignol, P.; Bracard, S.; Felblinger, J.; Fay, R.; Boivin, J.-M.; Lecompte, T.; Lacolley, P.; Benetos, A.; Zannad, F. Vascular Structure and Function Is Correlated to Cognitive Performance and White Matter Hyperintensities in Older Hypertensive Patients with Subjective Memory Complaints. Stroke 2009, 40, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Nagai, M.; Hoshide, S.; Kario, K. Association of Prothrombotic Status with Markers of Cerebral Small Vessel Disease in Elderly Hypertensive Patients. Am. J. Hypertens. 2012, 25, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Pescini, F.; Donnini, I.; Cesari, F.; Nannucci, S.; Valenti, R.; Rinnoci, V.; Poggesi, A.; Gori, A.M.; Giusti, B.; Rogolino, A.; et al. Circulating Biomarkers in Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy Patients. J. Stroke Cerebrovasc. Dis. 2017, 26, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Bevan, S.; Barrick, T.; Rich, P.; Markus, H.S.; Singhal, S. The influence of genetic and cardiovascular risk factors on the CADASIL phenotype. Brain 2004, 127, 2031–2038. [Google Scholar]
- Pescini, F.; Cesari, F.; Giusti, B.; Sarti, C.; Zicari, E.; Bianchi, S.; Dotti, M.T.; Federico, A.; Balestrino, M.; Enrico, A.; et al. Bone marrow-derived progenitor cells in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Stroke 2010, 41, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.L.; Li, J.H.; Wu, J.; Sun, W.J.; Liu, S.; Wang, Z.L.; Zhou, H.; Yang, J.-H.; Qu, L.-H. deepBase v2.0: Identification, expression, evolution and function of small RNAs, lncRNAs and circular RNAs from deep-sequencing data. Nucleic Acids Res. 2016, 44, D196–D202. [Google Scholar] [CrossRef]
- Vemuganti, R. The MicroRNAs and Stroke: No Need to be Coded to be Counted. Transl. Stroke Res. 2010, 1, 158–160. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Xin, X.; Kim, T.S.; Cabeza, E.A.; Ren, J.; Nielsen, R.; Wrana, J.L.; Zhang, Z. Evidence for Positive Selection on a Number of MicroRNA Regulatory Interactions during Recent Human Evolution. PLoS Genet. 2012, 8, 1002578. [Google Scholar] [CrossRef]
- Weidhaas, J. Using microRNAs to understand cancer biology. Lancet Oncol. 2010, 11, 106–107. [Google Scholar] [CrossRef]
- Li, M.; Zhang, J. Circulating microRNAs. Potential and emerging bio-markers for diagnosis of cardiovascular and cerebrovascular diseases. Biomed. Res. Int. 2015, 2015. [Google Scholar] [CrossRef]
- Chen, X.; Ba, Y.; Ma, L.; Cai, X.; Yin, Y.; Wang, K.; Guo, J.; Zhang, Y.; Chen, J.; Guo, X.; et al. Characterization of microRNAs in serum: A novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008, 18, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Sano, T.; Reynolds, J.P.; Jimenez-Mateos, E.M.; Matsushima, S.; Taki, W.; Henshall, D.C.; Henshall, D. MicroRNA-34a upregulation during seizure-induced neuronal death. Cell Death Dis. 2012, 3, e287. [Google Scholar] [CrossRef] [PubMed]
- Vemuganti, R. All’s Well That Transcribes Well: Non-coding RNAs and Post-Stroke Brain Damage. Neurochem. Int. 2013, 63, 438–449. [Google Scholar] [CrossRef]
- Ragusa, M.; Bosco, P.; Tamburello, L.; Barbagallo, C.; Condorelli, A.G.; Tornitore, M.; Spada, R.S.; Barbagallo, D.; Scalia, M.; Elia, M.; et al. miRNAs Plasma Profiles in Vascular Dementia: Biomolecular Data and Biomedical Implications. Front. Cell. Neurosci. 2016, 10, 1. [Google Scholar] [CrossRef]
- Sørensen, S.S.; Nygaard, A.B.; Christensen, T. miRNA expression profiles in cerebrospinal fluid and blood of patients with Alzheimer’s disease and other types of dementia—An exploratory study. Transl. Neurodegener. 2016, 5, 6. [Google Scholar]
| Author, Year [Reference] | Population | Biomakers | Outcome | Results |
|---|---|---|---|---|
| Murr J et al., 2014 [17] | 670 cognitively normal subjects | Plasma oxidized low-density lipoprotein (OxLDL) levels | Risk Factor follow-up: 4.8 years (2.2–10.3) | No association between OxLDL and risk of all-cause dementia, AD, and vascular dementia or subtypes was found. |
| Dong H et al., 2015 [18] | 127 AD patients, 30 MCI and 30 VaD patients. | Serum circulating miRNAs | Differential diagnosis | The panel of miR-31, miR-93 and miR-146a can be used to discriminate AD from VaD. |
| Duits FH et al., 2015 [19] | 52 AD patients, 24 VaD patients, 26 cognitively normal subjects. | Plasma and CSF levels of MMP2, MMP9, MMP10, TIMP1 and TIMP2. CSF levels of amyloid-β(1–42) (Aβ42), tau, and tau phosphorylated at threonine-181 (p-tau). | Differential diagnosis | AD patients showed higher plasma MMP2 levels compared to VaD patients (p < 0.05). |
| Hatanaka H et al., 2015 [20] | 72 AD, 27 VaD, 24 Mixed Dementia (MD) patients and 53 cognitively normal subjects. | Plasma levels of diacron reactive oxygen metabolite (dROM) and biological anti-oxidant potential (BAP). | Differential diagnosis | The dROM levels were significantly higher in the AD and MD groups than in the control group. The BAP levels were significantly lower in the MD group than in the control, AD and VaD groups. |
| Hilal S et al., 2015 [21] | 41 cognitive impairment with no dementia (CIND) subjects and 46 demented subjects with burden of cerebrovascular diseases (CeVD); 37 CIND and 34 cases with dementia without CeVD; 35 cognitively normal subjects. | Plasma NTpro-BNP and high sensitivity cardiac troponin T (hs-cTnT) levels. | Differential diagnosis | Plasma concentrations of hs-cTnT were associated significantly with CeVD in both CIND and dementia. NTpro-BNP was associated with dementia with CeVD |
| Teunissen CE et al., 2015 [22] | 295 subjects including healthy controls (n = 65), patients with subjective memory complaints (n = 99), patients with AD (n = 100), and patients with vascular dementia (n = 31). | Serum leptin levels | Differential diagnosis | Serum leptin levels are not altered in AD or vascular dementia patients compared to healthy controls and were not related to cognitive decline. |
| Wang C et al., 2015 [23] | 10 cognitively healthy subjects and 10 age-matched VaD subjects. | Differentially expressed proteins (DEPs) | Differential diagnosis | High-degree proteins were detected in the protein-protein (PPI) interaction network, such as ATP5B (ATP synthase subunit β) in VaD. |
| Castellazzi M et al., 2016 [24] | 232 MCI, 65 VaD, 175 AD, 88 MD, 104 Multiple Sclerosis (MS) patients, 165 cognitively healthy controls. | Serum High-density lipoprotein (HDL)-bound paraoxonase-1 (PON-1) levels | Differential diagnosis | Serum arylesterase, but not paraoxonase, levels were significantly lower in patients affected by MCI, VaD, AD, MD as well as those with MS as compared to healthy controls. |
| Chen Z et al., 2016 [25] | 52 subcortical ischemic vascular disease patients with no dementia (SIVDND), 55 patients with mild cognitive impairment (SVMCI), 54 patients with vascular dementia (VaD), 54 cognitively healthy controls. | Serum thyroid-stimulating hormone (TSH), total triiodothyronine (TT3), free triiodothyronine (FT3), total thyroxine (TT4) and free thyroxine (FT4), thyroglobulin antibody (TGA), and antithyroid peroxidase antibody (TPO-Abs). | Correlation with cognitive status | Serum TT3 and FT3 levels decreased, whereas serum TSH level increased, with the decline in cognitive functions in SIVD. |
| Dukic L et al., 2016 [26] | 235 participants, divided in 4 groups: AD (N = 70), VaD (N = 67), MCI (N = 48) and Cognitively Healthy Participants (N = 50). | Serum kallikrein 6 (KLK6), clusterin (CLU), adiponectin (ADPN) and interleukin-6 (IL-6) | Differential diagnosis | Serum concentrations of KLK6, CLU and ADPN did not differ between AD, VaD, MCI and cognitively healthy control group of participants, whereas IL-6 was significantly higher in VaD patients than in AD, MCI and healthy individuals. |
| Horvath I et al., 2016 [27] | 40 nondemented controls, 11 stable mild cognitive impairment (SMCI), 6 MCI due to AD (MCI-AD), 40 AD and 7 VaD.patients. | CSF concentration of Aβ1−42, S100A8, S100A9 and Tau. | Differential diagnosis | The S100A9 and Aβ1−42 levels correlated with each other: their CSF content decreased already at the SMCI stage and declined further under MCIAD, AD, and VaD conditions. |
| Kitagawa K et al., 2016 [28] | 466 cognitively healthy subjects | serum high-molecular-weight (HMW) adiponectin level | Risk factor (median follow-up period: 6.9 years) | Risks of dementia in patients with high versus low HMW adiponectin levels were almost identical. No association was found between adiponectin levels and Alzheimer’s disease dementia or vascular dementia in the whole group or amongst men and women separately. |
| Levada OA et al., 2016 [29] | 21 patients with AD; 22 patients with subcortical vascular dementia; 16 cognitively healthy subjects. | Plasma levels of brain-derived neurotrophic factor (BDNF) | Differential diagnosis | At baseline there was lower BDNF levels in both AD and VaD groups, which was significant only in subjects with AD. |
| Mirza SS et al., 2016 [30] | 357 AD patients; 32 VaD patients. | Serum N-terminal pro B-type natriuretic peptide (NT-proBNP) levels | Risk factor | Higher NT-proBNP was associated with a higher risk of dementia, with a particularly strong association with vascular dementia. |
| Nilsson ED et al., 2016 [31] | 374 incident dementia cases: 120 AD patients, 84 VaD and 102 MD patients. | Plasma level of copeptin | Risk factor (median follow-up period: 4.2 years) | Baseline level of copeptin predicted incident VaD. |
| Pan X et al., 2016 [32] | Exploration phase: 338 control subjects and 43 AD patients; Validation phase: 861 control subjects, 81 AD patients, 70 vascular dementia patients and 13 frontotemporal dementia patients. | Blood Thiamine diphosphate (TDP), thiamine monophosphate, and thiamine levels | Differential diagnosis | TDP exhibited significant and consistent values for AD diagnosis in both exploration and validation phases. TDP can effectively distinguish AD from vascular dementia. |
| Bednarska-Makaruk M et al., 2017 [33] | 205 patients with dementia (89 with AD, 47 with VaD, 69 with mixed dementia (MD)), 113 persons with MCI and 107 controls. | Serum adiponectin, leptin and resistin levels, IL-6, CRP, chitotriosidase, 25-OH vitamin D, HDL-cholesterol and paraoxonase 1, glucose, insulin and HOMA-IR. | Differential diagnosis | Vascular and mixed dementia are characterized by an increase of resistin. Positive correlation of resistin with inflammation indicators may suggest the potential pro-inflammatory role of resistin in the development of dementia, especially in dementia of vascular mechanism. |
| Busse M et al., 2017 [34] | 60 AD patients, 20 VaD patients, 12 frontotemporal dementia patients and 24 cognitively healthy persons. | Innate and adaptive cell populations in whole blood. | Differential diagnosis | Monocytes and NK cells were diminished in VaD, but not in AD and FTD. B cell and T cell numbers were decreased in all investigated forms of dementia. Changes in the contribution of naïve/memory T cells were only present in AD. |
| Holm H et al., 2017 [35] | 120 AD patients, 83 VaD, 102 MD and 68 other aetiology. | Plasma N-terminal prosomatostatin (NT-proSST) | Risk factor (follow-up period of 4.6 ± 1.3 years) | Higher levels of circulating NTproSST are associated with increased incidence of vascular dementia. |
| Holm H et al., 2017 [36] | A population-based cohort of 5347 individuals without prevalent dementia | Plasma midregional pro-atrial natriuretic peptide (MR-proANP), C-terminal endothelin-1 (CT-proET-1) and midregional proadrenomedullin (MR-proADM). | Risk factor (follow-up period of 4.6 ± 1.3 years) | Elevated plasma concentration of MR-proANP is an independent predictor of all-cause and vascular dementia. Pronounced increase in CT-proET-1 indicates higher risk of vascular dementia. |
| Hsu PF et al., 2017 [37] | 1436 individuals from a national representative sample in Taiwan. | CRP levels were determined. | Risk Factor (11.04 years (median) of follow-up) | 260 individuals (18.11%) were diagnosed with dementia. Those with high CRP had a 55% higher risk of dementia compared with those with normal CRP. After adjusting for possible confounding cardiovascular risk factors, high CRP was independently associated with VaD, but not AD. |
| Moretti R et al., 2017 [38] | 543 patients: 456 patients suffering from subcortical vascular dementia (sVAD), 87 AD and healthy age-matched controls | Clinical laboratory measurements, including serum total cholesterol, triglycerides, and high-density lipoprotein (HDL) cholesterol, have been determined enzymatically and low-density lipoprotein (LDL) cholesterol was calculated using Friedewald’s formula. Serum levels of 25(OH)D, the level of calcium and PTH were measured.The level of folate, vitamin B12 levels and Homocysteine were also tested. | Correlation | Vitamin D deficiency was present in demented cases, as well as low levels of folate and high levels of homocysteine, more pronounced in sVAD cases. |
| Prabhakar P et al., 2017 [39] | 204 patients with small vessel VaD; 200 cognitively normal subjects. | Plasma miRNA profiling. | Differential diagnosis | plasma miR-409-3p, miR-502-3p, miR-486-5p and miR-451a could be used to differentiate small vessel VaD patients from healthy controls. |
| Quinlan P et al., 2017 [40] | 342 patients with subjective or objective mild cognitive impairment | Serum IGF-I concentrations at baseline | Risk factor (mean follow-up: 3.6 years) | In a memory clinic population, low serum IGF-I was a risk marker for subsequent VaD whereas low IGF-I did not associate with the risk of AD. High serum IGF-I was not related to the risk of conversion to dementia. |
| Suridjan I et al., 2017 [41] | 29 possible vascular mild cognitive impairment patients and 89 controls) | Serum lipid peroxidation markers levels. Ratios of early- (lipid hydroperoxides, LPH) to late-stage (8-isoprostane, 8-ISO; 4-hydroxy-2-nonenal, 4-HNE) lipid peroxidation products were calculated. | Correlation with cognitive status | A global effect of group on lipid peroxidation markers was observed, adjusting for sex, years of education, and cardiopulmonary fitness. Lower lipid peroxidation at baseline, as determined by lower 8-ISO concentration, was associated with greater improvement in verbal memory (F (1, 64) = 4.738, p = 0.03) and executive function (F(1, 64) = 5.219, p = 0.026) performance. Higher ratios of 8-ISO/LPH and 8-ISO+4-HNE to LPH, were associated with less improvement in executive function performance over a 24-week exercise intervention. |
| Tang SC et al., 2017 [42] | 172 Ischemic Stroke (IS) patients, including 73 with CDR = 0, 63 with CDR = 0.5, and 36 with CDR ≥ 1 (VaD patients). | Plasma concentration of receptor for advanced glycation end products (soluble RAGE (sRAGE); endogenous soluble form of RAGE (esRAGE)) | Correlation with cognitive status | Plasma sRAGE and esRAGE were elevated in patients with dementia compared with those without dementia among IS patients. |
| Vishnu VY et al., 2017 [43] | 118 subects, 68 with dementia (MCI-AD and AD: 52; MCI VaSC and VaD:16). | Plasma IL-6; C-Reactive Protein (CRP); plasma fibrinogen; plasma D-dimer. | Differential diagnosis | Plasma Fibrinogen and D dimer: were higher in Vascular group; no difference in IL-6 and CRP. |
| Wang R et al.,2017 [44] | 88 patients with dementia (43 AD patients, 45 VaD patients) and 45 healthy age-matched controls | Plasma Cystatin C (Cys C) and HDL levels | Differential diagnosis | Plasma Cys C levels were higher in patients with AD/VaD than in healthy subjects. Plasma HDL levels were lower in patients with AD/VaD than in healthy subjects. |
| Yang R et al., 2017 [45] | 51 healthy subjects and 41 elderly patients (with 6 participants affected by vascular mild cognitive impairment (Va-MCI)), 9 with VaD, 8 with MCI due to AD and 18 with AD | Plasma GDF-11 and β2-MG levels | Differential diagnosis | No differences in circulating GDF-11 levels amongst the healthy advanced age and four cognitive impairment groups. β2-MG levels increased with age, but there was no significant difference between healthy elderly males and advanced age males. Increased levels of β2-MG were observed in the dementia group compared with the healthy advanced age group. |
| Brombo G et al., 2018 [46] | 320 elderly individuals (≥65 years old): 60 patients with normal cognition; 60 patients with VaD; 100 patients with AD; 100 patients with MCI. | Plasma Klotho levels | Differential diagnosis | Lower levels of plasma Klotho (1st tertile) were associated with higher prevalence of VaD, but not AD. |
| Latourte A et al., 2018 [47] | 1598 people. | Serum Uric Acid (SUA) | Risk Factor (median folow-up duration: 10.1 years) | Association for development of dementia was stronger with vascular or mixed dementia (HR = 3.66 (95% CI 1.29 to 10.41), p = 0.015) than Alzheimer’s disease (HR = 1.55 (95% CI 0.92 to 2.61), p = 0.10). |
| Lauriola M et al., 2018 [48] | 116 patients: 32 healthy controls, 39 with diagnosis of VaD, 14 MCI and 31 AD. | Erythrocyte associated Aβ (iAβ40 and iAβ42) levels | Differential diagnosis | AD showed different iAβ42 levels as compared to VaD. Conversely, no differences were found for iAβ40. |
| Shang J et al., 2018 [49] | 266 patients with AD, 44 MCI, 33 VaD and 200 ischemic stroke (IS) in comparison to 130 healthy controls. | Plasma fatty acids [eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)], adiponectin, reptin, plasma markers of inflammation [high-sensitivity C-reactive protein (hsCRP) and serum amyloid A (serum AA)], and plasma lipids [high-density lipoprotein and low-density lipoprotein (LDL)] | Differential disgnosis | Lower EPA and DHA levels and higher reptin and LDL levels were associated with AD and IS. The reptin/adiponectin ratio was strongly associated with IS. The hsCRP level was more strongly associated with VaD and IS, and the serum AA level was associated with all three cognitive diseases and IS. |
| Staszewski J et al., 2018 [50] | 123 patients (age, mean ± SD: 72.2 ± 8 years, 49% females), with lacunar stroke (n = 49), vascular dementia (n = 48), and vascular parkinsonism (n = 26). | Soluble intercellular cell adhesion molecule-1 (sICAM-1), soluble platelet selectin (sP-selectin), CD40 ligand (sCD40 L), platelet factor-4 (PF-4) and homocysteine; combined high-sensitivity C-reactive protein (hsCRP), interleukin-1α and -6 (IL-1α and IL-6, respectively) and tumor necrosis factor-α (TNF-α). | Correlation with radiological status | Lacunes are associated with different inflammatory markers. |
| Yang TT et al., 2018 [51] | 101 MCI, 107 AD, 30 Parkinson’s disease with dementia (PDD), 20 VaD patients | Serum levels of exosomal miR-135a, -193b, and -384 | Differential diagnosis | Both serum exosome miR-135a and miR-384 were up-regulated while miR-193b was down-regulated in AD patients compared with normal controls and non-AD dementias. |
| Staszewski et al., 2019 [52] | 130 patients with marked MRI features of SVD and recent lacunar stroke (n = 52,LS), vascular Parkinsonism (n = 28,VaP) or dementia (n = 50,VaD). | IL-1α, IL-6, hs-CRP, sICAM-1, sP-selectin, TNF-α, homocysteine, fibrinogen, D-dimer, serum total cholesterol (TC), high density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglycerides (TG), eGFR, serum FG, HbA1c, albumin and uric acid (UA). | Risk Factor (mean follow-up time: 22.3 ± 4.3 months) | IL-1α, IL-6, homocysteine, d-dimer were significantly associated with the event of death or stroke, even after adjusting for age, sex and SVD radiological markers. |
| Author, Year [Reference] | Population | Biomakers | Outcome | Results |
|---|---|---|---|---|
| Busse S et al., 2014 [53] | serum and CSF of 24 patients with AD, 20 patients with subcortical ischemic vascular dementia (SIVD) and 274 healthy volunteers | N-methyl-D-aspartate glutamate receptors (NMDA-R) autoantibodies directed against the NR1a subunit (NR1a NMDA-R autoantibodies) | Differential diagnosis | The overall seroprevalence was not statistically different between dementia patients and matched controls. CSF samples were negative for NMDA-R autoantibodies. |
| Herbert MK et al., 2014 [54] | 39 DLB, 110 AD, 24 VaD and 28 FTD patients. | CSF concentration of amyloid-b42 (Ab42), total tau protein (t-tau), and phosphorylated tau protein (p-tau) and 3-methoxy-4- hydroxyphenylethyleneglycol (MHPG) | Differential diagnosis | The used combination of Ab42, t-tau, and p-tau could not discriminate among DLB, VaD and FTD. The addition of MHPG to Ab42, t-tau, and p-tau improves the discrimination of DLB from AD, but could not distinguish DLB from other forms of dementia. |
| Hermann P et al., 2014 [55] | 32 patients with VaD with Cerebral Small Vessels Disease (CSVD); 27 patients with AD; 27 patients with AD + CSVD on MRI. | CSF albumin ratio | Differential diagnosis | VaD + CSVD and AD + CSVD had a higher albumin ratio, as an expression of BBB disruption. |
| Skillbäck T et al., 2014 [56] | 107 healthy controls, 223 early onset AD, 1194 late onset AD, 437 subjects with dementia with no other specification,146 FTD, 114 DLB, 517 MD, 45 PDD, 465 VaD, 108 Other) | CSF neurofilament light (NFL) levels | Differential diagnosis | CSF NFL differed among clinical diagnoses, with the highest levels seen in frontotemporal dementia, VaD, and mixed dementia |
| Ewers M et al., 2015 [57] | 55 healthy controls (HC) subjects, 167 patients with AD dementia, 172 subjects with MCI, 22 subjects with subjective memory impairment (SMI), 69 patients with VaD, 26 patients with Lewy body dementia (LBD), 39 patients with FTD, 39 patients with depression, and 86 patients with other neurological disorders (OND). | CSF concentrations of Ab1-42, p-tau181, and total tau. | Differential diagnosis | CSF Aβ1-42 showed the best diagnostic accuracy among the CSF biomarkers. CSF Aβ1-42 discriminates AD dementia from FTD but shows significant overlap with other non-AD forms of dementia, possibly reflecting the underlying mixed pathologies. |
| Liguori C et al., 2015 [58] | Patients with AD (N = 145), healthy controls (N = 80) and patients with VaD (N = 44). | CSF lactate concentrations, AD biomarker levels (τ-proteins and β-amyloid) | Differential diagnosis | AD patients showed a significant increase of CSF lactate concentration compared to controls and patients with VaD. |
| Rosenberg GA et al., 2015 [59] | 62 patients with Vascular Cognitive Impairment (VCI) | CSF measurements of albumin ratio, matrix metalloproteinases (MMPs), amyloid-β1-42 and phosphorylated-τ181. Patients were followed for an average of 2 years. | Predictor | Inflammatory biomarkers of increased BBB permeability, elevated albumin index and reduced MMP-2 index, predicted the diagnosis of the Binswanger disease (BD) type of subcortical ischaemic vascular disease. |
| Skillbäck T et al., 2015 [60] | 383 Early onset AD and 221 late onset AD patients, 759 vascular dementia, 982 mixed dementia, 232 frontotemporal dementia, 150 Parkinson’s disease dementia and 79 dementia with Lewy bodies (N = 79). | cerebrospinal fluid amyloid-β1-42, total tau and phosphorylated tau. | Differential diagnosis | In Parkinson’s disease dementia and vascular dementia low CSF amyloid-β1-42 was associated with low Mini-Mental State Examination score. |
| Struyfs H et al., 2015 [61] | Patients with AD (n = 50), MCI due to AD (n = 50) and patients with non-AD dementias (n = 50). The non-AD group consisted of 17 patients with FTD, 17 DLB patients, and 16 patients with vascular dementia (VaD). The Control group was composed of 35 subjects. | CSF levels of Aβ isoforms, Aβ(1-37), Aβ(1-38), and Aβ(1-40), as compared to the AD CSF biomarkers Aβ(1-42), T-tau, and P-tau(181P). | Differential diagnosis | Best biomarkers to distinguish AD and VaD were Aβ1-42/T-tau and Aβ 1-42/P-tau181P |
| Skillbäck T et al., 2017 [62] | Patients diagnosed with Alzheimer’s disease (AD, early onset [EAD, n = 130], late onset AD [LAD, n = 666]), vascular dementia (VaD, n = 255), mixed AD and VaD (MD, n = 362), Lewy body dementia (DLB, n = 50), frontotemporal dementia (FTD, n = 56), Parkinson’s disease dementia (PDD, n = 23), other dementias (other, n = 48), and dementia not otherwise specified (NOS, n = 271), two healthy control groups (n = 292, n = 20). | CSF/serum albumin ratio | Differential diagnnosis | Patients with DLB, LAD, VaD, MD, other, and NOS groups had higher CSF/serum albumin ratio than controls. CSF/serum albumin ratio correlated with CSF neurofilament light in LAD, MIX, VaD, and other groups but not with AD biomarkers. |
| Kiđemet-Piskač S et al., 2018 [63] | 152 patients with AD, 28 VaD, and 18 healthy controls (HC). | CSF levels of total tau protein (t-tau), tau protein phosphorylated at threonine 231 (p-tau231). | Differential diagnosis | Total tau levels were significantly elevated in subjects with AD compared to HC, as well as in VaD subjects compared to HC. p-tau231 levels were significantly higher in patients with AD vs. HC as well in patients with VaD vs. HC. p-tau231 levels did not distinguish AD from VaD patients. |
| Barry Erhardt E et al., 2018 [64] | 62 possible VCID patients | Matrix metalloproteinases-2 (MMP-2) and MMP-9 in the CSF and plasma and MMP-2 and MMP-9 indexes were calculated. Amyloid b1-42 (Ab42) and phosphoTau181 (PTau) in the CSF. | Diagnostic accuracy | MMP-2 was accurate in predicting VCID diagnosis |
| Chakraborty A et al., 2018 [65] | age-matched groups of controls with subjective cognitive decline (n = 21), AD without the presence of microbleeds (MB) (n = 25), AD with MB (n = 25), and VaD (n = 21) patients. | VEGF levels in CSF | Differential diagnosis | No significant differences were detected between groups |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cipollini, V.; Troili, F.; Giubilei, F. Emerging Biomarkers in Vascular Cognitive Impairment and Dementia: From Pathophysiological Pathways to Clinical Application. Int. J. Mol. Sci. 2019, 20, 2812. https://doi.org/10.3390/ijms20112812
Cipollini V, Troili F, Giubilei F. Emerging Biomarkers in Vascular Cognitive Impairment and Dementia: From Pathophysiological Pathways to Clinical Application. International Journal of Molecular Sciences. 2019; 20(11):2812. https://doi.org/10.3390/ijms20112812
Chicago/Turabian StyleCipollini, Virginia, Fernanda Troili, and Franco Giubilei. 2019. "Emerging Biomarkers in Vascular Cognitive Impairment and Dementia: From Pathophysiological Pathways to Clinical Application" International Journal of Molecular Sciences 20, no. 11: 2812. https://doi.org/10.3390/ijms20112812
APA StyleCipollini, V., Troili, F., & Giubilei, F. (2019). Emerging Biomarkers in Vascular Cognitive Impairment and Dementia: From Pathophysiological Pathways to Clinical Application. International Journal of Molecular Sciences, 20(11), 2812. https://doi.org/10.3390/ijms20112812




