Lymphocyte Subsets and Inflammatory Cytokines of Monoclonal Gammopathy of Undetermined Significance and Multiple Myeloma
Abstract
:1. Introduction
1.1. General Considerations for MGUS
1.2. MGUS Progression: Genetic and Microenvironment Factors
1.3. MGUS Progression and Immunosurveillance
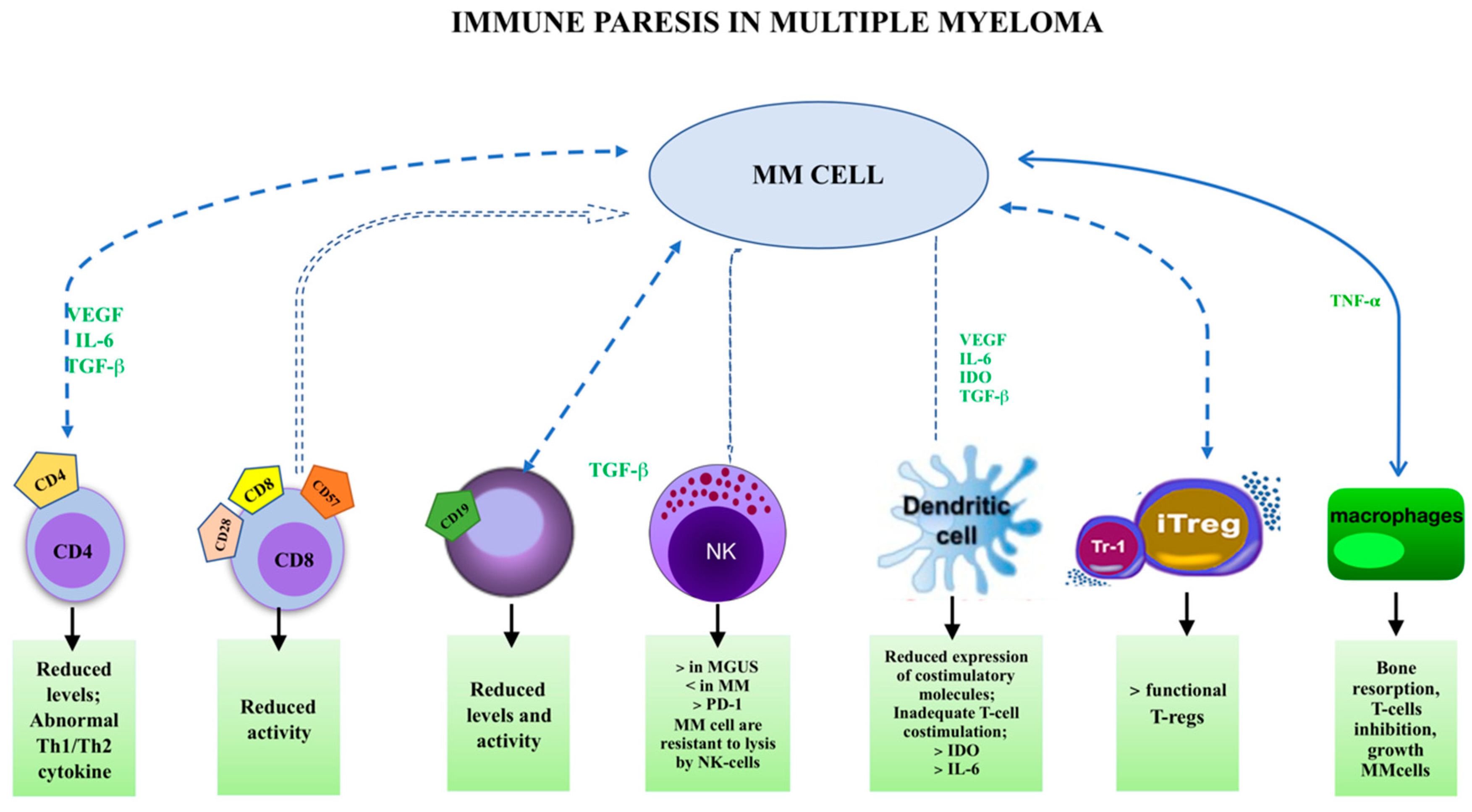
1.4. Immune Paresis and MGUS
1.5. Cellular Subsets
1.6. MGUS and Bone Marrow Cell Populations
1.7. Inflammation, Cytokines, and MGUS
1.8. Inflammatory Cytokines and MGUS
1.9. Anti-Inflammatory Cytokines
2. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kyle, R.A.; Rajkumar, S.V. Monoclonal gammopathies of undetermined significance. Hematol. Oncol. Clin. North Am. 1999, 13, 1181–1202. [Google Scholar] [CrossRef]
- Weiss, B.M.; Abadie, J.; Verma, P.; Howard, R.S.; Kuehl, W.M. A monoclonal gammopathy precedes multiple myeloma in most patients. Blood 2009, 113, 5418–5422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyle, R.A. Multiple myeloma: How did it begin? Mayo Clin. Proc. 1994, 69, 680–683. [Google Scholar] [CrossRef]
- Kyle, R.A. Monoclonal gammopathy of undetermined significance and solitary plasmacytoma. Implications for progression to overt multiple myeloma. Hematol. Oncol. Clin. North Am. 1997, 11, 71–87. [Google Scholar] [CrossRef]
- Bosseboeuf, A.; Feron, D.; Tallet, A.; Rossi, C.; Charlier, C.; Garderet, L.; Caillot, D.; Moreau, P.; Cardo-Vila, M.; Pasqualini, R.; et al. Monoclonal IgG in MGUS and multiple myeloma target infectious pathogens. J. Clin. Invest. Insight 2017, 2, 19. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Branagan, A.R.; Liu, J.; Boddupalli, C.S.; Mistry, P.K.; Dhodapkar, M.V. Clonal Immunoglobulin against Lysolipids in the Origin of Myeloma. N. Engl. J. Med. 2016, 374, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Sng, J.; Sekhar Boddupalli, C.; Seckinger, A.; Chesi, M.; Fulciniti, M.; Zhang, L.; Rauniyar, N.; Lopez, M.; Neparidze, N.; et al. Antigen-mediated regulation in monoclonal gammopathies and myeloma. J. Clin. Invest. Insight 2018, 3, e98259. [Google Scholar] [CrossRef] [PubMed]
- Sahota, S.S.; Leo, R.; Hamblin, T.J.; Stevenson, F.K. Ig VH gene mutational patterns indicate different tumor cell status in human myeloma and monoclonal gammopathy of undetermined significance. Blood 1996, 87, 746–755. [Google Scholar] [PubMed]
- Drach, J.; Angerler, J.; Schuster, J.; Rothermundt, C.; Thalhammer, R.; Haas, O.A.; Jäger, U.; Fiegl, M.; Geissler, K.; Ludwig, H.; et al. Interphase fluorescence in situ hybridization identifies chromosomal abnormalities in plasma cells from patients with monoclonal gammopathy of undetermined significance. Blood 1995, 86, 3915–3921. [Google Scholar]
- Landgren, O.; Kyle, R.A.; Pfeiffer, R.M.; Katzmann, J.A.; Caporaso, N.E.; Hayes, R.B.; Dispenzieri, A.; Kumar, S.; Clark, R.J.; Baris, D.; et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: A prospective study. Blood 2009, 113, 5412–5417. [Google Scholar] [CrossRef]
- Chiecchio, L.; Dagrada, G.P.; Ibrahim, A.H.; Dachs Cabanas, E.; Protheroe, R.K.; Stockley, D.M.; Orchard, K.H.; Cross, N.C.; Harrison, C.J.; Ross, F.M.; et al. Timing of acquisition of deletion 13 in plasma cell dyscrasias is dependent on genetic context. Haematologica 2009, 94, 1708–1713. [Google Scholar] [CrossRef]
- Landgren, O. Monoclonal gammopathy of undetermined significance and smoldering multiple myeloma: Biological insights and early treatment strategies. Hematology 2013, 2013, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Qian, L.; Yuan, X.; Hu, C.; Wang, L.; Huang, Q.; Miao, P.; Yu, Q.; Ma, Y.; Zhang, J.; et al. BlyS: A potential hallmark of multiple myeloma. Front. Biosci. Landmark 2013, 18, 324–331. [Google Scholar]
- McNee, G.; Eales, K.L.; Wei, W.; Williams, D.S.; Barkhuizen, A.; Bartlett, D.B.; Essex, S.; Anandram, S.; Filer, A.; Moss, P.A.H.; et al. Citrullination of histone H3 drives IL-6 production by bone marrow mesenchymal stem cells in MGUS and multiple myeloma. Leukemia 2017, 31, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Van Riet, I.; Van Camp, B. The involvement of adhesion molecules in the biology of multiple myeloma. Leuk. Lymphoma 1993, 9, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Sonneveld, P.; Durie, B.G.; Lokhorst, H.M.; Frutiger, Y.; Schoester, M.; Vela, E.E. Analysis of multidrug-resistance (MDR-1) glycoprotein and CD56 expression to separate monoclonal gammopathy from multiple myeloma. Br. J. Haematol. 1993, 83, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Harada, H.; Kawano, M.M.; Huang, N.; Harada, Y.; Iwato, K.; Tanabe, O.; Tanaka, H.; Sakai, A.; Asaoku, H.; Kuramoto, A. Phenotypic difference of normal plasma cells from mature myeloma cells. Blood 1993, 81, 2658–2663. [Google Scholar] [PubMed]
- San Miguel, J.F.; Almeida, J.; Ocqueteau, M. Detection of two subpopulations of plasma cells in MGUS patients: Utility in the differential diagnosis with multiple myeloma. Blood 1996, 88, 640a. [Google Scholar]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Sahota, S.S.; Leo, R.; Hamblin, T.J.; Stevenson, F.K. Myeloma VL and VH gene sequences reveal a complementary imprint of antigen selection in tumor cells. Blood 1997, 89, 219–226. [Google Scholar]
- Yi, Q.; Osterborg, A. Idiotype-specific T cells in multiple myeloma: Targets for an immunotherapeutic intervention? Med. Oncol. 1996, 13, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yi, Q.; Osterborg, A.; Bergenbrant, S.; Mellstedt, H.; Holm, G.; Lefvert, A.K. Idiotype-reactive T-cell subsets and tumor load in monoclonal gammopathies. Blood 1995, 86, 3043–3049. [Google Scholar] [PubMed]
- Hong, S.; Qian, J.; Yang, J.; Li, H.; Kwak, L.W.; Yi, Q. Roles of idiotype-specific t cells in myeloma cell growth and survival: Th1 and CTL cells are tumoricidal while Th2 cells promote tumor growth. Cancer Res. 2008, 68, 8456–8464. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, A.O.; Hansson, L.; Eriksson, I.; Näsman-Glaser, B.; Rossmann, E.D.; Rabbani, H.; Mellstedt, H.; Osterborg, A. Idiotype protein vaccination in combination with adjuvant cytokines in patients with multiple myeloma--evaluation of T-cell responses by different read-out systems. Haematologica 2007, 92, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Allegra, A.; Penna, G.; Innao, V.; Greve, B.; Maisano, V.; Russo, S.; Musolino, C. Vaccination of multiple myeloma: Current strategies and future prospects. Crit. Rev. Oncol. Hematol. 2015, 96, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Musolino, C.; Allegra, A.; Innao, V.; Allegra, A.G.; Pioggia, G.; Gangemi, S. Inflammatory and anti-inflammatory equilibrium, proliferative and anti-proliferative balance: The role of cytokines in multiple myeloma. Mediat. Inflamm. 2017. [Google Scholar] [CrossRef] [PubMed]
- Pika, T.; Lochman, P.; Sandecka, V.; Maisnar, V.; Minarik, J.; Tichy, M.; Zapletalova, J.; Solcova, L.; Scudla, V.; Hajek, R. Immunoparesis in MGUS - Relationship of uninvolved immunoglobulin pair suppression and polyclonal immunoglobuline levels to MGUS risk categories. Neoplasma 2015, 62, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Magnano, L.; Fernández de Larrea, C.; Elena, M.; Cibeira, M.T.; Tovar, N.; Aróstegui, J.I.; Pedrosa, F.; Rosiñol, L.; Filella, X.; Yagüe, J.; et al. Prognostic Impact of Serum Heavy/Light Chain Pairs in Patients With Monoclonal Gammopathy of Undetermined Significance and Smoldering Myeloma: Long-Term Results From a Single Institution. Clin. Lymphoma Myeloma Leuk. 2016, 16, e71–e77. [Google Scholar] [CrossRef]
- Cherry, B.M.; Costello, R.; Zingone, A.; Burris, J.; Korde, N.; Manasanch, E.; Kwok, M.; Annunziata, C.; Roschewski, M.J.; Engels, E.A.; et al. Immunoparesis and monoclonal gammopathy of undetermined significance are disassociated in advanced age. Am. J. Hematol. 2013, 88, 89–92. [Google Scholar] [CrossRef]
- Valkovic, T.; Gacic, V.; Nacinovic-Duletic, A. Multiple Myeloma Index for Risk of Infection. J. Cancer 2018, 9, 2211–2214. [Google Scholar]
- Grigorieva, I.; Thomas, X.; Epstein, J. The bone marrow stromal environment is a major factor in myeloma cell resistance to dexamethasone. Exp. Hematol. 1998, 6, 597–603. [Google Scholar]
- Nefedova, Y.; Landowski, T.H.; Dalton, W.S. Bone marrow stromal-derived soluble factors and direct cell contact contrib-ute to de novo drug resistance of myeloma cells by distinct mechanism. Leukemia 2003, 17, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Dhodapkar, M.V.; Krasovsky, J.; Olson, K.T. cells from the tumor microenvironment of patients with progressive myeloma can generate strong, tumor-specific cytolytic responses to autologous, tumor-loaded dendritic cells. Proc. Natl Acad. Sci. USA 2002, 99, 13009–13013. [Google Scholar] [CrossRef] [PubMed]
- Dhodapkar, M.V.; Krasovsky, J.; Osman, K.; Geller, M.D. Vigorous premalignancy-specific effector T cell response in the bone marrow of patients with monoclonal gammopathy. J Exp Med. 2003, 11, 1753–1757. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Badros, A.; Lue, C.; Bartologie, N. Distinct T-cell clonal expansions in the vicinity of tumor cells in plasmacytoma. Cancer 2001, 91, 900–908. [Google Scholar] [CrossRef]
- Raitakari, M.; Brown, R.D.; Gibson, J.; Joshua, D.E. T cells in myeloma. Hematol. Oncol. 2003, 21, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Moss, P.; Gillespie, G.; Frodsham, P.; Bell, J.; Reybrun, H. Clonal populations of CD4 and CD8 T cells in patients with multiple myeloma and paraproteinemia. Blood 1996, 8, 3297–3306. [Google Scholar]
- Brown, R.D.; Yuen, E.; Nelson, M.; Gibson, J.; Joshua, D.E. The prognostic significance of T cell receptor b gene rearrange-ments and idiotype-reactive T cells in multiple myeloma. Leukemia 1997, 11, 1312–1317. [Google Scholar] [CrossRef]
- Sze, D.M.; Giesajtis, G.; Brown, R.D.; Raitakari, M.; Gibson, J.; Ho, J.; Baxter, A.G.; Fazekas de St Groth, B.; Basten, A.; Joshua, D.E. Clonal cytotoxic T cells are expanded in myeloma and reside in the CD8+CD57+CD28− compartment. Blood 2001, 98, 2817–2827. [Google Scholar] [CrossRef]
- Kunzmann, V.; Bauer, E.; Feurle, J.; Weissinger, F.; Tony, P.; Wilheim, M. Stimulation of gammadelta T cells by aminophos-phonates and induction of antiplasma cell activity in mul-tiple myeloma. Blood 2000, 96, 384–392. [Google Scholar]
- Davies, F.E.; Raje, N.; Hideshima, T.; Lentzsch, S.; Young, G.; Tai, Y.T.; Lin, B.; Podar, K.; Gupta, D.; Chauhan, D.; et al. Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood 2001, 98, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Hideshima, T.; Akiyama, M.; Raje, N.; Richardson, P.; Chauhan, D.; Anderson, K.C. Ex vivo induction of multiple myeloma-specific cytotoxic T lymphocytes. Blood 2003, 102, 1435–1442. [Google Scholar] [CrossRef] [PubMed]
- Perez-Andres, M.; Almeida, J.; Martin-Ayuso, M.; Moro, M.J.; Martin-Nunez, G.; Galende, J.; Hernandez, J.; Mateo, G.; San Miguel, J.F.; Orfao, A. Characterization of Bone Marrow T Cells in Monoclonal Gammopathy of Undetermined Significance, Multiple Myeloma, and Plasma Cell Leukemia Demonstrates Increased Infiltration by Cytotoxic/Th1 T Cells Demonstrating a Squed TCR-V Repertoire. Cancer 2006, 106, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Ellis, T.M.; Le, P.T.; DeVries, G.; Stubbs, E.; Fisher, M.; Bhoopalam, N. Alterations in CD30 T Cells in Monoclonal Gammopathy of Undetermined Signicance. Clin. Immunol. 2001, 98, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, R.; Bailey, R.J.; Ahmann, G.J.; Rajkumar, S.V.; Hoyer, J.D.; Lust, J.A.; Kyle, R.A.; Gertz, M.A.; Greipp, P.R.; Dewald, G.W. Genomic abnormalities in monoclonal gammopathy of undetermined significance. Blood 2002, 100, 1417–1424. [Google Scholar] [PubMed]
- Kuehl, W.M.; Bergsagel, P.L. Multiple myeloma: Evolving genetic events and host interactions. Nat. Rev. Cancer 2002, 2, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Zhan, F.; Hardin, J.; Kordsmeier, B.; Bumm, K.; Zheng, M.; Tian, E.; Sanderson, R.; Yang, Y.; Wilson, C.; Zangari, M.; et al. Global gene expression profiling of multiple myeloma, monoclonal gammopathy of undetermined significance, and normal bone marrow plasma cells. Blood 2002, 99, 1745–1757. [Google Scholar] [CrossRef] [PubMed]
- Dhodapkar, M.V.; Geller, M.D.; Chang, D.H.; Shimizu, K.; Fujii, S.; Dhodapkar, K.M.; Krasovsky, J. A reversible defect in natural killer T-cell function characterizes the progression of premalignant to malignant multiple myeloma. J. Exp. Med. 2003, 197, 1667–1676. [Google Scholar] [CrossRef]
- Massaia, M.; Dianzani, U.; Bianchi, A.; Camponi, A.; Boccadoro, M.; Pileri, A. Defective generation of alloreactive cytotoxic T lymphocytes (CTL) in human monoclonal gammopathies. Clin. Exp. Immunol. 1988, 73, 214–218. [Google Scholar]
- Choi, C.; Witzens, M.; Bucur, M.; Feuerer, M.; Sommerfeldt, N.; Trojan, A.; Ho, A.; Schirrmacher, V.; Goldschmidt, H.; Beckhove, P. Enrichment of functional CD8 memory T cells specific for MUC1 in bone marrow of patients with multiple myeloma. Blood 2005, 105, 2132–2134. [Google Scholar] [CrossRef]
- Finn, O.J. Premalignant lesions as targets for cancer vaccines. J. Exp. Med. 2003, 198, 1623–1626. [Google Scholar] [CrossRef] [PubMed]
- Racanelli, V.; Leone, P.; Frassanito, M.A.; Brunetti, C.; Perosa, F.; Ferrone, S.; Dammacco, F. Alterations in the antigen processing-presenting machinery of transformed plasma cells are associated with reduced recognition by CD8 T cells and characterize the progression of MGUS to multiple myeloma. Blood 2009. [Google Scholar] [CrossRef] [PubMed]
- Tsung, K.; Meko, J.B.; Peplinski, G.R.; Tsung, Y.L.; Norton, J.A. IL-12 induces T helper-1 directed antitumor response. J. Immunol. 1997, 158, 3359–3365. [Google Scholar] [PubMed]
- Nishioka, Y.; Hirao, M.; Robbins, P.D.; Lotze, M.T.; Tahara, H. Induction of systemic and therapeutic antitumor immunity using intratumoral injection of dendritic cells genetically modified to express interleukin. Cancer Res. 1999, 59, 4035–4041. [Google Scholar] [PubMed]
- Nastala, C.L.; Edington, H.D.; McKinney, T.G.; Tahara, H.; Nalesnik, M.A.; Brunda, M.J.; Gately, M.K.; Wolf, S.F.; Schreiber, R.D.; Storkus, W.J. Recombinant IL-12 administration induces tumor regression in association with IFN-g production. J. Immunol. 1994, 153, 1697–1706. [Google Scholar] [PubMed]
- Coughlin, C.M.; Salhany, K.E.; Wysocka, M.; Aruga, E.; Kurzawa, H.; Chang, A.E.; Hunter, C.A.; Fox, J.C.; Trinchieri, G.; Lee, W.M. Interleukin-12 and interleukin-18 synergistically induce murine tumor regression which involves inhibition of angiogenesis. J. Clin. Invest. 1998, 101, 1441–1452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, L.X.; Zhang, X.; Xiao, C.; Chang, C.K. Function of peripheral blood Th17 cells in patients with multiple myeloma. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2013, 21, 1187–1189. [Google Scholar] [PubMed]
- Atanackovic, T.; Cao, Y.; Luetkens, T.; Panse, J.; Faltz, C.; Arfsten, J.; Bartels, K.; Wolschke, C.; Eiermann, T.; Zander, A.R.; et al. CD4+CD25+ FOXP3+ T regulatory cells reconstitute and accumulate in the bone marrow of patients with multiple myeloma following allogeneic stem cell transplantation. Haematologica 2008, 3, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Prabhala, R.H.; Neri, P.; Bae, J.; Tassone, P.; Shammas, M.A.; Allam, C.K.; Daley, J.F.; Chauhan, D.; Blanchard, E.; Thatte, H.S.; et al. Dysfunctional T regulatory cells in multiple myeloma. Blood 2006, 1, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Yan, R.; Dai, X.; Xie, X.; Wen, H.; Yang, S. The alteration and clinical significance of h1/Th2/Th17/Treg cells in patients with multiple myeloma. Inflammation 2015, 38, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, P.; Jia, Y.; He, N.; Li, D.; Ji, C.; Ma, D. Elevated Th22 as well as Th17 cells associated with therapeutic outcome and clinical stage are potential targets in patients with multiple myeloma. Oncotarget 2015, 6, 17958–17967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takikawa, O.; Yoshida, R.; Kido, R.; Hayaishi, O. Tryptophan degradation in mice initiated by indoleamine 2,3-dioxygenase. J. Biol. Chem. 1986, 261, 3648–3653. [Google Scholar] [PubMed]
- Bonanno, G.; Mariotti, A.; Procoli, A.; Folgiero, V.; Natale, D.; De Rosa, L.; Majolino, I.; Novarese, L.; Rocci, A.; Gambella, M.; et al. Indoleamine 2,3-dioxygenase 1 (IDO1) activity correlates with immune system abnormalities in multiple myeloma. J. Transl. Med. 2012, 10, 247. [Google Scholar] [CrossRef] [PubMed]
- Beyer, M.; Schultze, J.L. Regulatory T cells: Major players in the tumor microenvironment. Curr. Pharm. Design 2009, 15, 1879–1892. [Google Scholar] [CrossRef]
- Beyer, M.; Classen, S.; Endl, E.; Kochanek, M.; Weihrauch, M.R.; Debey-Pascher, S.; Knolle, P.A.; Schultze, J.L. Comparative approach to define increased regulatory T cells in different cancer subtypes by combined assessment of CD127 and FOXP3. Clin. Dev. Immunol. 2011, 2011, 734036. [Google Scholar] [CrossRef] [PubMed]
- Castella, B.; Melaccio, A.; Foglietta, M.; Riganti, C.; Massaia, M. Vγ9Vδ2 T Cells as Strategic Weapons to Improve the Potency of Immune Checkpoint Blockade and Immune Interventions in Human Myeloma. Front. Oncol. 2018, 8, 508. [Google Scholar] [CrossRef] [PubMed]
- Di Lullo, G.; Marcatti, M.; Heltai, S.; Brunetto, E.; Tresoldi, C.; Bondanza, A.; Bonini, C.; Ponzoni, M.; Tonon, G.; Ciceri, F.; et al. Th22 cells increase in poor prognosis multiple myeloma and promote tumor cell growth and survival. Oncoimmunology 2015, 4, 1005460. [Google Scholar] [CrossRef]
- Fridman, W.H.; Pages, F.; Sautes-Fridman, C.; Galon, J. The immune contexture in human tumours: Impact on clinical outcome. Nat. Rev. Cancer 2012, 12, 298–306. [Google Scholar] [CrossRef]
- Protti, M.P.; Monte, L.D.; Lullo, G.D. Tumor antigen-specific CD4(+) T cells in cancer immunity: From antigen identification to tumor prognosis and development of therapeutic strategies. Tissue Antigens 2014, 83, 237–246. [Google Scholar] [CrossRef]
- Galon, J.; Costes, A.; Sanchez-Cabo, F.; Kirilovsky, A.; Mlecnik, B.; Lagorce-Pages, C.; Tosolini, M.; Camus, M.; Berger, A.; Wind, P.; et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006, 313, 1960–1964. [Google Scholar] [CrossRef]
- Garderet, L.; Mazurier, C.; Chapel, A.; Ernou, I.; Boutin, L.; Holy, X.; Gorin, N.C.; Lopez, M.; Doucet, C.; Lataillade, J.J. Mesenchymal stem cell abnormalities in patients with multiple myeloma. Leuk. Lymphoma 2007, 48, 2032–2041. [Google Scholar] [CrossRef] [PubMed]
- Wallace, S.R.; Oken, M.M.; Lunetta, K.L.; Panoskaltsis-Mortari, A.; Masellis, A.M. Abnormalities of bone marrow mesenchymal cells in multiple myeloma patients. Cancer 2001, 91, 1219–1230. [Google Scholar] [CrossRef]
- Zdzisinska, B.; Bojarska-Junak, A.; Dmoszynska, A.; Kandefer-Szerszen, M. Abnormal cytokine production by bone marrow stromal cells of multiple myeloma patients in response to RPMI8226 myeloma cells. Arch. Immunol. Ther. Exp. 2008, 56, 207–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnulf, B.; Lecourt, S.; Soulier, J.; Ternaux, B.; Lacassagne, M.N.; Crinquette, A.; Dessoly, J.; Sciaini, A.K.; Benbunan, M.; Chomienne, C.; et al. Phenotypic and functional characterization of bone marrow mesenchymal stem cells derived from patients with multiple myeloma. Leukemia 2007, 21, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Corre, J.; Mahtouk, K.; Attal, M.; Gadelorge, M.; Huynh, A.; Fleury-Cappellesso, S.; Danho, C.; Laharrague, P.; Klein, B.; Rème, T.; et al. Bone marrow mesenchymal stem cells are abnormal in multiple myeloma. Leukemia 2007, 21, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Garayoa, M.; Garcia, J.L.; Santamaria, C.; Garcia-Gomez, A.; Blanco, J.F.; Pandiella, A.; Hernández, J.M.; Sanchez-Guijo, F.M.; del Cañizo, M.C.; Gutiérrez, N.C.; et al. Mesenchymal stem cells from multiple myeloma patients display distinct genomic profile as compared with those from normal donors. Leukemia 2009, 23, 1515–1527. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Campbell, R.A.; Chang, Y.; Li, M.; Wang, C.S.; Li, J.; Sanchez, E.; Share, M.; Steinberg, J.; Berenson, A.; et al. Pleiotrophin produced by multiple myeloma induces transdifferentiation of monocytes into vascular endothelial cells: A novel mechanism of tumor-induced vasculo-genesis. Blood 2009, 113, 1992–2002. [Google Scholar] [CrossRef]
- Sponaas, A.M.; Moen, S.H.; Liabakk, N.B.; Feyzi, E.; Holien, T.; Kvam, S.; Grøseth, L.A.; Størdal, B.; Buene, G.; Espevik, T.; et al. The proportion of CD16(C)CD14(dim) monocytes increases with tumor cell load in bone marrow of patients with multiple myeloma. Immun. Inflamm. Dis. 2015, 3, 94–102. [Google Scholar] [CrossRef]
- Sedlarikova, L.; Sadilkova, K.; Kubiczkova, L.; Hajek, R.; Sevcikova, S. Citokines profiles of multiple myeloma and Waldenstrom macroglobulinemia. Klin. Oncol. 2014, 27, 18–23. [Google Scholar] [CrossRef]
- Mehtap, O.; Atesoglu, E.B.; Tarkun, A.; Hacihanefioglu, A.; Dolasik, I.; Musul, M.M. IL-21 and other serum proinflammatory cytokine levels in patients with multiple myeloma at diagnosis. J. Postgrad Med. 2014, 60, 141–144. [Google Scholar] [CrossRef]
- Owagara, H.; Handa, H.; Yamazaki, T.; Toda, T.; Yoshida, K.; Nishimoto, N.; Al-ma’Quol, W.H.; Kaneko, Y.; Matsushima, T.; Tsukamoto, N.; et al. High Th1/Th2 ratio in patients with multiple myeloma. Leuk Res. 2005, 29, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Hideshima, T.; Chauhan, D.; Hayashi, T.; Podar, K.; Akiyama, M.; Gupta, D.; Richardson, P.; Munshi, N.; Anderson, K.C. The biological sequelae of stromal cell-derived factor-1alpha in multiple myeloma. Mol. Cancer Ther. 2002, 1, 539–544. [Google Scholar] [PubMed]
- Podar, K.; Tai, Y.T.; Davies, F.E.; Lentzsch, S.; Sattler, M.; Hideshima, T.; Lin, B.K.; Gupta, D.; Shima, Y.; Chauhan, D.; et al. Vascular endothelial growth factor triggers signalling cascades mediating multiple myeloma cell growth and migration. Blood 2001, 98, 428–435. [Google Scholar] [CrossRef]
- Derksen, P.W.; de Gorter, D.J.; Meijer, H.P.; Bende, R.J.; van Dijk, M.; Lokhorst, H.M.; Bloem, A.C.; Spaargaren, M.; Pals, S.T. The hepatocyte growth factor/Met pathway controls proliferation and apoptosis in multiple myeloma. Leukemia 2003, 17, 764–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, W.C.; Van Ness, B. Distinct IL-6 signal transduction leads to growth arrest and death in B cells or growth promotion and cell survival in myeloma cells. Leukemia 2002, 16, 1182–1188. [Google Scholar] [CrossRef] [Green Version]
- Dezorella, N.; Pevsner-Fischer, M.; Deutsch, V.; Kay, S.; Baron, S.; Stern, R.; Tavor, S.; Nagler, A.; Naparstek, E.; Zipori, D.; et al. Mesenchymal stromal cells revert multiple myeloma cells to less differentiated phenotype by the combined activities of adhesive interactions and interleukin-6. Exp. Cell Res. 2009, 315, 1904–1913. [Google Scholar] [CrossRef] [PubMed]
- Bosseboeuf, A.; Allain-Maillet, S.; Mennesson, N.; Tallet, A.; Rossi, C.; Garderet, L.; Caillot, D.; Moreau, P.; Piver, E.; Girodon, F.; et al. Pro-inflammatory State in Monoclonal Gammopathy of Undetermined Significance and in Multiple Myeloma Is haracterized by Low Sialylation of Pathogen-Specific and Other Monoclonal Immunoglobulins. Front. Immunol. 2017, 8, 1347. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, A.; Moschetta, M.; Frassanito, M.A.; Berardi, S.; Catacchio, I.; Ria, R.; Racanelli, V.; Caivano, A.; Solimando, A.G.; Vergara, D.; et al. A HGF/cMET autocrine loop is operative in multiple myeloma bone marrow endothelial cells and may represent a novel therapeutic target. Clin. Cancer Res. 2014, 205, 796–807. [Google Scholar] [CrossRef]
- Koerber, R.M.; Held, S.A.E.; Heine, A.; Kotthoff, P.; Daecke, S.N.; Bringmann, A.; Brossart, P. Analysis of the anti-proliferative and the pro-apoptotic efficacy of Syk inhibition in multiple myeloma. Exp. Hematol. Oncol. 2015, 4, 21. [Google Scholar] [CrossRef]
- Westendorf, J.J.; Ahmann, G.J.; Greipp, P.R.; Witzig, T.E.; Kyle, R.A.; Lust, J.A.; Jelinek, D.F. Establishment and characterization of three myeloma cell lines that demonstrate variable cytokine responses and abilities to produce autocrine interleukin. Leukemia 1996, 10, 866–876. [Google Scholar] [PubMed]
- Lichtenstein, A.; Berenson, J.; Norman, D.; Chang, M.P.; Carlile, A. Production of cytokines by bone marrow cells obtained from patients with multiple myeloma. Blood 1989, 74, 1266–1273. [Google Scholar] [PubMed]
- Klein, B.; Lu, Z.Y.; Gaillard, J.P.; Harousseau, J.L.; Bataille, R. Inhibiting IL-6 in human multiple myeloma. Curr. Top. Microbiol. Immunol. 1992, 182, 237–244. [Google Scholar] [PubMed]
- Cozzolino, F.; Torcia, M.; Aldinucci, D.; Rubartelli, A.; Miliani, A.; Shaw, A.R.; Lansdorp, P.M.; Di Guglielmo, R. Production of interleukin-1 by bone marrow myeloma cells. Blood 1989, 74, 380–387. [Google Scholar] [PubMed]
- Donovan, K.A.; Lacy, M.Q.; Kline, M.P. Contrast in cytokine expression between patients with monoclonal gammopathy of undetermined significance or multiple myeloma. Leukemia 1998, 12, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Kyle, R.A.; Lust, J.A. Monoclonal gammopathies of undetermined significance. Semin. Hematol. 1989, 26, 176–200. [Google Scholar] [PubMed]
- Hawley, T.S.; Lach, B.; Burns, B.F.; May, L.T.; Sehgal, P.B.; Hawley, R.G. Expression of retrovirally transduced IL-1alpha in IL-6-dependent B cells: A murine model of aggressive multiple myeloma. Growth Factors. 1991, 5, 327–338. [Google Scholar] [CrossRef]
- Hawley, R.G.; Wang, M.H.; Fong, A.Z.; Hawley, T.S. Association between ICAM-1 expression and metastatic capacity of murine B-cell hybridomas. Clin. Exp. Metastasis 1993, 11, 213–226. [Google Scholar] [CrossRef]
- Hamilton, M.S.; Ball, J.; Bromidge, E.; Franklin, I.M. Surface antigen expression of human neoplastic plasma cells includes molecules associated with lymphocyte recirculation and adhesion. Br. J. Haematol. 1991, 78, 60–65. [Google Scholar] [CrossRef]
- Van Camp, B.; Durie, B.G.; Spier, C.; De Waele, M.; Van Riet, I.; Vela, E.; Frutiger, Y.; Richter, L.; Grogan, T.M. Plasma cells in multiple myeloma express a natural killer cell-associated antigen: CD56 (NKH-1; Leu-19). Blood 1990, 6, 377–382. [Google Scholar]
- Lewinsohn, D.M.; Nagler, A.; Ginzton, N.; Greenberg, P.; Butcher, E.C. Hematopoietic progenitor cell expression of the H-CAM (CD44) homing-associated adhesion molecule. Blood 1990, 75, 589–595. [Google Scholar]
- Drach, J.; Gattringer, C.; Huber, H. Expression of the neural cell adhesion molecule (CD56) by human myeloma cells. Clin. Exp. Immunol. 1991, 83, 418–422. [Google Scholar] [CrossRef]
- Dinarello, C.A. Biologic basis for interleukin-1 in disease. Blood 1996, 87, 2095–2147. [Google Scholar] [Green Version]
- Rettig, M.B.; Ma, H.J.; Vescio, R.A.; Põld, M.; Schiller, G.; Belson, D.; Savage, A.; Nishikubo, C.; Wu, C.; Fraser, J.; et al. Kaposi’s sarcoma-associated herpesvirus infection of bone marrow dendritic cells from multiple myeloma patients. Science 1997, 276, 1851–1854. [Google Scholar] [CrossRef]
- Corley, P.A. Induction of interleukin-1 and glucocorticoid hormones by HIV promotes viral replication and links human chromosome 2 to AIDS pathogenesis: Genetic mechanisms and therapeutic implications. Med. Hypotheses 1997, 48, 415–421. [Google Scholar] [CrossRef]
- Beaulieu, A.D.; Paquin, R.; Gosselin, J. Epstein-Barr virus modulates de novo protein synthesis in human neutrophils. Blood 1995, 86, 2789–2798. [Google Scholar] [PubMed]
- Bitko, V.; Velazquez, A.; Yang, L.; Yang, Y.C.; Barik, S. Transcriptional induction of multiple cytokines by human respiratory syncytial virus requires activation of NF-kappa B and is inhibited by sodium salicylate and aspirin. Virology 1997, 232, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Vacca, R.; Di Stefano, A.; Frassanito, G.; Iodice, A.; Dammacco, F. A disturbance of the IL-2/IL-2 receptor system parallels the activity of multiple myeloma. Clin. Exp. Immunol. 1991, 84, 429–443. [Google Scholar]
- Goto, H.; Shimazaki, C.; Ashikara, E.; Ohkawa, K.; Oku, N.; Inaba, T.; Murakami, S.; Fujita, S.; Nakagawa, M. Effects of interleukin-3 and interleukin-6 on peripheral blood cells from mul-tiple myeloma patients and their clinical significance. Acta Haematol. 1992, 88, 129–135. [Google Scholar] [CrossRef]
- Treon, S.P.; Anderson, K.C. Interleukin-6 in multiple myeloma and related plasma cell dyscrasias. Curr. Opin. Haem. 1998, 5, 42–48. [Google Scholar] [CrossRef]
- Teoh, G.; Anderson, K.C. Interaction of tumor and host cells with adhesion and extracellular matrix molecules in the development of multiple myeloma. Hematol Oncol Clin North Am. 1997, 11, 27–42. [Google Scholar] [CrossRef]
- Chauhan, D.; Uchiyama, H.; Akbarali, Y.; Urashima, D.; Yamamoto, K.I.; Libermann, T.A.; Anderson, K.C. Multiple myeloma cell adhesion induced interleukin-6 expression in bone marrow stromal cells involves activation of NF-κB. Blood 1996, 87, 1104–1112. [Google Scholar] [PubMed]
- Lokhorst, H.M.; Lemme, T.; De Smet, M.; Klein, S.; De Weger, R.A.; Van Oers, R.; Bloem, A.C. Primary tumor cells of myeloma patients induce interleukin-6 secretion in long-term bone marrow cultures. Blood 1994, 84, 2269–2277. [Google Scholar] [PubMed]
- Frassanito, M.A.; Cusmai, A.; Dammacco, F. Deregulated cytokine network and defective Th1 immune response in multiple myeloma. Clin. Exp. Immunol. 2001, 125, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Nachbaur, D.M.; Herold, M.; Maneschg, A.; Huber, H. Serum levels of interleukin 6 in multiple myeloma and other hematological disorders: Correlation with disease activity and other prognostic parameters. Ann. Haematol. 1991, 62, 54–58. [Google Scholar] [CrossRef]
- Duvillard, L.; Guiguet, M.; Casanovas, R.O.; Caillot, D.; Monnier-Zeller, V.; Bernard, A.; Guy, H.; Solary, E. Diagnostic value of serum interleukin 6 levels in monoclonal gammopathies. Br. J. Haematol. 1995, 89, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Bataille, R.; Jourdan, M.; Zhang, X.G.; Klein, B. Serum levels of interleukin 6, a potent myeloma cell growth factor, as a reflect of disease severity in plasma cell dyscrasias. J. Clin. Investig. 1989, 84, 2008–2011. [Google Scholar] [CrossRef]
- Papadaki, H.; Kyriakou, D.; Foudoulakis, A.; Markidou, F.; Alexandrakis, M.; Eliopoulos, G.D. Serum levels of soluble IL-6 receptor in multiple myeloma as indicator of disease activity. Acta Haematol. 1997, 97, 191–195. [Google Scholar] [CrossRef]
- Soutar, R.L.; Dillon, J.M.; Brown, D.; Ralston, S.H. Cytokine expression in multiple myeloma and monoclonal gammopathy: Analysis by reverse transcription/polymerase chain reaction and quantitation PCR. Leuk. Lymphoma 1996, 24, 111–120. [Google Scholar] [CrossRef]
- Emile, C.; Fermand, J.P.; Danon, F. Interleukin-6 serum levels in patients with multiple myeloma. Br. J. Haematol. 1994, 86, 439–440. [Google Scholar] [CrossRef]
- Blade, J.; Filella, X.; Montoto, S.; Bosch, F.; Rosinol, L.; Coca, F.; Gine, E.; Nadal, E.; Aymerich, M.; Rozman, M.; et al. Interleukin 6 and tumour necrosis factor alpha serum levels in monoclonal gammopathy of undetermined significance. Br. J. Haematol. 2002, 117, 387–389. [Google Scholar] [CrossRef]
- Stasi, R.; Brunetti, M.; Parma, A.; Di Giulio, C.; Terzoli, E.; Pagano, A. The prognostic value of soluble interleukin-6 receptor in patients with multiple myeloma. Cancer 1998, 82, 1860–1866. [Google Scholar] [CrossRef]
- Gaillard, J.P.; Bataille, R.; Brailly, H.; Zuber, C.; Yasukawa, K.; Attal, M.; Maruo, N.; Taga, T.; Kishimoto, T.; Klein, B. Increased and highly stable levels of functional soluble interleukin-6 receptor in sera of patients with monoclonal gammopathy. Eur. J. Immunol. 1993, 23, 820–824. [Google Scholar] [CrossRef] [PubMed]
- Salem, D.A.; Korde, N.; Venzon, D.J.; Liewehr, D.J.; Maric, I.; Calvo, K.R.; Braylan, R.; Tembhare, P.R.; Yuan, C.M.; Landgren, C.O.; et al. Expression of the IL-6 receptor alpha-chain (CD126) in normal and abnormal plasma cells in monoclonal gammopathy of undetermined significance and smoldering myeloma. Leuk. Lymphoma 2018, 59, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Lamarthée, B.; de Vassoigne, F.; Malard, F.; Stocker, N.; Boussen, I.; Médiavilla, C.; Tang, R.; Fava, F.; Garderet, L.; Marjanovic, Z.; et al. Quantitative and functional alterations of 6-sulfo LacNac dendritic cells in multiple myeloma. OncoImmunology 2018, 7, 7. [Google Scholar]
- Dhodapkar, K.M.; Barbuto, S.; Matthews, P.; Kukreja, A.; Mazumder, A.; Vesole, D.; Jagannath, S.; Dhodapkar, M.V. Dendritic cells mediate the induction of polyfunctional human IL17-producing cells (Th17-1 cells) enriched in the bone marrow of patients with myeloma. Blood 2008, 112, 2878–2885. [Google Scholar] [CrossRef] [PubMed]
- Musolino, C.; Allegra, A.; Profita, M.; Alonci, A.; Saitta, S.; Russo, S.; Bonanno, A.; Innao, V.; Gangemi, S. Reduced IL-33 plasma levels in multiple myeloma patients are associated with more advanced stage of disease. Br. J. Haematol. 2013, 160, 709–710. [Google Scholar] [CrossRef] [PubMed]
- Musolino, C.; Allegra, A.; Profita, M.; Alonci, A.; Saitta, S.; Bonanno, A.; Gerace, D.; Calabrò, L.; Gangemi, S. Reduction in IL-33 plasma levels might be involved in T cell dysregulation in chronic lymphocytic leukemia. Acta Haematol. 2014, 131, 165–166. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, G.; Munshi, N.C. Pathogenesis beyond the cancer clone(s) in multiple myeloma. Blood 2015, 125, 3049–3058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roussou, M.; Tasidou, A.; Dimopoulos, M.A.; Kastritis, E.; Migkou, M.; Christoulas, D.; Gavriatopoulou, M.; Zagouri, F.; Matsouka, C.; Anagnostou, D.; et al. Increased expression of macrophage inflammatory protein-1 alpha on trephine biopsies correlates with extensive bone disease, increased angiogenesis and advanced stage in newly diagnosed patients with multiple myeloma. Leukemia 2009, 23, 2177–2181. [Google Scholar] [CrossRef] [PubMed]
- Bladè, J.; Filella, X.; Montoto, S.; Bosch, F.; Molina, R.; Coca, F.; Lopez-Guillermo, A.; Cid, J.; Ballesta, A.; Montserrat, E. Clinical relevance of interleukin 6 and tumor necrosis factor-alpha serum levels in monoclonal gammopathy of undetermined significance. Blood 1997, 90, 351. [Google Scholar]
- Allegra, A.; Alonci, A.; Bellomo, G.; Granata, A.; Tolomeo, A.; Penna, G.; Russo, S.; Sabattini, E.; Musolino, C. Serum levels of interleukin-16 in a multiple myeloma patient with cutaneous involvement. Int. J. Derm. 2010, 49, 435–437. [Google Scholar] [CrossRef] [PubMed]
- Hermann, F.; Andreeff, M.; Gruss, H.J.; Brach, M.A.; Lubbert, M.; Mertelsmann, R. Interleukin-4 inhibits growth of multiple myelomas by suppressing interleukin-6 expression. Blood 1991, 78, 2070–2074. [Google Scholar] [Green Version]
- Sawamura, M.; Murakami, H.; Tamura, J.; Matsushima, T.; Sato, S.; Naruse, T.; Tsuchiya, J. Tumor necrosis factor- and interleukin-4 promote the differentiation of myeloma cell precursors in multiple myeloma. Br. J. Haematol. 1994, 88, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Sawamura, M.; Murakami, H.; Tsuchiya, J. Tumor necrosis factor- and interleukin-4 in myeloma cell precursor differentiation. Leuk. Lymph. 1996, 21, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Rousset, F. Human B lymphocytes: Phenotype, proliferation and differentiation. Adv. Immunol. 1992, 52, 125–130. [Google Scholar] [PubMed]
- Merico, F.; Bergui, L.; Gregoretti, M.G.; Ghia, P.; Aimo, G.; Lindley, I.J.O.; Caligaris-Cappio, F. Cytokines involved in the progression of multiple myeloma. Clin. Exp. Immunol. 1993, 92, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Stasi, R.; Brunetti, M.; Bussa, S.; Pagano, A. Serum interleukin-10 in plasma cell dyscrasias. Am. J. Hematol. 1997, 54, 335–337. [Google Scholar] [CrossRef]
- Yoshida, H.; Hunter, C.A. The immunobiology of interleukin. Annu. Rev. Immunol. 2015, 33, 417–443. [Google Scholar] [CrossRef] [PubMed]
- Murugaiyan, G.; Saha, B. IL-27 in tumor immunity and immunotherapy. Trends Mol. Med. 2013, 19, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Carbotti, G.; Barisione, G.; Airoldi, I.; Mezzanzanica, D.; Bagnoli, M.; Ferrero, S.; Petretto, A.; Fabbi, M.; Ferrini, S. IL-27 induces the expression of IDO and PD-L1 in human cancer cells. Oncotarget 2015, 6, 43267–43280. [Google Scholar] [CrossRef] [Green Version]
- Pesce, S.; Greppi, M.; Tabellini, G.; Rampinelli, F.; Parolini, S.; Olive, D.; Moretta, L.; Moretta, A.; Marcenaro, E. Identification of a subset of human natural killer cells expressing high levels of programmed death 1: A phenotypic and functional characterization. J. Allergy Clin. Immunol. 2017, 139, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Dondero, A.; Casu, B.; Bellora, F.; Vacca, A.; De Luisi, A.; Frassanito, M.A.; Cantoni, C.; Gaggero, S.; Olive, D.; Moretta, A.; et al. NK cells and multiple myeloma-associated endothelial cells: Molecular interactions and influence of IL-27. Oncotarget 2017, 8, 35088–35102. [Google Scholar] [CrossRef] [PubMed]
- Greco, C.; Vitelli, G.; Vercillo, G.; Vona, R.; Giannarelli, D.; Sperduti, I.; Pisani, F.; Capoluongo, E.; Petti, M.C.; Ameglio, F. Reduction of serum IGF-1 levels in patients affected with monoclonal gammopathies of undetermined significance or multiple myeloma. Comparison with bFGF, VEGF and κ-ras gene mutation. J. Exp. Clin. Cancer Res. 2009, 28, 35. [Google Scholar] [CrossRef] [PubMed]
- Diamond, T.; Levy, A.; Smith, A.; Day, P.; Manoharan, A. Non-invasive markers of bone turnover and plasma cytokines differ in osteoporotic patients with multiple myeloma and monoclonal gammopathies of undetermined significance. Intern. Med. J. 2001, 31, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Allegra, A.; Sant’antonio, E.; Penna, G.; Alonci, A.; D’Angelo, A.; Russo, S.; Cannavò, A.; Gerace, D.; Musolino, C. Novel therapeutic strategies in multiple myeloma: Role of the heat shock protein inhibitors. Eur. J. Haematol. 2011, 86, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Pinder, J.B.; Attwood, K.M.; Dellaire, G. Reading, writing, and repair: The role of ubiquitin and the ubiquitin-like proteins in DNA damage signaling and repair. Front. Genet. 2013, 4, 45. [Google Scholar] [CrossRef]
- Bologna, S.; Ferrari, S. It takes two to tango: Ubiquitin and SUMO in the DNA damage response. Front. Genet. 2013, 4, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Hakim, A.; Escribano-Diaz, C.; Landry, M.C.; O’Donnell, L.; Panier, S.; Szilard, R.K.; Durocher, D. The ubiquitous role of ubiquitin in the DNA damage response. DNA Repair 2010, 9, 1229–1240. [Google Scholar] [CrossRef]
- Matunis, M.J.; Coutavas, E.; Blobel, G. A novel ubiq-uitin-like modification modulates the partition-ing of the Ran-GTPase-activating protein Ran-GAP1 between the cytosol and the nuclear pore complex. J. Cell Biol. 1996, 135, 1457–1470. [Google Scholar] [CrossRef]
- Mahajan, R.; Delphin, C.; Guan, T.; Gerace, L.; Melchior, F. A small ubiquitin-related polypeptide involved in targeting RanGAP1 to nuclear pore complex protein Ranbp. Cell 1997, 88, 97–107. [Google Scholar] [CrossRef]
- Janka, C.; Selmi, C.; Gershwin, M.E.; Will, H.; Sternsdorf, T. Small ubiquitin-related modifiers: A novel and independent class of autoantigens in primary biliary cirrhosis. Hepatology 2005, 41, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Preuss, K.D.; Pfreundschuh, M.; Fadle, N.; Regitz, E.; Kubuschok, B. Sumoylated HSP90 is a dominantly inherited plasma cell dyscrasias risk factor. J. Clin. Invest. 2015, 125, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Josselin, N.; Libouban, H.; Dib, M.; Ifrah, N.; Legrand, E.; Baslé, M.F.; Audran, M.; Chappard, D. Quantification of dendritic cells and osteoclasts in the bone marrow of patients with monoclonal gammopathy. Pathol. Oncol. Res. 2009, 15, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Boissinot, M.; Vilaine, M.; Hermouet, S. The hepatocyte growth factor (HGF)/ met axis: A neglected target in the treatment of chronic myeloproliferative neoplasms? Cancers 2014, 6, 1631–1669. [Google Scholar] [CrossRef] [PubMed]
- Klintrup, K.; Mäkinen, J.M.; Kauppila, S.; Väre, P.O.; Melkko, J.; Tuominen, H.; Tuppurainen, K.; Mäkelä, J.; Karttunen, T.J.; Mäkinen, M.J. Inflammation and prognosis in colorectal cancer. Eur. J. Cancer 2005, 41, 2645–2654. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.H.; Gillett, E.C.; Ryder, K.; Fentiman, I.S.; Miles, D.W.; Millis, R.R. Different patterns of inflammation and prognosis in invasive carcinoma of the breast. Histopathology 2006, 48, 692–701. [Google Scholar] [CrossRef]
- Cai, R.T.; Nesi, G.; Boddi, V.; Mazzoli, S.; Dal Canto, M.; Bartoletti, R. Prognostic role of the tumor-associated tissue inflammatory reaction in transitional bladder cell carcinoma. Oncol. Rep. 2006, 16, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Di Salvo, E.; Casciaro, M.; Quartuccio, S.; Genovese, L.; Gangemi, S. Do Alarmins have a potential role in autism spectrum disorders pathogenesis and progression? Biomulecules 2019, 9, 2. [Google Scholar] [CrossRef]
- Melo-Gonzalez, F.; Hepworth, M.R. Functional and phenotypic heterogeneity of group 3 innate lymphoid cells. Immunology 2017, 150, 265–275. [Google Scholar] [CrossRef]
- Whiters, D.R.; Hepworth, M.R. Group 3 Innate Lymphoid Cells: Communications Hubs of the Intestinal Immune System. Front. Immunol. 2017, 8, 1298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, A.I.; Di Santo, J.P. ILC-poiesis: Ensuring tissue ILC differentiation at the right place and time. Eur. J. Immunol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.J.; Mebius, R.E.; et al. Innate Lymphoid Cells: 10 Years On. Cell 2018, 174, 1054–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montaldo, E.; Vacca, P.; Vitale, C.; Moretta, F.; Locatelli, F.; Mingari, M.C.; Moretta, L. Human innate lymphoid cells. Immunol. Lett. 2016, 179, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Kabata, H.; Moro, K.; Koyasu, S. The group 2 innate lymphoid cell (ILC2) regulatory network and its underlying mechanisms. Immunol. Rev. 2018, 286, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Kawano, Y.; Roccaro, A.M.; Ghobrial, I.M.; Azzi, J. Multiple Myeloma and the Immune Microenvironment. Curr. Cancer Drug Targets 2017, 17, 806–818. [Google Scholar] [CrossRef] [PubMed]
- Spisek, R.; Kukreja, A.; Chen, L.C.; Matthews, P.; Mazumder, A.; Vesole, D.; Jagannath, S.; Zebroski, H.A.; Simpson, A.J.; Ritter, G.; et al. Frequent and specific immunity to the embryonal stem cell-associated antigen SOX2 in patients with monoclonal gammopathy. J. Exp. Med. 2007, 204, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Lesokhin, A.M.; Ansell, S.M.; Armand, P.; Scott, E.C.; Halwani, A.; Gutierrez, M.; Millenson, M.M.; Cohen, A.D.; Schuster, S.J.; Lebovic, D. Nivolumab in Patients With Relapsed or Refractory Hematologic Malignancy: Preliminary Results of a Phase Ib Study. J. Clin. Oncol. 2016, 34, 2698–2704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pianko, M.J.; Funt, S.A.; Page, D.B.; Cattry, D.; Scott, E.C.; Ansell, S.M.; Borrello, I.M.; Gutierrez, M.; Lendvai, N.; Hassoun, H.; et al. Efficacy and toxicity of therapy immediately after treatment with nivolumab in relapsed multiple myeloma. Leuk. Lymphoma. 2017, 59, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, G.; Dinarello, C.A. Anakinra Therapy for Non-cancer Inflammatory Diseases. Front. Pharmacol. 2018, 9, 1157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, A.; Asli, B.; Braun-Falco, M.; De Koning, H.; Fermand, J.-P.; Grattan, C.; Krause, K.; Lachmann, H.; Lenormand, C.; Martinez-Taboada, V.; et al. Schnitzler’s syndrome: Diagnosis, treatment, and follow-up. Allergy 2013, 68, 562–568. [Google Scholar] [CrossRef] [PubMed]



| Authors | Year | MGUS | MM | Humans | Animals | In Vivo | In Vitro | Ex Vivo | N. Pz. | Values |
|---|---|---|---|---|---|---|---|---|---|---|
| Landgren O. et al. [10] | 2009 | x | x | x | x | x | 71 | +M protein | ||
| Chiecchio L. et al. [11] | 2009 | x | x | x | x | 716 | +deletion/monosomy 13 (Δ13) | |||
| Garayoa M. et al. [76] | 2009 | x | x | x | 26 | distinct genomic profile | ||||
| Chen H. et al. [77] | 2009 | x | x | BM | x | +PTN | ||||
| Dezorella N. et al. [86] | 2009 | x | x | BM | x | x | −CD38; −CD138 | |||
| Greco C. et al. [143] | 2009 | x | x | x | BM | x | x | 71 with MGUS; 77 with MM | −IGF-I | |
| Josselin N et al. [153] | 2009 | x | x | x | BM | x | 53 with MGUS; 46 with MM | +dendritic cells; +osteoclasts | ||
| Racanelli V. et al. [52] | 2010 | x | x | x | BM | x | 20 with MGUS; 20 with MM | MM → APM components | ||
| Bonanno G. et al. [63] | 2012 | x | x | x | x | x | 7 with MGUS; 25 with MM | +IDO activity | ||
| Wang P. et al. [13] | 2013 | x | x | x | BM | x | x | 11 for MGUS; 13 for MM | +BAFFR; +TACI; +BCMA in MGUS; −BCMA in MM | |
| Mehtap O. et al. [80] | 2014 | x | x | BM | 44 | −IL-21; +IL-6, +IL-1β, +TNF-α | ||||
| Ferrucci A. et al. [88] | 2014 | x | x | x | BM | x | 24 with MGUS; 32 with MM | +HGF/cMET | ||
| Feng P. et al. [60] | 2015 | x | x | x | x | 33 | +Th1; +Th17; −Treg; +IL-6; +IL17A; +IFN-γ; −Foxp3 | |||
| Wang M. et al. [61] | 2015 | x | x | BM | x | 55 | +Th17; +Th22 | |||
| Di Lullo G. et al. [67] | 2015 | x | x | x | BM | x | x | 5 with MGUS; 37 with MM | +IL-13; +IL-17; +IL-22 | |
| Sponaas A.M. et al. [78] | 2015 | x | x | BM | +CD14⁺; +CD16⁺ | |||||
| Koerber R.M et al. [89] | 2015 | x | x | x | −Syk | |||||
| Nair S. et al. [6] | 2016 | x | x | x | x | BM | x | 20 Gaucher’s disease in humans and 6 GBA 1 mice | +LGL1 | |
| Bosseboeuf A. et al. [5] | 2017 | x | x | x | x | x | 101 for MGUS; 135 for MM | +IL-6, + IL-10, +IL-33 in MGUS; −IL-33 in MM | ||
| McNee G. et al. [14] | 2017 | x | x | x | BM | x | 30 | +IL-6; +CXCL12; +cMET | ||
| Bosseboeuf A. et al. [87] | 2017 | x | x | x | x | 68 with MGUS; 74 with MM | +IL-17, +IFN-α2, +IL-33, + IL-13 | |||
| Dondero A. et al. [142] | 2017 | x | x | x | BM | x | 9 | +NK; +IL-27 | ||
| Salem D. et al. [123] | 2018 | x | x | x | BM | 24 with MGUS; 35 with MM | +CD126 | |||
| Lamarthée B. et al. [124] | 2018 | x | x | x | BM | x | 21 with MGUS; 24 with MM | −Slan-DC for MM; +Slan-DC for MGUS; -IL-12 | ||
| Nair S. et al. [7] | 2018 | x | x | x | x | x | x | cohort 1 (76); cohort 2 (274) | +Ig G; +LPC |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allegra, A.; Innao, V.; Allegra, A.G.; Pugliese, M.; Di Salvo, E.; Ventura-Spagnolo, E.; Musolino, C.; Gangemi, S. Lymphocyte Subsets and Inflammatory Cytokines of Monoclonal Gammopathy of Undetermined Significance and Multiple Myeloma. Int. J. Mol. Sci. 2019, 20, 2822. https://doi.org/10.3390/ijms20112822
Allegra A, Innao V, Allegra AG, Pugliese M, Di Salvo E, Ventura-Spagnolo E, Musolino C, Gangemi S. Lymphocyte Subsets and Inflammatory Cytokines of Monoclonal Gammopathy of Undetermined Significance and Multiple Myeloma. International Journal of Molecular Sciences. 2019; 20(11):2822. https://doi.org/10.3390/ijms20112822
Chicago/Turabian StyleAllegra, Alessandro, Vanessa Innao, Andrea Gaetano Allegra, Marta Pugliese, Eleonora Di Salvo, Elvira Ventura-Spagnolo, Caterina Musolino, and Sebastiano Gangemi. 2019. "Lymphocyte Subsets and Inflammatory Cytokines of Monoclonal Gammopathy of Undetermined Significance and Multiple Myeloma" International Journal of Molecular Sciences 20, no. 11: 2822. https://doi.org/10.3390/ijms20112822
APA StyleAllegra, A., Innao, V., Allegra, A. G., Pugliese, M., Di Salvo, E., Ventura-Spagnolo, E., Musolino, C., & Gangemi, S. (2019). Lymphocyte Subsets and Inflammatory Cytokines of Monoclonal Gammopathy of Undetermined Significance and Multiple Myeloma. International Journal of Molecular Sciences, 20(11), 2822. https://doi.org/10.3390/ijms20112822





