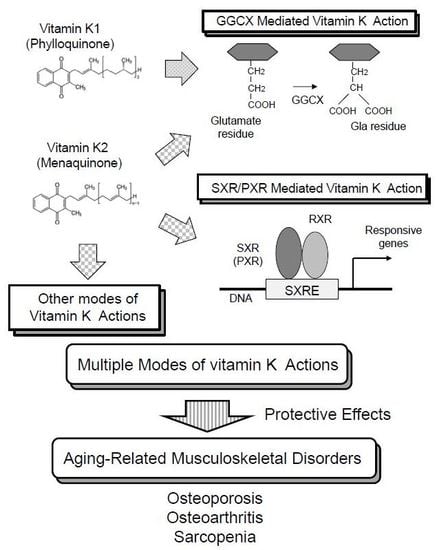
Multiple Modes of Vitamin K Actions in Aging-Related Musculoskeletal Disorders
Abstract
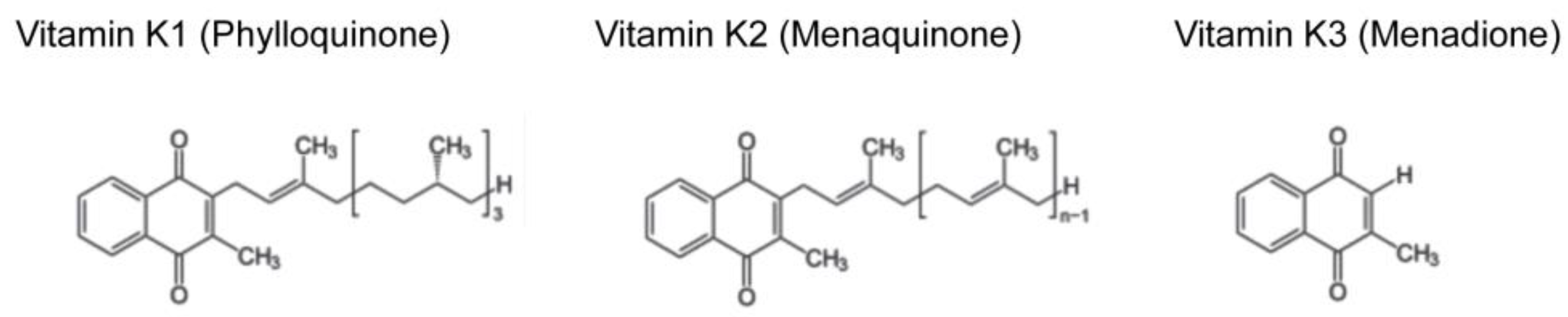
:1. Introduction
2. Vitamin K Function Mediated by γ-Carboxylation of Proteins
3. Vitamin K Function as a Ligand of SXR/PXR
4. Other Modes of Vitamin K Actions
5. Roles of Vitamin K on Osteoporosis
6. Roles of Vitamin K on Osteoarthritis
7. Roles of Vitamin K on Sarcopenia
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Suttie, J.W.; Booth, S.L. Vitamin K. Adv Nutr. 2011, 2, 440–441. [Google Scholar] [CrossRef]
- Tarvainen, M.; Fabritius, M.; Yang, B. Determination of vitamin K composition of fermented food. Food Chem. 2019, 275, 515–522. [Google Scholar] [CrossRef]
- Fu, X.; Harshman, S.G.; Shen, X.; Haytowitz, D.B.; Karl, J.P.; Wolfe, B.E.; Booth, S.L. Multiple Vitamin K Forms Exist in Dairy Foods. Curr. Dev. Nutr. 2017, 1, e000638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakagawa, K.; Hirota, Y.; Sawada, N.; Yuge, N.; Watanabe, M.; Uchino, Y.; Okuda, N.; Shimomura, Y.; Suhara, Y.; Okano, T. Identification of UBIAD1 as a novel human menaquinone-4 biosynthetic enzyme. Nature 2010, 468, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Sawada, N.; Hirota, Y.; Uchino, Y.; Suhara, Y.; Hasegawa, T.; Amizuka, N.; Okamoto, T.; Tsugawa, N.; Kamao, M.; et al. Vitamin K2 biosynthetic enzyme, UBIAD1 is essential for embryonic development of mice. PLoS ONE 2014, 9, e104078. [Google Scholar] [CrossRef]
- Dam, H. The antihemorrhagic vitamin of the chick: Occurrence and chemical nature. Nature 1935, 135, 652–653. [Google Scholar] [CrossRef]
- Doisy, E.A.; Binkley, S.B.; Thayer, S.A.; McKee, R.W. VITAMIN K. Science 1940, 91, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Nelsestuen, G.L.; Zytkovicz, T.H.; Howard, J.B. The mode of action of vitamin K. Identification of gamma-carboxyglutamic acid as a component of prothrombin. J. Biol. Chem. 1974, 249, 6347–6350. [Google Scholar]
- Stenflo, J.; Fernlund, P.; Egan, W.; Roepstorff, P. Vitamin K dependent modifications of glutamic acid residues in prothrombin. Proc. Natl. Acad. Sci. USA 1974, 71, 2730–2733. [Google Scholar] [CrossRef] [PubMed]
- Czogalla, K.J.; Biswas, A.; Höning, K.; Hornung, V.; Liphardt, K.; Watzka, M.; Oldenburg, J. Warfarin and vitamin K compete for binding to Phe55 in human VKOR. Nat. Struct. Mol. Biol. 2017, 24, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Stafford, D.W. The vitamin K cycle. J. Thromb Haemost. 2005, 3, 1873–1878. [Google Scholar] [CrossRef] [PubMed]
- Dissing, C.; Persson, E. Factor VII Tokushima (Cys22→Gly) is not -carboxylated due to a disrupted γ-carboxylase recognition site. Thromb Res. 2017, 158, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Bucher, D.; Nebelin, E.; Thomsen, J.; Stenflo, J. Identification of gamma-carboxyglutamic acid residues in bovine factors IX and X, and in a new vitamin K-dependent protein. FEBS Lett. 1976, 68, 293–296. [Google Scholar] [CrossRef]
- Tabb, M.M.; Sun, A.; Zhou, C.; Grün, F.; Errandi, J.; Romero, K.; Pham, H.; Inoue, S.; Mallick, S.; Lin, M.; et al. Vitamin K2 regulation of bone homeostasis is mediated by the steroid and xenobiotic receptor SXR. J. Biol. Chem. 2003, 278, 43919–43927. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.A.; Moore, L.B.; Shenk, J.L.; Wisely, G.B.; Hamilton, G.A.; McKee, D.D.; Tomkinson, N.C.; LeCluyse, E.L.; Lambert, M.H.; Willson, T.M.; et al. The pregnane X receptor: A promiscuous xenobiotic receptor that has diverged during evolution. Mol. Endocrinol. 2000, 14, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Suhara, Y.; Hanada, N.; Okitsu, T.; Sakai, M.; Watanabe, M.; Nakagawa, K.; Wada, A.; Takeda, K.; Takahashi, K.; Tokiwa, H.; et al. Structure-activity relationship of novel menaquinone-4 analogues: Modification of the side chain affects their biological activities. J. Med. Chem. 2012, 55, 1553–1558. [Google Scholar] [CrossRef] [PubMed]
- Karasawa, S.; Azuma, M.; Kasama, T.; Sakamoto, S.; Kabe, Y.; Imai, T.; Yamaguchi, Y.; Miyazawa, K.; Handa, H. Vitamin K2 covalently binds to Bak and induces Bak-mediated apoptosis. Mol. Pharmacol. 2013, 83, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, M.; Kato, N.; Ichimura, T.; Abe, S.; Tanaka, Y.; Taniguchi, H.; Hoshida, Y.; Moriyama, M.; Wang, Y.; Shao, R.X.; et al. Vitamin K2 binds 17beta-hydroxysteroid dehydrogenase 4 and modulates estrogen metabolism. Life Sci. 2005, 76, 2473–2482. [Google Scholar] [CrossRef]
- Ichikawa, T.; Horie-Inoue, K.; Ikeda, K.; Blumberg, B.; Inoue, S. Vitamin K2 induces phosphorylation of protein kinase A and expression of novel target genes in osteoblastic cells. J. Mol. Endocrinol. 2007, 39, 239–247. [Google Scholar] [CrossRef]
- Tie, J.; Wu, S.M.; Jin, D.; Nicchitta, C.V.; Stafford, D.W. A topological study of the human gamma-glutamyl carboxylase. Blood 2000, 96, 973–978. [Google Scholar]
- Kulman, J.D.; Harris, J.E.; Xie, L.; Davie, E.W. Identification of two novel transmembrane gamma-carboxyglutamic acid proteins expressed broadly in fetal and adult tissues. Proc. Natl. Acad. Sci. USA 2001, 98, 1370–1375. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.P.; Stevens, R.D.; Wright, D.J.; Stafford, D.W. Processive post-translational modification. Vitamin K-dependent carboxylation of a peptide substrate. J. Biol. Chem. 1995, 270, 30491–30498. [Google Scholar] [CrossRef] [PubMed]
- Hauschka, P.V.; Lian, J.B.; Gallop, P.M. Direct identification of the calcium-binding amino acid, gamma-carboxyglutamate, in mineralized tissue. Proc. Natl. Acad. Sci. USA 1975, 72, 3925–3929. [Google Scholar] [CrossRef] [PubMed]
- Price, P.A.; Otsuka, A.A.; Poser, J.W.; Kristaponis, J.; Raman, N. Characterization of a gamma-carboxyglutamic acid-containing protein from bone. Proc. Natl. Acad. Sci. USA 1976, 73, 1447–1451. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, M.; Shinar, D.; Rodan, G.A. Different pattern of alkaline phosphatase, osteopontin, and osteocalcin expression in developing rat bone visualized by in situ hybridization. J. Bone Miner. Res. 1990, 5, 831–842. [Google Scholar] [CrossRef]
- Zhang, M.; Xuan, S.; Bouxsein, M.L.; von Stechow, D.; Akeno, N.; Faugere, M.C.; Malluche, H.; Zhao, G.; Rosen, C.J.; Efstratiadis, A.; et al. Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J. Biol. Chem. 2002, 277, 44005–44012. [Google Scholar] [CrossRef] [PubMed]
- Hoang, Q.Q.; Sicheri, F.; Howard, A.J.; Yang, D.S. Bone recognition mechanism of porcine osteocalcin from crystal structure. Nature 2003, 425, 977–980. [Google Scholar] [CrossRef]
- Szulc, P.; Chapuy, M.C.; Meunier, P.J.; Delmas, P.D. Serum undercarboxylated osteocalcin is a marker of the risk of hip fracture in elderly women. J. Clin. Investig. 1993, 91, 1769–1774. [Google Scholar] [CrossRef]
- Azuma, K.; Shiba, S.; Hasegawa, T.; Ikeda, K.; Urano, T.; Horie-Inoue, K.; Ouchi, Y.; Amizuka, N.; Inoue, S. Osteoblast-Specific γ-Glutamyl Carboxylase-Deficient Mice Display Enhanced Bone Formation With Aberrant Mineralization. J. Bone Miner. Res. 2015, 30, 1245–1254. [Google Scholar] [CrossRef]
- Ducy, P.; Desbois, C.; Boyce, B.; Pinero, G.; Story, B.; Dunstan, C.; Smith, E.; Bonadio, J.; Goldstein, S.; Gundberg, C.; et al. Increased bone formation in osteocalcin-deficient mice. Nature 1996, 382, 448–452. [Google Scholar] [CrossRef] [Green Version]
- Price, P.A.; Urist, M.R.; Otawara, Y. Matrix Gla protein, a new gamma-carboxyglutamic acid-containing protein which is associated with the organic matrix of bone. Biochem. Biophys. Res. Commun. 1983, 117, 765–771. [Google Scholar] [CrossRef]
- den Hollander, W.; Boer, C.G.; Hart, D.J.; Yau, M.S.; Ramos, Y.F.M.; Metrustry, S.; Broer, L.; Deelen, J.; Cupples, L.A.; Rivadeneira, F.; et al. Genome-wide association and functional studies identify a role for matrix Gla protein in osteoarthritis of the hand. Ann. Rheum. Dis. 2017, 76, 2046–2053. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Ducy, P.; McKee, M.D.; Pinero, G.J.; Loyer, E.; Behringer, R.R.; Karsenty, G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature 1997, 386, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Wallin, R.; Schurgers, L.J.; Loeser, R.F. Biosynthesis of the vitamin K-dependent matrix Gla protein (MGP) in chondrocytes: A fetuin-MGP protein complex is assembled in vesicles shed from normal but not from osteoarthritic chondrocytes. Osteoarthr. Cartil. 2010, 18, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Varnum, B.C.; Young, C.; Elliott, G.; Garcia, A.; Bartley, T.D.; Fridell, Y.W.; Hunt, R.W.; Trail, G.; Clogston, C.; Toso, R.J.; et al. Axl receptor tyrosine kinase stimulated by the vitamin K-dependent protein encoded by growth-arrest-specific gene 6. Nature 1995, 373, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Nagata, K.; Ohashi, K.; Nakano, T.; Arita, H.; Zong, C.; Hanafusa, H.; Mizuno, K. Identification of the product of growth arrest-specific gene 6 as a common ligand for Axl, Sky, and Mer receptor tyrosine kinases. J. Biol. Chem. 1996, 271, 30022–30027. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.S.; Hakeda, Y.; Takakura, N.; Kameda, T.; Hamaguchi, I.; Miyamoto, T.; Kakudo, S.; Nakano, T.; Kumegawa, M.; Suda, T. Tyro 3 receptor tyrosine kinase and its ligand, Gas6, stimulate the function of osteoclasts. Stem Cells 1998, 16, 229–238. [Google Scholar] [CrossRef]
- Motomura, H.; Niimi, H.; Sugimori, K.; Ohtsuka, T.; Kimura, T.; Kitajima, I. Gas6, a new regulator of chondrogenic differentiation from mesenchymal cells. Biochem. Biophys. Res. Commun. 2007, 357, 997–1003. [Google Scholar] [CrossRef]
- Stenflo, J.; Jönsson, M. Protein S, a new vitamin K-dependent protein from bovine plasma. FEBS Lett. 1979, 101, 377–381. [Google Scholar] [CrossRef] [Green Version]
- Manfioletti, G.; Brancolini, C.; Avanzi, G.; Schneider, C. The protein encoded by a growth arrest-specific gene (gas6) is a new member of the vitamin K-dependent proteins related to protein S, a negative coregulator in the blood coagulation cascade. Mol. Cell Biol. 1993, 13, 4976–4985. [Google Scholar] [CrossRef]
- Stitt, T.N.; Conn, G.; Gore, M.; Lai, C.; Bruno, J.; Radziejewski, C.; Mattsson, K.; Fisher, J.; Gies, D.R.; Jones, P.F.; et al. The anticoagulation factor protein S and its relative, Gas6, are ligands for the Tyro 3/Axl family of receptor tyrosine kinases. Cell 1995, 80, 661–670. [Google Scholar] [CrossRef] [Green Version]
- Coutu, D.L.; Wu, J.H.; Monette, A.; Rivard, G.E.; Blostein, M.D.; Galipeau, J. Periostin, a member of a novel family of vitamin K-dependent proteins, is expressed by mesenchymal stromal cells. J. Biol. Chem. 2008, 283, 17991–18001. [Google Scholar] [CrossRef] [PubMed]
- Rios, H.; Koushik, S.V.; Wang, H.; Wang, J.; Zhou, H.M.; Lindsley, A.; Rogers, R.; Chen, Z.; Maeda, M.; Kruzynska-Frejtag, A.; et al. Periostin null mice exhibit dwarfism, incisor enamel defects, and an early-onset periodontal disease-like phenotype. Mol. Cell Biol. 2005, 25, 11131–11144. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wergedal, J.E.; Zhao, Y.; Mohan, S. Targeted disruption of TGFBI in mice reveals its role in regulating bone mass and bone size through periosteal bone formation. Calcif Tissue Int. 2012, 91, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Stewart, H.; Black, G.C.; Donnai, D.; Bonshek, R.E.; McCarthy, J.; Morgan, S.; Dixon, M.J.; Ridgway, A.A. A mutation within exon 14 of the TGFBI (BIGH3) gene on chromosome 5q31 causes an asymmetric, late-onset form of lattice corneal dystrophy. Ophthalmology 1999, 106, 964–970. [Google Scholar] [CrossRef]
- Tagariello, A.; Luther, J.; Streiter, M.; Didt-Koziel, L.; Wuelling, M.; Surmann-Schmitt, C.; Stock, M.; Adam, N.; Vortkamp, A.; Winterpacht, A. Ucma--A novel secreted factor represents a highly specific marker for distal chondrocytes. Matrix Biol. 2008, 27, 3–11. [Google Scholar] [CrossRef]
- Viegas, C.S.; Simes, D.C.; Laizé, V.; Williamson, M.K.; Price, P.A.; Cancela, M.L. Gla-rich protein (GRP), a new vitamin K-dependent protein identified from sturgeon cartilage and highly conserved in vertebrates. J. Biol. Chem. 2008, 283, 36655–36664. [Google Scholar] [CrossRef]
- Rafael, M.S.; Cavaco, S.; Viegas, C.S.; Santos, S.; Ramos, A.; Willems, B.A.; Herfs, M.; Theuwissen, E.; Vermeer, C.; Simes, D.C. Insights into the association of Gla-rich protein and osteoarthritis, novel splice variants and γ-carboxylation status. Mol. Nutr. Food Res. 2014, 58, 1636–1646. [Google Scholar] [CrossRef]
- Tew, B.Y.; Hong, T.B.; Otto-Duessel, M.; Elix, C.; Castro, E.; He, M.; Wu, X.; Pal, S.K.; Kalkum, M.; Jones, J.O. Vitamin K epoxide reductase regulation of androgen receptor activity. Oncotarget 2017, 8, 13818–13831. [Google Scholar] [CrossRef] [Green Version]
- Kawano, H.; Sato, T.; Yamada, T.; Matsumoto, T.; Sekine, K.; Watanabe, T.; Nakamura, T.; Fukuda, T.; Yoshimura, K.; Yoshizawa, T.; et al. Suppressive function of androgen receptor in bone resorption. Proc. Natl. Acad. Sci. USA 2003, 100, 9416–9421. [Google Scholar] [CrossRef] [Green Version]
- Falahati-Nini, A.; Riggs, B.L.; Atkinson, E.J.; O’Fallon, W.M.; Eastell, R.; Khosla, S. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J. Clin. Investig. 2000, 106, 1553–1560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhasin, S.; Storer, T.W.; Berman, N.; Callegari, C.; Clevenger, B.; Phillips, J.; Bunnell, T.J.; Tricker, R.; Shirazi, A.; Casaburi, R. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N. Engl. J. Med. 1996, 335, 1–7. [Google Scholar] [CrossRef] [PubMed]
- MacLean, H.E.; Chiu, W.S.; Notini, A.J.; Axell, A.M.; Davey, R.A.; McManus, J.F.; Ma, C.; Plant, D.R.; Lynch, G.S.; Zajac, J.D. Impaired skeletal muscle development and function in male, but not female, genomic androgen receptor knockout mice. FASEB J. 2008, 22, 2676–2689. [Google Scholar] [CrossRef] [PubMed]
- Berkner, K.L.; Pudota, B.N. Vitamin K-dependent carboxylation of the carboxylase. Proc. Natl. Acad. Sci. USA 1998, 95, 466–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Annis, D.S.; Ma, H.; Balas, D.M.; Kumfer, K.T.; Sandbo, N.; Potts, G.K.; Coon, J.J.; Mosher, D.F. Absence of Vitamin K-Dependent γ-Carboxylation in Human Periostin Extracted from Fibrotic Lung or Secreted from a Cell Line Engineered to Optimize γ-Carboxylation. PLoS ONE 2015, 10, e0135374. [Google Scholar] [CrossRef] [PubMed]
- Sultana, H.; Watanabe, K.; Rana, M.M.; Takashima, R.; Ohashi, A.; Komai, M.; Shirakawa, H. Effects of Vitamin K2 on the Expression of Genes Involved in Bile Acid Synthesis and Glucose Homeostasis in Mice with Humanized PXR. Nutrients 2018, 10, 982. [Google Scholar] [CrossRef]
- Blumberg, B.; Sabbagh, W., Jr.; Juguilon, H.; Bolado, J., Jr.; van Meter, C.M.; Ong, E.S.; Evans, R.M. SXR, a novel steroid and xenobiotic-sensing nuclear receptor. Genes Dev. 1998, 12, 3195–3205. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Verma, S.; Blumberg, B. The steroid and xenobiotic receptor (SXR), beyond xenobiotic metabolism. Nucl. Recept. Signal. 2009, 7, e001. [Google Scholar] [CrossRef] [Green Version]
- Staudinger, J.L.; Goodwin, B.; Jones, S.A.; Hawkins-Brown, D.; MacKenzie, K.I.; LaTour, A.; Liu, Y.; Klaassen, C.D.; Brown, K.K.; Reinhard, J.; et al. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc. Natl. Acad. Sci. USA 2001, 98, 3369–3374. [Google Scholar] [CrossRef] [Green Version]
- Xie, W.; Radominska-Pandya, A.; Shi, Y.; Simon, C.M.; Nelson, M.C.; Ong, E.S.; Waxman, D.J.; Evans, R.M. An essential role for nuclear receptors SXR/PXR in detoxification of cholestatic bile acids. Proc. Natl. Acad. Sci. USA 2001, 98, 3375–3380. [Google Scholar] [CrossRef] [Green Version]
- Synold, T.W.; Dussault, I.; Forman, B.M. The orphan nuclear receptor SXR coordinately regulates drug metabolism and efflux. Nat. Med. 2001, 7, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Miki, Y.; Suzuki, T.; Tazawa, C.; Blumberg, B.; Sasano, H. Steroid and xenobiotic receptor (SXR), cytochrome P450 3A4 and multidrug resistance gene 1 in human adult and fetal tissues. Mol. Cell Endocrinol. 2005, 231, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Albermann, N.; Schmitz-Winnenthal, F.H.; Z’graggen, K.; Volk, C.; Hoffmann, M.M.; Haefeli, W.E.; Weiss, J. Expression of the drug transporters MDR1/ABCB1, MRP1/ABCC1, MRP2/ABCC2, BCRP/ABCG2, and PXR in peripheral blood mononuclear cells and their relationship with the expression in intestine and liver. Biochem. Pharmacol. 2005, 70, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Suhara, Y.; Watanabe, M.; Motoyoshi, S.; Nakagawa, K.; Wada, A.; Takeda, K.; Takahashi, K.; Tokiwa, H.; Okano, T. Synthesis of new vitamin K analogues as steroid and xenobiotic receptor (SXR) agonists: Insights into the biological role of the side chain part of vitamin K. J. Med. Chem. 2011, 54, 4918–4922. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.L.; Limacher, M.; Assaf, A.R.; Bassford, T.; Beresford, S.A.; Black, H.; Bonds, D.; Brunner, R.; Brzyski, R.; Caan, B.; et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The Women’s Health Initiative randomized controlled trial. JAMA 2004, 291, 1701–1712. [Google Scholar] [PubMed]
- Nagai, S.; Ikeda, K.; Horie-Inoue, K.; Shiba, S.; Nagasawa, S.; Takeda, S.; Inoue, S. Estrogen modulates exercise endurance along with mitochondrial uncoupling protein 3 downregulation in skeletal muscle of female mice. Biochem. Biophys. Res. Commun. 2016, 480, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Jilka, R.L.; O’Brien, C.A.; Roberson, P.K.; Bonewald, L.F.; Weinstein, R.S.; Manolagas, S.C. Dysapoptosis of osteoblasts and osteocytes increases cancellous bone formation but exaggerates cortical porosity with age. J. Bone Miner. Res. 2014, 29, 103–117. [Google Scholar] [CrossRef]
- Bouckaert, J.H.; Said, A.H. Fracture healing by vitamin K. Nature 1960, 185, 849. [Google Scholar] [CrossRef]
- Hart, J.P.; Shearer, M.J.; Klenerman, L.; Catterall, A.; Reeve, J.; Sambrook, P.N.; Dodds, R.A.; Bitensky, L.; Chayen, J. Electrochemical detection of depressed circulating levels of vitamin K1 in osteoporosis. J. Clin. Endocrinol. Metab. 1985, 60, 1268–1269. [Google Scholar] [CrossRef]
- Booth, S.L.; Tucker, K.L.; Chen, H.; Hannan, M.T.; Gagnon, D.R.; Cupples, L.A.; Wilson, P.W.; Ordovas, J.; Schaefer, E.J.; Dawson-Hughes, B.; et al. Dietary vitamin K intakes are associated with hip fracture but not with bone mineral density in elderly men and women. Am. J. Clin. Nutr. 2000, 71, 1201–1208. [Google Scholar] [CrossRef]
- Kaneki, M.; Hodges, S.J.; Hosoi, T.; Fujiwara, S.; Lyons, A.; Crean, S.J.; Ishida, N.; Nakagawa, M.; Takechi, M.; Sano, Y.; et al. Japanese fermented soybean food as the major determinant of the large geographic difference in circulating levels of vitamin K2: Possible implications for hip-fracture risk. Nutrition 2001, 17, 315–321. [Google Scholar] [CrossRef]
- Yaegashi, Y.; Onoda, T.; Tanno, K.; Kuribayashi, T.; Sakata, K.; Orimo, H. Association of hip fracture incidence and intake of calcium, magnesium, vitamin D, and vitamin K. Eur. J. Epidemiol. 2008, 23, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Mott, A.; Bradley, T.; Wright, K.; Cockayne, E.S.; Shearer, M.J.; Adamson, J.; Lanham-New, S.A.; Torgerson, D.J. Effect of vitamin K on bone mineral density and fractures in adults: An updated systematic review and meta-analysis of randomised controlled trials. Osteoporos Int. 2019. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Fujita, T.; Kishimoto, H.; Makino, T.; Nakamura, T.; Nakamura, T.; Sato, T.; Yamazaki, K. Randomized controlled study on the prevention of osteoporotic fractures (OF study): A phase IV clinical study of 15-mg menatetrenone capsules. J. Bone Miner. Metab. 2009, 27, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, H.; Nakagawa, K.; Narusawa, K.; Goseki-Sone, M.; Fukushi-Irie, M.; Mizoi, L.; Yoshida, H.; Okano, T.; Nakamura, T.; Suzuki, T.; et al. A functional single nucleotide polymorphism in the vitamin-K-dependent gamma-glutamyl carboxylase gene (Arg325Gln) is associated with bone mineral density in elderly Japanese women. Bone 2007, 40, 451–456. [Google Scholar] [CrossRef]
- Ichikawa, T.; Horie-Inoue, K.; Ikeda, K.; Blumberg, B.; Inoue, S. Steroid and xenobiotic receptor SXR mediates vitamin K2-activated transcription of extracellular matrix-related genes and collagen accumulation in osteoblastic cells. J. Biol. Chem. 2006, 281, 16927–16934. [Google Scholar] [CrossRef]
- Ohta, K.; Lupo, G.; Kuriyama, S.; Keynes, R.; Holt, C.E.; Harris, W.A.; Tanaka, H.; Ohnuma, S. Tsukushi functions as an organizer inducer by inhibition of BMP activity in cooperation with chordin. Dev. Cell 2004, 7, 347–358. [Google Scholar] [CrossRef]
- Wagener, R.; Ehlen, H.W.; Ko, Y.P.; Kobbe, B.; Mann, H.H.; Sengle, G.; Paulsson, M. The matrilins--adaptor proteins in the extracellular matrix. FEBS Lett. 2005, 579, 3323–3329. [Google Scholar] [CrossRef]
- Roman-Roman, S.; Garcia, T.; Jackson, A.; Theilhaber, J.; Rawadi, G.; Connolly, T.; Spinella-Jaegle, S.; Kawai, S.; Courtois, B.; Bushnell, S.; et al. Identification of genes regulated during osteoblastic differentiation by genome-wide expression analysis of mouse calvaria primary osteoblasts in vitro. Bone 2003, 32, 474–482. [Google Scholar] [CrossRef]
- Filipp, D.; Alizadeh-Khiavi, K.; Richardson, C.; Palma, A.; Paredes, N.; Takeuchi, O.; Akira, S.; Julius, M. Soluble CD14 enriched in colostrum and milk induces B cell growth and differentiation. Proc. Natl. Acad. Sci. USA 2001, 98, 603–608. [Google Scholar] [CrossRef] [Green Version]
- Manabe, N.; Kawaguchi, H.; Chikuda, H.; Miyaura, C.; Inada, M.; Nagai, R.; Nabeshima, Y.; Nakamura, K.; Sinclair, A.M.; Scheuermann, R.H.; et al. Connection between B lymphocyte and osteoclast differentiation pathways. J. Immunol. 2001, 167, 2625–2631. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Casey, S.C.; Ito, M.; Urano, T.; Horie, K.; Ouchi, Y.; Kirchner, S.; Blumberg, B.; Inoue, S. Pregnane X receptor knockout mice display osteopenia with reduced bone formation and enhanced bone resorption. J. Endocrinol. 2010, 207, 257–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konno, Y.; Moore, R.; Kamiya, N.; Negishi, M. Nuclear xenobiotic receptor PXR-null mouse exhibits hypophosphatemia and represses the Na/Pi-cotransporter SLC34A2. Pharm. Genom. 2010, 20, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Urano, T.; Shiraki, M.; Ouchi, Y.; Inoue, S. Association of a single nucleotide polymorphism in the steroid and xenobiotic receptor (SXR) gene (IVS1-579A/G) with bone mineral density. Geriatr. Gerontol. Int. 2007, 7, 104–109. [Google Scholar] [CrossRef]
- Neogi, T.; Booth, S.L.; Zhang, Y.Q.; Jacques, P.F.; Terkeltaub, R.; Aliabadi, P.; Felson, D.T. Low vitamin K status is associated with osteoarthritis in the hand and knee. Arthritis Rheum. 2006, 54, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Oka, H.; Akune, T.; Muraki, S.; En-yo, Y.; Yoshida, M.; Saika, A.; Sasaki, S.; Nakamura, K.; Kawaguchi, H.; Yoshimura, N. Association of low dietary vitamin K intake with radiographic knee osteoarthritis in the Japanese elderly population: Dietary survey in a population-based cohort of the ROAD study. J. Orthop. Sci. 2009, 14, 687–692. [Google Scholar] [CrossRef]
- Misra, D.; Booth, S.L.; Tolstykh, I.; Felson, D.T.; Nevitt, M.C.; Lewis, C.E.; Torner, J.; Neogi, T. Vitamin K deficiency is associated with incident knee osteoarthritis. Am. J. Med. 2013, 126, 243–248. [Google Scholar] [CrossRef]
- Neogi, T.; Felson, D.T.; Sarno, R.; Booth, S.L. Vitamin K in hand osteoarthritis: Results from a randomized clinical trial. Ann. Rheum. Dis. 2008, 67, 1570–1573. [Google Scholar] [CrossRef]
- Azuma, K.; Casey, S.C.; Urano, T.; Horie-Inoue, K.; Ouchi, Y.; Blumberg, B.; Inoue, S. Pregnane X receptor knockout mice display aging-dependent wearing of articular cartilage. PLoS ONE 2015, 10, e0119177. [Google Scholar] [CrossRef] [PubMed]
- Koike, T.; Izumikawa, T.; Tamura, J.; Kitagawa, H. FAM20B is a kinase that phosphorylates xylose in the glycosaminoglycan-protein linkage region. Biochem. J. 2009, 421, 157–162. [Google Scholar] [CrossRef]
- Oya, K.; Ishida, K.; Nishida, T.; Sato, S.; Kishino, M.; Hirose, K.; Ogawa, Y.; Ikebe, K.; Takeshige, F.; Yasuda, H.; et al. Immunohistochemical analysis of dentin matrix protein 1 (Dmp1) phosphorylation by Fam20C in bone: Implications for the induction of biomineralization. Histochem. Cell Biol. 2017, 147, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, I.H. Sarcopenia: Origins and clinical relevance. J. Nutr. 1997, 127, 990S–991S. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Shea, M.K.; Loeser, R.F.; Hsu, F.C.; Booth, S.L.; Nevitt, M.; Simonsick, E.M.; Strotmeyer, E.S.; Vermeer, C.; Kritchevsky, S.B. Health ABC Study. Vitamin K Status and Lower Extremity Function in Older Adults: The Health Aging and Body Composition Study. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 1348–1355. [Google Scholar] [CrossRef] [PubMed]
- Van Ballegooijen, A.J.; van Putten, S.R.; Visser, M.; Beulens, J.W.; Hoogendijk, E.O. Vitamin K status and physical decline in older adults-The Longitudinal Aging Study Amsterdam. Maturitas 2018, 113, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Shea, M.K.; Dawson-Hughes, B.; Gundberg, C.M.; Booth, S.L. Reducing Undercarboxylated Osteocalcin With Vitamin K Supplementation Does Not Promote Lean Tissue Loss or Fat Gain Over 3 Years in Older Women and Men: A Randomized Controlled Trial. J. Bone Miner. Res. 2017, 32, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Fulton, R.L.; McMurdo, M.E.; Hill, A.; Abboud, R.J.; Arnold, G.P.; Struthers, A.D.; Khan, F.; Vermeer, C.; Knapen, M.H.; Drummen, N.E.; et al. Effect of Vitamin K on Vascular Health and Physical Function in Older People with Vascular Disease—A Randomised Controlled Trial. J. Nutr. Health Aging 2016, 20, 325–333. [Google Scholar] [CrossRef]


| Proteins | Expression | Function | References |
|---|---|---|---|
| Osteocalcin (Bone Gla protein, BGP) | Osteoblast | Regulation of bone calcification, Regulation of glucose metabolism? Enhancing male fertility? | [23,24,25,26,27,28,29,30] |
| Matrix Gla protein (MGP) | Chondrocyte, vascular smooth muscle cell, etc. | Regulation of calcification | [31,32,33,34] |
| Growth arrest specific-6 (GAS6) | Lung, heart, kidney, intestine, endothelium, vascular smooth muscle cell, bone marrow, osteoblast, osteoclast, monocyte, etc. | Thrombus formation; Inflammation; Cell proliferation; Enhancing osteoclast activity | [35,36,37,38] |
| Protein S | Liver, endothelium, monocyte, etc. | Anti-coagulation; Enhancing osteoclast activity | [37,39,40,41] |
| Periostin | Periosteum, periodontal ligament, heart valve, adrenal gland, lung, thyroid, intestine, ovary, testis, prostate | Maintenance of periodontal ligament; Bone development and maintenance | [42,43] |
| TGFβ induced (TGFBI) | Bone, joint, skin, cornea, kidney. | Bone development; Maintenance of cornea | [42,44,45] |
| Gla-rich protein (GRP)/Upper zone of growth plate and cartilage matrix associated protein (Ucma) | Chondrocyte, osteoblast, osteocyte, vascular smooth muscle cell, skin | Suppression of inflammation; Suppression of calcification in blood vessels and articular cartilages | [46,47,48] |
| Androgen receptor | Testis, prostate, muscle, brain, prostate cancer | Induction of male sexual characteristics; Promotion of prostate cancer; Bone protection; Enhancing skeletal muscle mass | [49,50,51,52,53] |
| γ-glutamyl carboxylase (GGCX) | Systemic (High expression in liver) | γ-carboxylation of proteins | [54] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azuma, K.; Inoue, S. Multiple Modes of Vitamin K Actions in Aging-Related Musculoskeletal Disorders. Int. J. Mol. Sci. 2019, 20, 2844. https://doi.org/10.3390/ijms20112844
Azuma K, Inoue S. Multiple Modes of Vitamin K Actions in Aging-Related Musculoskeletal Disorders. International Journal of Molecular Sciences. 2019; 20(11):2844. https://doi.org/10.3390/ijms20112844
Chicago/Turabian StyleAzuma, Kotaro, and Satoshi Inoue. 2019. "Multiple Modes of Vitamin K Actions in Aging-Related Musculoskeletal Disorders" International Journal of Molecular Sciences 20, no. 11: 2844. https://doi.org/10.3390/ijms20112844
APA StyleAzuma, K., & Inoue, S. (2019). Multiple Modes of Vitamin K Actions in Aging-Related Musculoskeletal Disorders. International Journal of Molecular Sciences, 20(11), 2844. https://doi.org/10.3390/ijms20112844