Non-Ionic Deep Eutectic Liquids: Acetamide–Urea Derived Room Temperature Solvents
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals
3.2. Instrumentation and Protocols
3.2.1. Molecular Dynamics Study of Acetamide (A)-urea (U) Interaction
3.2.2. General Procedure for Preparation of Eutectic Mixtures
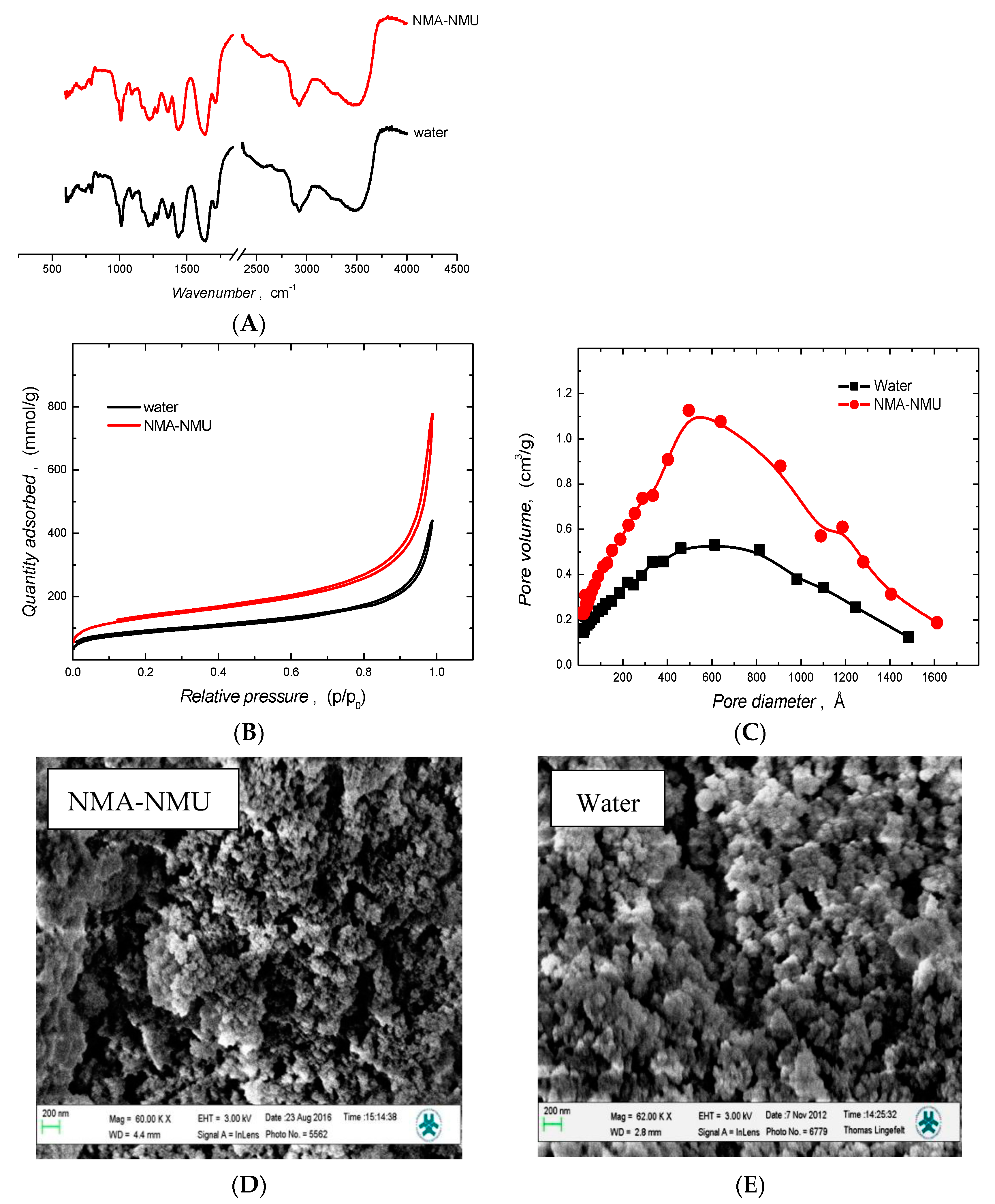
3.2.3. Characterization of Eutectic Mixtures
3.2.4. Synthesis of Methacrylic Acid (MAA)–bisacryloylpiperazine (BAP) Co-Polymer
3.2.5. Characterization of Polymers
Brunauer-Emmett-Teller (BET) and Barrett-Joyner-Halenda (BJH) Analyses
Scanning Electron Microscopy (SEM) Analysis
Fourier Transform infrared Spectrometry (FT-IR) Analysis
3.2.6. Extraction of Betulin from Birch Bark
3.2.7. General Procedure for Copper Catalyzed Click Reaction in Eutectic Mixture
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Atkins, P. Physical Chemistry; Oxford University Press: Oxford, UK, 1980. [Google Scholar]
- Issac, M.F. On the spontaneous crystallisation and the melting and freezing point curves of mixtures of two substances which form mixed crystals and posses a minimum or eutectic freezing point—Mixtures of azobenzene and benzylaniline. Proc. R. Soc. Lond. Ser. A 1910, 84, 344–369. [Google Scholar] [CrossRef]
- Francisco, M.; van den Bruinhorst, A.; Kroon, M.C. Low-Transition-Temperature Mixtures (LTTMs): A New Generation of Designer Solvents. Angew. Chem. 2013, 52, 3074–3085. [Google Scholar] [CrossRef] [PubMed]
- Plass, K.E.; Kim, K.; Matzger, A.J. Two-Dimensional Crystallization: Self-Assembly, Pseudopolymorphism, and Symmetry-Independent Molecules. J. Am. Chem. Soc. 2004, 126, 9042–9053. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Ji, X.Y.; Lu, X.H. Choline-based deep eutectic solvents for CO2 separation: Review and thermodynamic analysis. Renew. Sustain. Energy Rev. 2018, 97, 436–455. [Google Scholar] [CrossRef]
- Longo, L.S.; Craveiro, M.V. Deep Eutectic Solvents as Unconventional Media for Multicomponent Reactions. J. Braz. Chem. Soc. 2018, 29, 1999–2025. [Google Scholar] [CrossRef]
- Cunha, S.C.; Fernandes, J.O. Extraction techniques with deep eutectic solvents. Trends Anal. Chem. 2018, 105, 225–239. [Google Scholar] [CrossRef]
- Brett, C.M.A. Deep eutectic solvents and applications in electrochemical sensing. Curr. Opin. Electrochem. 2018, 10, 143–148. [Google Scholar] [CrossRef]
- Wazeer, I.; Hayyan, M.; Hadj-Kali, M.K. Deep eutectic solvents: Designer fluids for chemical processes. J. Chem. Technol. Biotechnol. 2018, 93, 945–958. [Google Scholar] [CrossRef]
- Mota-Morales, J.D.; Sanchez-Leija, R.J.; Carranza, A.; Pojman, J.A.; del Monte, F.; Luna-Barcenas, G. Free-radical polymerizations of and in deep eutectic solvents: Green synthesis of functional materials. Prog. Polym. Sci. 2018, 78, 139–153. [Google Scholar] [CrossRef]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.N.; Pauli, G.F. Natural Deep Eutectic Solvents: Properties, Applications, and Perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef]
- Li, X.; Choi, J.; Ahn, W.S.; Row, K.H. Preparation and Application of Porous Materials based on Deep Eutectic Solvents. Crit. Rev. Anal. Chem. 2018, 48, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.C.; Yao, C.F.; Wu, W.Z. Deep Eutectic Solvents: Green Solvents for Separation Applications. Acta Phys. Chim. Sin. 2018, 34, 873–885. [Google Scholar]
- Fernandez, M.D.; Boiteux, J.; Espino, M.; Gomez, F.J.V.; Silva, M.F. Natural deep eutectic solvents-mediated extractions: The way forward for sustainable analytical developments. Anal. Chim. Acta 2018, 1038, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jérôme, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef] [PubMed]
- Cooper, E.R.; Andrews, C.D.; Wheatley, P.S.; Webb, P.B.; Wormald, P.; Morris, R.E. Ionic liquids and eutectic mixtures as solvent and template in synthesis of zeolite analogues. Nature 2004, 430, 1012–1016. [Google Scholar] [CrossRef] [PubMed]
- Leuner, C.; Dressman, J. Improving drug solubility for oral delivery using solid dispersions. Eur. J. Pharm. Biopharm. 2000, 50, 47–60. [Google Scholar] [CrossRef]
- Bowmaker, G.A. Solvent-assisted mechanochemistry. Chem. Commun. 2013, 49, 334–348. [Google Scholar] [CrossRef] [PubMed]
- Usanovich, M. On the “deviations” from Raoult’s law due to chemical interaction between the components. Dok. Acad. Nauk. SSSR 1958, 120, 1304–1306. [Google Scholar]
- Hollis, J.M.; Lovas, F.J.; Anthony, J.R.; Jewell, P.R.; Ilyushin, V.V.; Kleiner, I. Detection of Acetamide (CH3CONH2): The Largest Interstellar Molecule with a Peptide Bond. Astrophys. J. 2006, 643, L25–L28. [Google Scholar] [CrossRef]
- Das, A.; Das, S.; Biswas, R. Density relaxation and particle motion characteristics in a non-ionic deep eutectic solvent (acetamide + urea): Time-resolved fluorescence measurements and all-atom molecular dynamics simulations. J. Chem. Phys. 2015, 142, 034505. [Google Scholar] [CrossRef] [PubMed]
- Zhou, E.; Liu, H. A Novel Deep Eutectic Solvents Synthesized by Solid Organic Compounds and Its Application on Dissolution for Cellulose. Asian, J. Chem. 2014, 26, 3626–3630. [Google Scholar] [CrossRef]
- Smallwood, I.M. n-Octanol. In Handbook of Organic Solvent Properties; Smallwood, I.M., Ed.; Butterworth-Heinemann: Oxford, UK, 1996; pp. 101–103. [Google Scholar]
- Sun, T.; Teja, A.S. Density, Viscosity, and Thermal Conductivity of Aqueous Ethylene, Diethylene, and Triethylene Glycol Mixtures between 290 K and 450 K. J. Chem. Eng. Data 2003, 48, 198–202. [Google Scholar] [CrossRef]
- Sodium Chloride. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Sodium-chloride (accessed on 29 May 2019).
- Sucrose. Available online: https://eu.mpbio.com/0290471305-sucrose-cf (accessed on 29 May 2019).
- Zein from Maize. Available online: https://www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/Sigma/Product_Information_Sheet/z3625pis.pdf (accessed on 29 May 2019).
- D-Biotin. Available online: https://eu.mpbio.com/02101023-1-d-biotin-cf (accessed on 29 May 2019).
- Theophylline. Available online: https://www.sigmaaldrich.com/catalog/product/sigma/t1633?lang=en®ion=SE (accessed on 29 May 2019).
- 9,10-Phenanthrenequinone. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Phenanthrenequinone (accessed on 29 May 2019).
- Green, B.; Bentley, M.D.; Chung, B.Y.; Lynch, N.G.; Jensen, B.L. Isolation of betulin and rearrangement to allobetulin—A biomimetic natural product synthesis. J. Chem. Educ. 2007, 84, 1985–1987. [Google Scholar] [CrossRef]
- Case, D.A.; Darden, T.A.; Cheatham, T.E., III; Simmerling, C.L.; Wang, J.; Duke, R.E.; Luo, R.; Crowley, M.; Walker, R.C.; Zhang, W.; et al. Amber 10; University of California: San Francisco, CA, USA, 2008. [Google Scholar]
- Martínez, L.; Andrade, R.; Birgin, E.G.; Martínez, J.M. PACKMOL: A package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef] [PubMed]
- Mario, M.J.; Leandro, M. Packing optimization for automated generation of complex system’s initial configurations for molecular dynamics and docking. J. Comput. Chem. 2003, 24, 819–825. [Google Scholar]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Wu, C.; Chowdhury, S.; Lee, M.C.; Xiong, G.; Zhang, W.; Yang, R.; Cieplak, P.; Luo, R.; Lee, T.; et al. A point-charge force field for molecular mechanics simulations of proteins based on condensed-phase quantum mechanical calculations. J. Comput. Chem. 2003, 24, 1999–2012. [Google Scholar] [CrossRef]
- Karlsson, J.G.; Andersson, L.I.; Nicholls, I.A. Probing the molecular basis for ligand-selective recognition in molecularly imprinted polymers selective for the local anaesthetic bupivacaine. Anal. Chem. Acta 2001, 435, 57–64. [Google Scholar] [CrossRef]
- Shao, C.; Wang, X.; Zhang, Q.; Luo, S.; Zhao, J.; Hu, Y. Acid–Base Jointly Promoted Copper(I)-Catalyzed Azide–Alkyne Cycloaddition. J. Org. Chem. 2011, 76, 6832–6836. [Google Scholar] [CrossRef]
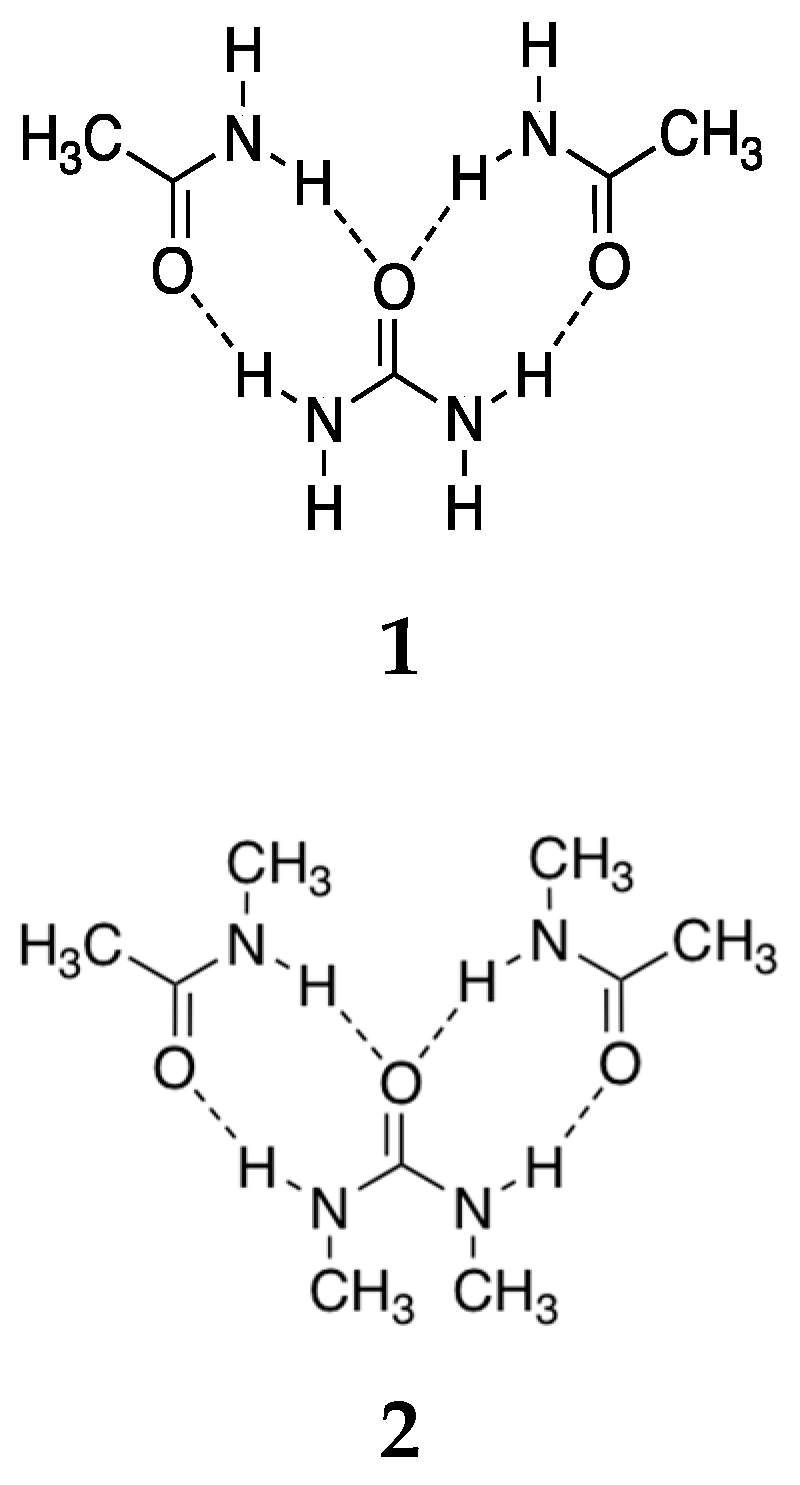

| A § | U § | |
|---|---|---|
| A | 40.3 | 65.8 |
| U | – | 50.9 |
| System a | R 1 | R 2 | R 3 | R 4 | R 5 | R 6 | R 7 | R 8 | R 9 | R 10 | Eutectic Point °C | Composition A:B ± 3 | Conductivity μS/cm b | Viscosity cP c |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A–U | H | H | - | - | - | - | H | H | H | H | 56 ± 2 | 65:35 | 126 | 10.8 |
| A–NMU | H | H | - | - | - | - | CH3 | H | H | H | 42 ± 3 | 50:50 | 30 | 9.2 |
| A–NNDMU | H | H | - | - | - | - | CH3 | CH3 | H | H | 68 ± 3 | 80:20 | n.d. | 8.5 |
| A–NN’DMU | H | H | - | - | - | - | CH3 | H | CH3 | H | 43 ± 3 | 50:50 | 64 | 10.1 |
| NMA–NMU | CH3 | H | - | - | - | - | H | H | CH3 | H | 14 ± 2 | 80:20 | 12 | 7.1 |
| NMA–NN’DMU | CH3 | H | - | - | - | - | CH3 | H | CH3 | H | 12 ± 4 | 70:30 | 25 | 7.2 |
| U–NMU | - | - | H | H | H | H | CH3 | H | H | H | 61 ± 2 | 70:30 | 27 | 14.8 |
| U–NN’DMU | - | - | H | H | H | H | CH3 | H | CH3 | H | 69 ± 3 | 70:30 | n.d. | 12.7 |
| NMU–NNDMU | - | - | CH3 | H | H | H | CH3 | CH3 | H | H | 76 ± 4 | 80:20 | n.d. | 8.1 |
| NMU–NN’DMU | - | - | CH3 | H | H | H | CH3 | H | CH3 | H | 49 ± 3 | 50:50 | 47 | 15.1 |
| Solutes | Solvents (mg/mL) | |||
|---|---|---|---|---|
| Water (pH 5.9) | NMA–NMU | Methanol | Chloroform | |
| NaCl [26] | 360 | 12 | 15 | Insoluble |
| Sucrose [27] | 2000 | 9 | 10 | Insoluble |
| Zein [28] | Insoluble | 80 | 90 | Insoluble |
| Biotin [29] | 0.5 | 2 | 1 | Insoluble |
| Theophylline [30] | 8.3 | 5 | 12.5 | 9.1 |
| o-phenanthroquinone [31] | 0.4 | 50 | 2 | 2.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suriyanarayanan, S.; Olsson, G.D.; Kathiravan, S.; Ndizeye, N.; Nicholls, I.A. Non-Ionic Deep Eutectic Liquids: Acetamide–Urea Derived Room Temperature Solvents. Int. J. Mol. Sci. 2019, 20, 2857. https://doi.org/10.3390/ijms20122857
Suriyanarayanan S, Olsson GD, Kathiravan S, Ndizeye N, Nicholls IA. Non-Ionic Deep Eutectic Liquids: Acetamide–Urea Derived Room Temperature Solvents. International Journal of Molecular Sciences. 2019; 20(12):2857. https://doi.org/10.3390/ijms20122857
Chicago/Turabian StyleSuriyanarayanan, Subramanian, Gustaf D. Olsson, Subban Kathiravan, Natacha Ndizeye, and Ian A. Nicholls. 2019. "Non-Ionic Deep Eutectic Liquids: Acetamide–Urea Derived Room Temperature Solvents" International Journal of Molecular Sciences 20, no. 12: 2857. https://doi.org/10.3390/ijms20122857
APA StyleSuriyanarayanan, S., Olsson, G. D., Kathiravan, S., Ndizeye, N., & Nicholls, I. A. (2019). Non-Ionic Deep Eutectic Liquids: Acetamide–Urea Derived Room Temperature Solvents. International Journal of Molecular Sciences, 20(12), 2857. https://doi.org/10.3390/ijms20122857






