A Profile of the In Vitro Anti-Tumor Activity and In Silico ADME Predictions of Novel Benzothiazole Amide-Functionalized Imidazolium Ionic Liquids
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. In Silico Predictions
2.2.1. Physicochemical Properties
2.2.2. Pharmacokinetic/ADME and Drug Likeness Properties
2.3. Biological Study
2.3.1. Anticancer Activity
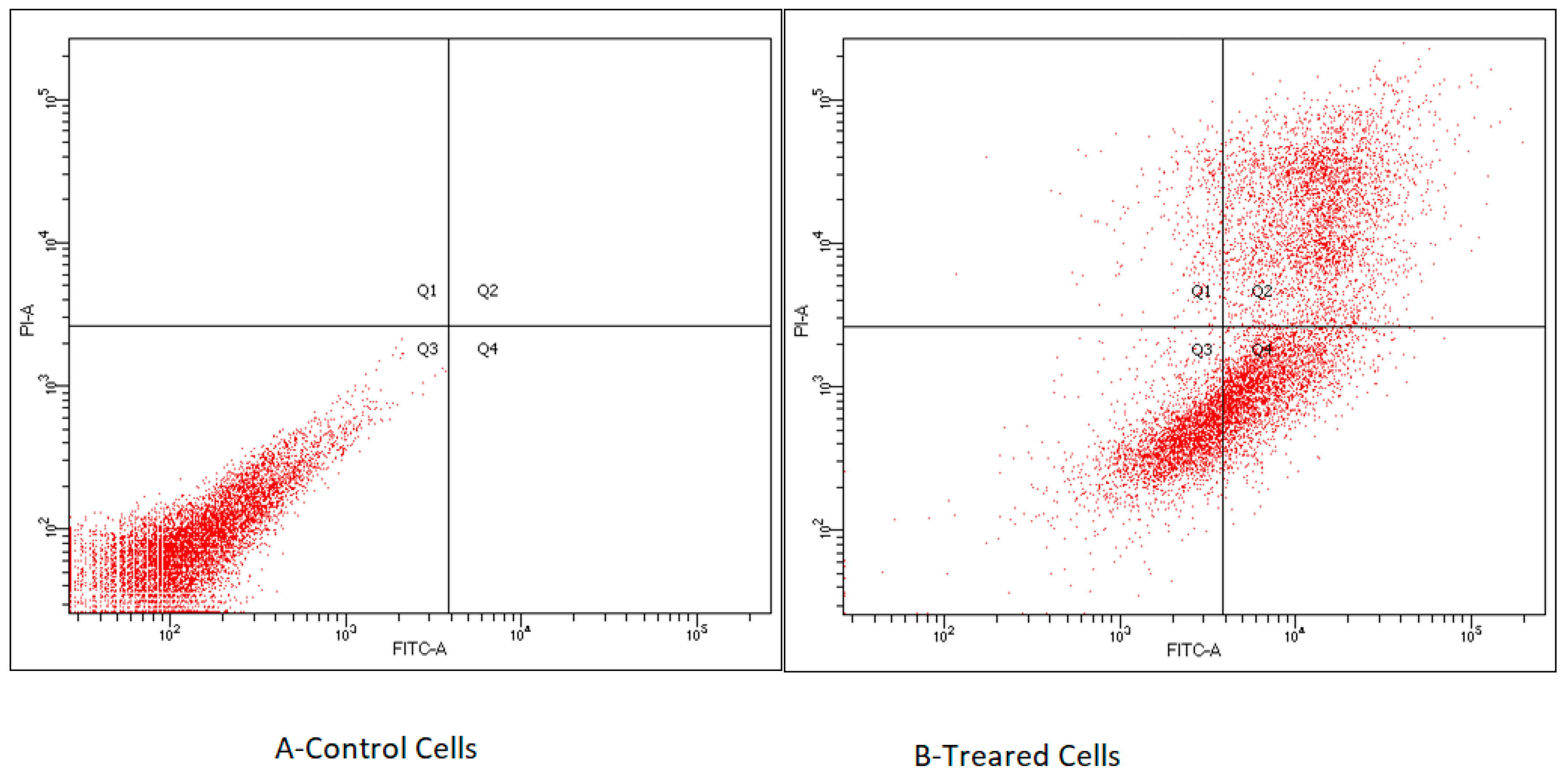
2.3.2. The Effects of ILs on cell Apoptosis
3. Experimental Section
3.1. Chemical Characterization and Synthesis
3.1.1. General Procedure for the Quaternization of Imidazoles
3.1.2. 3-(2-(Benzo[d]thiazol-2-ylamino)-2-oxoethyl)-1-methyl-1H-imidazol-3-ium bromide (6).
3.1.3. 3-(2-(Benzo[d]thiazol-2-ylamino)-2-oxoethyl)-1,2-dimethyl-1H-imidazol-3-ium bromide (7).
3.1.4. 1-Methyl-3-(2-((6-methylbenzo[d]thiazol-2-yl)amino)-2-oxoethyl)-1H-imidazol-3-ium bromide (8).
3.1.5. 1,2-Dimethyl-3-(2-((6-methylbenzo[d]thiazol-2-yl)amino)-2-oxoethyl)-1H-imidazol-3-ium bromide (9).
3.1.6. 1-Methyl-(2-((6-(methylsulfonyl)benzo[d]thiazol-2-yl)amino)-2-oxoethyl)-1H-imidazol-3-ium bromide (10).
3.1.7. 1,2-Dimethyl-3-(2-((6-(methylsulfonyl)benzo[d]thiazol-2-yl)amino)-2-oxoethyl)-1H-imidazol-3-ium bromide (11).
3.2. General Procedure for the Metathesis Reaction
3.2.1. 3-(2-(Benzo[d]thiazol-2-ylamino)-2-oxoethyl)-1-methyl-1H-imidazol-3-ium hexafluorophosphate (12).
3.2.2. 3-(2-(Benzo[d]thiazol-2-ylamino)-2-oxoethyl)-1-methyl-1H-imidazol-3-ium tetrafluoroborate (13).
3.2.3. 3-(2-Benzo[d]thiazol-2-ylamino)-2-oxoethyl)-1-methyl-1H-imidazol-3-ium 2,2,2-trifluoroacetate (14).
3.2.4. 3-(2-(Benzo[d]thiazol-2-ylamino)-2-oxoethyl)-1,2-dimethyl-1H-imidazol-3-ium hexafluorophosphate (15).
3.2.5. 3-(2-(Benzo[d]thiazol-2-ylamino)-2-oxoethyl)-1,2-dimethyl-1H-imidazol-3-ium tetrafluoroborate (16).
3.2.6. 3-(2-Benzo[d]thiazol-2-ylamino)-2-oxoethyl)-1,2-dimethyl-1H-imidazol-3-ium 2,2,2-trifluoroacetate (17).
3.2.7. 1-Methyl-3-(2-((6-methylbenzo[d]thiazol-2-yl)amino)-2-oxoethyl)-1H-imidazol-3-ium hexafluorophosphate (18).
3.2.8. 1-Methyl-3-(2-((6-methylbenzo[d]thiazol-2-yl)amino)-2-oxoethyl)-1H-imidazol-3-ium tetrafluoroborate (19).
3.2.9. 1-Methyl-3-(2-((6-methylbenzo[d]thiazol-2-yl)amino)-2-oxoethyl)-1H-imidazol-3-ium 2,2,2-tri-fluoroacetate (20).
3.2.10. 1,2-Dimethyl-3-(2-((6-methylbenzo[d]thiazol-2-yl)amino)-2-oxoethyl)-1H-imidazol-3-ium hexafluorophosphate (21).
3.2.11. 1,2-Dimethyl-3-(2-((6-methylbenzo[d]thiazol-2-yl)amino)-2-oxoethyl)-1H-imidazol-3-ium tetrafluoroborate (22).
3.2.12. 1,2-Dimethyl-3-(2-((6-methylbenzo[d]thiazol-2-yl)amino)-2-oxoethyl)-1H-imidazol-3-ium 2,2,2-trifluoroacetate (23).
3.2.13. 1-Methyl-3-(2-((6-(methylsulfonyl)benzo[d]thiazol-2-yl)amino)-2-oxoethyl)-1H-imidazol-3-ium hexafluorophosphate (24).
3.2.14. 1-Methyl-3-(2-((6-(methylsulfonyl)benzo[d]thiazol-2-yl)amino)-2-oxoethyl)-1H-imidazol-3-ium tetrafluoroborate (25).
3.2.15. 1-Methyl-3-(2-((6-(methylsulfonyl)benzo[d]thiazol-2-yl)amino)-2-oxoethyl)-1H-imidazol-3-ium 2,2,2-trifluoroacetate (26).
3.2.16. 1,2-Dimethyl-3-(2-((6-(methylsulfonyl)benzo[d]thiazol-2-yl)amino)-2-oxoethyl)-1H-imidazol-3-ium hexafluorophosphate (27).
3.2.17. 1,2-Dimethyl-3-(2-((6-(methylsulfonyl)benzo[d]thiazol-2-yl)amino)-2-oxoethyl)-1H-imidazol-3- ium tetrafluoroborate (28).
3.2.18. -1,2-Dimethyl-3-(2-((6-(methylsulfonyl)benzo[d]thiazol-2-yl)amino)-2-oxoethyl)-1H-imidazol-3-ium 2,2,2-trifluoroacetate (29).
3.3. Method of Computation: In Silico Study
3.4. Biological Study
3.4.1. Anticancer Activity
Cell Line and Culture Conditions
In Vitro Cytotoxicity
Flow Cytometry Analysis:
4. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
Abbreviations
| ILs | Ionic Liquids |
| ADME | Absorption, Distribution, Metabolism and Excretion |
| DCM | Dichloromethane |
| TPSA | Topological Polar Surface Area |
| ABS | Absorption |
| HBA | Number of hydrogen bond acceptor |
| HBD | Number of hydrogen bond donor |
| GI | Gastro intestinal |
| P-gp | P-glycoprotein |
| BBB | Blood-brain barrier |
| HIA | Human gastrointestinal absorption |
| RO5 | Rule-of-five |
| ROS | Reactive oxygen species |
| HRMS | High resolution mass spectroscopy |
| TMS | Tetramethylsilane |
| DMSO | Dimethylsufoxide |
| S | Singlet |
| D | Doublet |
| T | Triplet |
| M | Multiplet |
References
- Thurston, D.E. Chemistry and Pharmacology of Anticancer Drugs., 1st ed.; CRS Press; Taylor and Francis group: Abingdon, UK, 2006. [Google Scholar] [CrossRef]
- Dias, A.R.; Costa-Rodrigues, J.; Fernandes, M.H.; Ferraz, R.; Prudencio, C. The Anticancer Potential of Ionic Liquids. Chem. Med. Chem. 2017, 12, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Branco, L.C.; Carrera, G.V.S.M.; Aires-de-Sousa, J.; Martin, I.L.; Frade, R.; Afonso, C.A.M. Physico-Chemical Properties of Task-Specific Ionic Liquids. In Ionic Liquids: Theory, Properties, New Approaches; Kokorin, A., Ed.; In Tech Publishing: Rijeka, Croatia, 2011; pp. 61–94. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Attri, P.; Kaushik, N.; Choi, E.H. Synthesis and antiproliferative activity of ammonium and imidazolium ionic liquids against T98G brain cancer cells. Molecules 2012, 17, 13727–13739. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Malhotra, S.V. Study on the potential anti-cancer activity of phosphonium and ammonium-based ionic liquids. Bioorg. Med. Chem. Lett. 2009, 19, 4643–4646. [Google Scholar] [CrossRef]
- Ferraz, R.; Branco, L.C.; PrudÞncio, C.; Noronha, J.P.; Petrovski, Z. Ionic liquids as active pharmaceutical ingredients. Chem. Med. Chem. 2011, 6, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, R.; Costa-Rodrigues, J.; Fernandes, M.H.; Santos, M.M.; Marrucho, I.M.; Rebelo, L.P.; Prudêncio, C.; Noronha, J.P.; Petrovski, Ž.; Branco, L.C. Antitumor Activity of Ionic Liquids Based on Ampicillin. Chem. Med. Chem. 2015, 10, 1480–1483. [Google Scholar] [CrossRef] [PubMed]
- Marrucho, I.M.; Branco, L.C.; Rebelo, L.P.N. Ionic Liquids in Pharmaceutical Applications. Annu. Rev. Chem. Biomol. Eng. 2014, 5, 527–546. [Google Scholar] [CrossRef] [PubMed]
- Dobler, D.; Schmidts, T.; Klingenhçfer, I.; Runke, F. Ionic liquids as ingredients in topical drug delivery systems. Int. J. Pharm. 2013, 441, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Shamshina, J.L.; Kelley, S.P.; Gurau, G.; Rogers, R.D. Chemistry: Develop ionic liquid drugs. Nature 2015, 528, 188–189. [Google Scholar] [CrossRef]
- Malhotra, S.V.; Kumar, V. A profile of the in vitro anti-tumor activity of imidazolium-based ionic liquids. Bioorg. Med. Chem. Lett. 2010, 20, 581–585. [Google Scholar] [CrossRef]
- Wang, X.; Ohlin, C.A.; Lu, Q.; Fei, Z.; Hu, J.; Dyson, P.J. Cytotoxicity of ionic liquids and precursor compounds towards human cell line HeLa. Green Chem. 2007, 9, 1191–1197. [Google Scholar] [CrossRef]
- Jing, C.; Li, X.; Zhang, J.; Wang, J. Responses of the Antioxidant System in QGY-7701 Cells to the Cytotoxicity and Apoptosis Induced by 1-Octyl-3-methylimidazolium Chloride. J. Biochem. Mol. Toxicol. 2013, 27, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ma, J.; Wang, J. Cytotoxicity, oxidative stress, and apoptosis in HepG2 cells induced by ionic liquid 1-methyl-3-octylimidazolium bromide. Ecotoxicol. Environ. Saf. 2015, 120, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Iwai, N.; Nakayama, K.; Kitazume, T. Antibacterial activities of imidazolium, pyrrolidinium and piperidinium salts. Bioorg. Med. Chem. Lett. 2011, 21, 1728–1730. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-L.; Kao, H.-F.; Wang, J.-Y.; Wei, G.-T. Cytotoxicity of Imidazole Ionic Liquids in Human Lung Carcinoma A549 Cell Line. J. Chin. Chem. Soc. 2014, 61, 763–769. [Google Scholar] [CrossRef]
- Malhotra, S.V.; Kumar, V.; Velez, C.; Zayas, B. Imidazolium-Derived Ionic Salts Induce Inhibition of Cancerous Cell Growth through Apoptosis. MedChemComm 2014, 5, 1404–1409. [Google Scholar] [CrossRef]
- Miskiewicz, A.; Ceranowicz, P.; Szymczak, M.; Bartus, K.; Kowalczyk, P. The Use of Liquids Ionic Fluids as Pharmaceutically Active Substances Helpful in Combating Nosocomial Infections Induced by Klebsiella Pneumoniae New Delhi Strain, Acinetobacter Baumannii and Enterococcus Species. Int. J. Mol. Sci. 2018, 19, 2779. [Google Scholar] [CrossRef] [PubMed]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological activity of ionic liquids and their application in pharmaceutics and medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef]
- Rezki, N.; Al-Sodies, S.A.; Aouad, M.R.; Bardaweel, S.K.; Messali, M.; El Ashry, S.H. An Eco-Friendly Ultrasound-Assisted Synthesis of Novel Fluorinated Pyridinium Salts-Based and Antimicrobial and Antitumor Screening. Inter. J. Mol. Sci. 2016, 17, 766. [Google Scholar] [CrossRef]
- Rezki, N.; Al-Sodies, S.A.; Shreaz, S.; Shiekh, R.A.; Messali, M.; Raja, V.; Aouad, M.R. Green ultrasound versus conventional synthesis of specific task pyridinium ionic liquid hydrazones tethering fluorinated counter anions: Novel inhibitors of fungal ergosterol biosynthesis. Molecules 2017, 22, 1532. [Google Scholar] [CrossRef]
- Aljuhani, A.; El-Sayed, W.S.; Sahu, P.K.; Rezki, N.; Aouad, M.R.; Salghi, R.; Messali, M. Microwave-assisted synthesis of novel imidazolium, pyridinium and pyridazinium-based ionic liquids and prediction of physico-chemical properties for their toxicity and antibacterial activity. J. Mol. Liq. 2018, 249, 747–753. [Google Scholar] [CrossRef]
- Rezki, N.; Messali, M.; Al-Sodies, S.A.; Naqvi, A.; Bardaweel, S.K.; Al-blewi, F.F.; Aouad, M.R.; El Ashry, S.H. Design, Synthesis, in-silico molecular docking and evaluation of di-cationic pyridinium ionic liquids as potential anticancer scaffolds. J. Mol. Liq. 2018, 265, 428–441. [Google Scholar] [CrossRef]
- Albalawi, A.H.; El-Sayed, W.S.; Aljuhani, A.; Almutairi, S.M.; Rezki, N.; Aouad, M.R.; Messali, M. Microwave-assisted synthesis of some potential bioactive imidazolium-based room temperature ionic liquids. Molecules 2018, 23, 1727. [Google Scholar] [CrossRef] [PubMed]
- Rezki, N.; Al-Sodies, S.A.; Messali, M.; Bardaweel, S.K.; Sahu, P.K.; Al-blewi, F.F.; Sahu, P.K.; Aouad, M.R. Identification of new pyridinium ionic liquids tagged Schiff base: Design, synthesis, in silico ADMET prediction and biological evaluation. J. Mol. Liq. 2018, 264, 367–374. [Google Scholar] [CrossRef]
- Messali, M. Conventional versus Ultrasound and Microwave assisted synthesis, characterization and ecotoxicity of some new eco-friendly functionalized picolinium-based ionic liquids. Acta Pharmaceutica. 2015, 65, 253–270. [Google Scholar] [CrossRef] [PubMed]
- Messali, M. Eco-friendly synthesis of a new class of pyridinium-based ionic liquids with attractive antimicrobial activity. Molecules 2015, 20, 14936–14949. [Google Scholar] [CrossRef] [PubMed]
- Al-blewi, F.F.; Rezki, N.; Al-Sodies, S.A.; Bardaweel, S.K.; Sabbah, D.A.; Messali, M.; Aouad, M.R. Novel cationic amphiphilic fluorinated pyridinium hydrazones: Conventional versus green ultrasound assisted synthesis, characterization, molecular docking, and anticancer evaluation. Chem. Cent. J. 2018, 12, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Rezki, N.; Aouad, M.R. Green ultrasound-assisted three-component click synthesis of novel 1H-1,2,3-triazole carrying benzothiazoles and fluorinated-1,2,4-triazole conjugates and their antimicrobial evaluation. Acta pharmaceutica. 2017, 67, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Rezki, N. A Green ultrasound synthesis, characterization and antibacterial evaluation of 1,4-disubstituted 1,2,3-triazoles tethering bioactive benzothiazole nucleus. Molecules 2016, 21, 505. [Google Scholar] [CrossRef] [PubMed]
- Kramer, S.D.; Wunderli-Allenspach, H. Physicochemical properties in pharmacokinetic lead optimization. Farmaco 2001, 56, 145–148. [Google Scholar] [CrossRef]
- Neervannan, S. Preclinical formulations for discovery and toxicology: Physicochemical challenges. Expert Opin. Drug Metab. Toxicol. 2006, 2, 715–731. [Google Scholar] [CrossRef] [PubMed]
- Azam, F.; Madi, A.M.; Ali, H.I. Molecular Docking and Prediction of Pharmacokinetic Properties of Dual Mechanism Drugs that Block MAO-B and Adenosine A2A Receptors for the Treatment of Parkinson’s Disease. J. Young Pharm. 2012, 4, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Doogue, M.P.; Polasek, T.M. The ABCD of clinical pharmacokinetics. Ther. Adv. Drug Saf. 2013, 4, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Kassel, D.B. Applications of high-throughput ADME in drug discovery. Curr. Opin. Chem. Biol. 2004, 8, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Del. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. Prediction of hydrophobic (lipophilic) properties of small organic molecules using fragmental methods: An analysis of ALOGP and CLOGP methods. J. Phys. Chem. A 1998, 102, 3762–3772. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Egan, W.J.; Lauri, G. Prediction of intestinal permeability. Adv. Drug Del. Rev. 2002, 54, 273–289. [Google Scholar] [CrossRef]
- Muegge, I.; Heald, S.L.; Brittelli, D. Simple selection criteria for drug-like chemical matter. J. Med. Chem. 2001, 44, 1841–1846. [Google Scholar] [CrossRef] [PubMed]
- The SIB Swiss Institute of Bioinformatics’ resources: Focus on curated databases. Nucleic Acids Res. 2015, 44(D1), D27–D37. [CrossRef]
- ISO–International Organization for Standardization. ISO 10993-5–Biological Evaluation of Medical Devices. Part 5: Testes for in vitro cytotoxicity, 3ª Ed, ISO, 2009; pp. 1–34, website, access date: day month year.
- Bardaweel, S.K.; Alsalamat, H.A.; Aleidi, S.M.; Bashatwah, R.M. Glucose deprivation enhances the antiproliferative effects of oral hypoglycemic biguanides in different molecular subtypes of breast cancer: An in vitro study. Acta Pharmaceutica. 2018, 68, 517–524. [Google Scholar] [CrossRef]









| Comp.No. | Fraction Csp3 a | No. of Rotatable Bonds | HBA b | HBD c | iLogP d | Molar Refractivity | Log S e | TPSA f | In-Silico % Absorption |
|---|---|---|---|---|---|---|---|---|---|
| 6 | 0.15 | 4 | 2 | 1 | −5.18 | 85.22 | S | 79.04 | 81.75 |
| 7 | 0.21 | 4 | 2 | 1 | −2.34 | 90.19 | S | 79.04 | 81.75 |
| 8 | 0.21 | 4 | 2 | 1 | −4.45 | 90.19 | S | 79.04 | 81.75 |
| 9 | 0.27 | 4 | 2 | 1 | −2.88 | 95.16 | MS | 79.04 | 81.75 |
| 10 | 0.21 | 5 | 4 | 1 | −5.71 | 98.32 | S | 121.56 | 67.06 |
| 11 | 0.27 | 5 | 4 | 1 | −4.77 | 103.28 | MS | 121.56 | 67.06 |
| 12 | 0.15 | 4 | 8 | 1 | 0 | 89.86 | S | 92.63 | 77.04 |
| 13 | 0.15 | 4 | 6 | 1 | 0 | 86.48 | S | 79.04 | 81.75 |
| 14 | 0.2 | 5 | 7 | 1 | −4.3 | 88.05 | S | 119.17 | 67.89 |
| 15 | 0.21 | 4 | 8 | 1 | 0 | 94.83 | S | 92.63 | 77.04 |
| 16 | 0.21 | 4 | 6 | 1 | 0 | 91.45 | S | 79.04 | 81.75 |
| 17 | 0.25 | 5 | 7 | 1 | −3.36 | 93.01 | S | 119.17 | 67.89 |
| 18 | 0.21 | 4 | 8 | 1 | 0 | 94.83 | S | 92.63 | 77.04 |
| 19 | 0.21 | 4 | 6 | 1 | 0 | 91.45 | S | 79.04 | 81.75 |
| 20 | 0.25 | 5 | 7 | 1 | −3.57 | 93.01 | S | 119.17 | 67.89 |
| 21 | 0.27 | 4 | 8 | 1 | 0 | 99.8 | MS | 92.63 | 77.04 |
| 22 | 0.27 | 4 | 6 | 1 | 0 | 96.41 | MS | 79.04 | 81.75 |
| 23 | 0.29 | 5 | 7 | 1 | −4.17 | 97.98 | MS | 119.17 | 67.89 |
| 24 | 0.21 | 5 | 10 | 1 | 0 | 102.96 | S | 135.15 | 62.37 |
| 25 | 0.21 | 5 | 8 | 1 | 0 | 99.58 | S | 121.56 | 67.06 |
| 26 | 0.25 | 6 | 9 | 1 | −5.2 | 101.14 | S | 161.69 | 53.22 |
| 27 | 0.27 | 5 | 10 | 1 | 0 | 107.92 | MS | 135.15 | 62.37 |
| 28 | 0.27 | 5 | 8 | 1 | 0 | 104.54 | MS | 121.56 | 67.06 |
| 29 | 0.29 | 6 | 9 | 1 | −4.83 | 106.11 | MS | 161.69 | 53.22 |
| Comp. No | Pharmacokinetic/ADME Properties | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| GI Abs a | BBB Permeant b | P-Gpsubstrate c | CYP1A2 Inhibitor d | CYP2C19 Inhibitor e | CYP2C9 Inhibitor f | CYP2D6 Inhibitor g | CYP3A4 Inhibitor h | Log Kp i | |
| 6 | High | No | Yes | No | No | No | No | No | −6.39 |
| 7 | High | No | Yes | No | No | No | No | No | −6.19 |
| 8 | High | No | Yes | No | No | No | No | No | −6.22 |
| 9 | High | No | Yes | No | No | No | No | No | −6.02 |
| 10 | High | No | Yes | No | No | No | No | No | −7.4 |
| 11 | High | No | Yes | No | No | No | No | No | −7.21 |
| 12 | Low | No | No | No | Yes | No | No | Yes | −5.19 |
| 13 | Low | No | No | No | Yes | No | No | Yes | −4.99 |
| 14 | High | No | No | No | No | No | No | No | −5.54 |
| 15 | High | No | No | No | No | No | No | No | −5.34 |
| 16 | High | No | Yes | No | No | No | No | No | −6.64 |
| 17 | High | No | Yes | No | No | No | No | No | −6.44 |
| 18 | Low | No | No | No | Yes | No | No | Yes | −5.02 |
| 19 | High | No | No | No | No | No | No | No | −5.37 |
| 20 | High | No | Yes | No | No | No | No | No | −6.46 |
| 21 | Low | No | Yes | No | Yes | No | No | Yes | −4.82 |
| 22 | High | No | No | No | No | No | No | No | −5.17 |
| 23 | High | No | Yes | No | No | No | No | No | −6.26 |
| 24 | Low | No | No | No | No | No | No | Yes | −6.21 |
| 25 | Low | No | No | No | No | No | No | No | −6.56 |
| 26 | Low | No | Yes | No | No | No | No | No | −7.66 |
| 27 | Low | No | No | No | No | No | No | Yes | −6.01 |
| 28 | Low | No | No | No | No | No | No | No | −6.36 |
| 29 | Low | No | Yes | No | No | No | No | No | −7.46 |
| Comp. No. | Lipinski Violations | Ghose Violations | Veber Violations | Egan Violations | Muegge Violations | Bioavailability Score |
|---|---|---|---|---|---|---|
| 6 | 0 | 1 | 0 | 0 | 0 | 0.55 |
| 7 | 0 | 1 | 0 | 0 | 0 | 0.55 |
| 8 | 0 | 1 | 0 | 0 | 0 | 0.55 |
| 9 | 0 | 1 | 0 | 0 | 0 | 0.55 |
| 10 | 0 | 1 | 0 | 0 | 0 | 0.55 |
| 11 | 0 | 1 | 0 | 0 | 0 | 0.55 |
| 12 | 0 | 1 | 0 | 1 | 1 | 0.55 |
| 13 | 0 | 0 | 0 | 0 | 0 | 0.55 |
| 14 | 0 | 0 | 0 | 0 | 0 | 0.55 |
| 15 | 0 | 1 | 0 | 1 | 1 | 0.55 |
| 16 | 0 | 0 | 0 | 0 | 0 | 0.55 |
| 17 | 0 | 0 | 0 | 0 | 0 | 0.55 |
| 18 | 0 | 1 | 0 | 1 | 1 | 0.55 |
| 19 | 0 | 0 | 0 | 0 | 0 | 0.55 |
| 20 | 0 | 0 | 0 | 0 | 0 | 0.55 |
| 21 | 0 | 1 | 0 | 1 | 1 | 0.55 |
| 22 | 0 | 0 | 0 | 0 | 0 | 0.55 |
| 23 | 0 | 0 | 0 | 0 | 0 | 0.55 |
| 24 | 0 | 2 | 0 | 2 | 0 | 0.55 |
| 25 | 0 | 0 | 0 | 0 | 0 | 0.55 |
| 26 | 0 | 0 | 1 | 1 | 1 | 0.55 |
| 27 | 1 | 2 | 0 | 2 | 0 | 0.55 |
| 28 | 0 | 0 | 0 | 0 | 0 | 0.55 |
| 29 | 0 | 0 | 1 | 1 | 1 | 0.55 |
| Comp. N | HCT | Caco-2 | T47D | MCF-7 |
|---|---|---|---|---|
| 6 | >500 | >500 | >500 | >500 |
| 7 | >500 | >500 | >500 | >500 |
| 8 | 143 ± 5 | 122 ± 3 | 162 ± 6 | 153 ± 8 |
| 9 | 210 ± 8 | 189 ± 7 | 230 ± 11 | 194 ± 6 |
| 10 | 246 ± 12 | 229 ± 8 | 237 ± 12 | 241 ± 7 |
| 11 | 187 ± 8 | 178 ± 11 | 198 ± 7 | 193 ± 6 |
| 12 | >500 | >500 | >500 | >500 |
| 13 | >500 | >500 | >500 | >500 |
| 14 | >500 | >500 | >500 | >500 |
| 15 | >500 | >500 | >500 | >500 |
| 16 | >500 | >500 | >500 | >500 |
| 17 | >500 | >500 | >500 | >500 |
| 18 | 183 ± 7 | 179 ± 5 | 188 ± 9 | 177 ± 4 |
| 19 | 132 ± 9 | 112 ± 5 | 139 ± 7 | 125 ± 6 |
| 20 | 190 ± 11 | 181 ± 9 | 187 ± 9 | 188 ± 6 |
| 21 | 168 ± 7 | 156 ± 3 | 167 ± 8 | 143 ± 7 |
| 22 | 98 ± 9 | 88 ± 4 | 115 ± 5 | 89 ± 3 |
| 23 | 149 ± 6 | 137 ± 5 | 154 ± 7 | 155 ± 4 |
| 24 | 246 ± 9 | 260 ± 11 | 293 ± 5 | 287 ± 10 |
| 25 | 211 ± 5 | 199 ± 4 | 200 ± 8 | 205 ± 6 |
| 26 | 210 ± 9 | 219 ± 3 | 213 ± 7 | 218 ± 4 |
| 27 | 239 ± 11 | 248 ± 8 | 241 ± 9 | 251 ± 7 |
| 28 | 186 ± 7 | 179 ± 7 | 178 ± 4 | 168 ± 6 |
| 29 | 174 ± 10 | 169 ± 7 | 167 ± 8 | 171 ± 9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-blewi, F.; Rezki, N.; Naqvi, A.; Qutb Uddin, H.; Al-Sodies, S.; Messali, M.; Aouad, M.R.; Bardaweel, S. A Profile of the In Vitro Anti-Tumor Activity and In Silico ADME Predictions of Novel Benzothiazole Amide-Functionalized Imidazolium Ionic Liquids. Int. J. Mol. Sci. 2019, 20, 2865. https://doi.org/10.3390/ijms20122865
Al-blewi F, Rezki N, Naqvi A, Qutb Uddin H, Al-Sodies S, Messali M, Aouad MR, Bardaweel S. A Profile of the In Vitro Anti-Tumor Activity and In Silico ADME Predictions of Novel Benzothiazole Amide-Functionalized Imidazolium Ionic Liquids. International Journal of Molecular Sciences. 2019; 20(12):2865. https://doi.org/10.3390/ijms20122865
Chicago/Turabian StyleAl-blewi, Fawzia, Nadjet Rezki, Arshi Naqvi, Husna Qutb Uddin, Salsabeel Al-Sodies, Mouslim Messali, Mohamed Reda Aouad, and Sanaa Bardaweel. 2019. "A Profile of the In Vitro Anti-Tumor Activity and In Silico ADME Predictions of Novel Benzothiazole Amide-Functionalized Imidazolium Ionic Liquids" International Journal of Molecular Sciences 20, no. 12: 2865. https://doi.org/10.3390/ijms20122865
APA StyleAl-blewi, F., Rezki, N., Naqvi, A., Qutb Uddin, H., Al-Sodies, S., Messali, M., Aouad, M. R., & Bardaweel, S. (2019). A Profile of the In Vitro Anti-Tumor Activity and In Silico ADME Predictions of Novel Benzothiazole Amide-Functionalized Imidazolium Ionic Liquids. International Journal of Molecular Sciences, 20(12), 2865. https://doi.org/10.3390/ijms20122865






