Antimicrobial Gold Nanoclusters: Recent Developments and Future Perspectives
Abstract
1. Introduction
2. Small Molecule-Conjugated AuNCs
3. Macromolecule-Conjugated AuNCs
4. Challenges and Opportunities
Acknowledgements
Conflicts of Interest
References
- Holmes, A.H.; Moore, L.S.P.; Sundsfjord, A.; Steinbakk, M.; Regmi, S.; Karkey, A.; Guerin, P.J.; Piddock, L.J.V. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016, 387, 176–187. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Laxminarayan, R.; Matsoso, P.; Pant, S.; Brower, C.; Rottingen, J.A.; Klugman, K.; Davies, S. Access to effective antimicrobials: A worldwide challenge. Lancet 2016, 387, 168–175. [Google Scholar] [CrossRef]
- Duran, N.; Duran, M.; de Jesus, M.B.; Seabra, A.B.; Favaro, W.J.; Nakazato, G. Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity. Nanomed.-Nanotechnol. Biol. Med. 2016, 12, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, S. Understanding the contribution of environmental factors in the spread of antimicrobial resistance. Environ. Health Prev. Med. 2015, 20, 243–252. [Google Scholar] [CrossRef]
- Andersson, D.I.; Hughes, D.; Kubicek-Sutherland, J.Z. Mechanisms and consequences of bacterial resistance to antimicrobial peptides. Drug Resist. Updat. 2016, 26, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.G.; Zhao, Y.; Li, B.; Huang, C.L.; Zhang, S.Y.; Yu, S.; Chen, Y.S.; Zhang, T.; Gillings, M.R.; Su, J.Q. Continental-scale pollution of estuaries with antibiotic resistance genes. Nat. Microbiol. 2017, 2, 16270. [Google Scholar] [CrossRef]
- Kraker, M.E.A.; Stewardson, A.J.; Harbarth, S. Will 10 million people die a year due to antimicrobial resistance by 2050? PLOS Med. 2016, 13, e1002184. [Google Scholar] [CrossRef]
- O’Neill, J. Review on antimicrobial resistance antimicrobial resistance: Tackling a crisis for the health and wealth of nations. Review on Antimicrobial Resistance: London, UK, 2014. [Google Scholar]
- Rasool, K.; Helal, M.; Ali, A.; Ren, C.E.; Gogotsi, Y.; Mahmoud, K.A. Antibacterial activity of ti3c2tx mxene. ACS Nano 2016, 10, 3674–3684. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef]
- Czaplewski, L.; Bax, R.; Clokie, M.; Dawson, M.; Fairhead, H.; Fischetti, V.A.; Foster, S.; Gilmore, B.F.; Hancock, R.E.W.; Harper, D.; et al. Alternatives to antibiotics-a pipeline portfolio review. Lancet Infect. Dis. 2016, 16, 239–251. [Google Scholar] [CrossRef]
- Shen, K.; Chen, X.D.; Chen, J.Y.; Li, Y.W. Development of mof-derived carbon-based nanomaterials for efficient catalysis. ACS Catal. 2016, 6, 5887–5903. [Google Scholar] [CrossRef]
- Tan, C.L.; Cao, X.H.; Wu, X.J.; He, Q.Y.; Yang, J.; Zhang, X.; Chen, J.Z.; Zhao, W.; Han, S.K.; Nam, G.H.; et al. Recent advances in ultrathin two-dimensional nanomaterials. Chem. Rev. 2017, 117, 6225–6331. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.Y.; Coleman, J.N.; Zhang, H.; Shin, H.; Chhowalla, M.; Zheng, Z.J. Production of two-dimensional nanomaterials via liquid-based direct exfoliation. Small 2016, 12, 272–293. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.-R.; Liao, H.-J.; Chen, Y.-T.; Wei, C.-Y.; Chang, C.-C.; Chen, Y.-C.; Chang, Y.-H.; Lin, J.-C.; Lee, Y.-C.; Wen, C.-Y. Extended visible to near-infrared harvesting of earth-abundant fes2-tio2 heterostructures for highly active photocatalytic hydrogen evolution. Green Chem. 2018, 20, 1640–1647. [Google Scholar] [CrossRef]
- Choi, S.; Lee, H.; Ghaffari, R.; Hyeon, T.; Kim, D.H. Recent advances in flexible and stretchable bio-electronic devices integrated with nanomaterials. Adv. Mater. 2016, 28, 4203–4218. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Hersam, M.C. Assembly and electronic applications of colloidal nanomaterials. Adv. Mater. 2017, 29, 1603895. [Google Scholar] [CrossRef]
- Shin, T.H.; Cheon, J. Synergism of nanomaterials with physical stimuli for biology and medicine. Acc. Chem. Res. 2017, 50, 567–572. [Google Scholar] [CrossRef]
- Wen, A.M.; Steinmetz, N.F. Design of virus-based nanomaterials for medicine, biotechnology, and energy. Chem. Soc. Rev. 2016, 45, 4074–4126. [Google Scholar] [CrossRef]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371. [Google Scholar] [CrossRef]
- Zazo, H.; Colino, C.I.; Lanao, J.M. Current applications of nanoparticles in infectious diseases. J. Control. Release 2016, 224, 86–102. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.L.; Mahendra, S.; Lyon, D.Y.; Brunet, L.; Liga, M.V.; Li, D.; Alvarez, P.J.J. Antimicrobial nanomaterials for water disinfection and microbial control: Potential applications and implications. Water Res. 2008, 42, 4591–4602. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.; Teng, Z.; Wang, C.; Chong, H.; Wang, G. Recent progress in two-dimensional antimicrobial nanomaterials. Chem. Euro. J. 2019, 25, 929–944. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.W.; Sun, H.J.; Qu, X.G. Antibacterial applications of graphene-based nanomaterials: Recent achievements and challenges. Adv. Drug Deliv. Rev. 2016, 105, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Umar, A.; Kumar, G.; Nalwa, H.S. Antimicrobial properties of zno nanomaterials: A review. Ceram. Int. 2017, 43, 3940–3961. [Google Scholar] [CrossRef]
- Zou, X.F.; Zhang, L.; Wang, Z.J.; Luo, Y. Mechanisms of the antimicrobial activities of graphene materials. J. Am. Chem. Soc. 2016, 138, 2064–2077. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ali, S.F.; Dervishi, E.; Xu, Y.; Li, Z.; Casciano, D.; Biris, A.S. Cytotoxicity effects of graphene and single-wall carbon nanotubes in neural phaeochromocytoma-derived pc12 cells. ACS Nano 2010, 4, 3181–3186. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Bharate, P.; Lai, C.-H.; Ziem, B.; Böttcher, C.; Schulz, A.; Beckert, F.; Hatting, B.; Mülhaupt, R.; Seeberger, P.H. Multivalency at interfaces: Supramolecular carbohydrate-functionalized graphene derivatives for bacterial capture, release, and disinfection. Nano Lett. 2015, 15, 6051–6057. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Zhan, S.; Ma, S.; Zhou, Q. Fabrication of tio2–bi2wo6 binanosheet for enhanced solar photocatalytic disinfection of e. Coli: Insights on the mechanism. ACS Appl. Mater. Interfaces 2016, 8, 6841–6851. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cao, L.; Lu, F.; Meziani, M.J.; Li, H.; Qi, G.; Zhou, B.; Harruff, B.A.; Kermarrec, F.; Sun, Y.-P. Photoinduced electron transfers with carbon dots. Chem. Commun. 2009, 3774–3776. [Google Scholar] [CrossRef] [PubMed]
- Kittler, S.; Greulich, C.; Diendorf, J.; Koller, M.; Epple, M. Toxicity of silver nanoparticles increases during storage because of slow dissolution under release of silver ions. Chem. Mater. 2010, 22, 4548–4554. [Google Scholar] [CrossRef]
- Zhu, W.; Li, Z.; Zhou, Y.; Yan, X. Deposition of silver nanoparticles onto two dimensional biocl nanodiscs for enhanced visible light photocatalytic and biocidal activities. RSC Adv. 2016, 6, 64911–64920. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Hua, X.-W.; Wu, F.-G.; Li, B.; Liu, P.; Gu, N.; Wang, Z.; Chen, Z. Synthesis of ultrastable copper sulfide nanoclusters via trapping the reaction intermediate: Potential anticancer and antibacterial applications. ACS Appl. Mater. Interfaces 2015, 7, 7082–7092. [Google Scholar] [CrossRef] [PubMed]
- Javani, S.; Lorca, R.; Latorre, A.; Flors, C.; Cortajarena, A.L.; Somoza, Á. Antibacterial activity of DNA-stabilized silver nanoclusters tuned by oligonucleotide sequence. ACS Appl. Mater. Interfaces 2016, 8, 10147–10154. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.Y.; Setyawati, M.I.; Lim, T.P.; Leong, D.T.; Xie, J.P. Antimicrobial cluster bombs: Silver nanoclusters packed with daptomycin. ACS Nano 2016, 10, 7934–7942. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.F.; Wang, L.; Bu, C.P.; Wang, G.Q.; Zhang, Y.H.; Fang, S.M.; Shi, W.Z. Synthesis of luminescent ag nanoclusters with antibacterial activity. J. Nanomater. 2015, 2015, 792095. [Google Scholar] [CrossRef]
- Vimbela, G.V.; Ngo, S.M.; Fraze, C.; Yang, L.; Stout, D.A. Antibacterial properties and toxicity from metallic nanomaterials. Int. J. Nanomed. 2017, 12, 3941–3965. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Zhu, M.; Wu, Z.; Jin, R. Quantum sized gold nanoclusters with atomic precision. Acc. Chem. Res. 2012, 45, 1470–1479. [Google Scholar] [CrossRef]
- Jin, R.; Zeng, C.; Zhou, M.; Chen, Y. Atomically precise colloidal metal nanoclusters and nanoparticles: Fundamentals and opportunities. Chem. Rev. 2016, 116, 10346–103413. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, W.; Liu, G.; Panda, D.; Chen, P. Size-dependent catalytic activity and dynamics of gold nanoparticles at the single-molecule level. J. Am. Chem. Soc. 2010, 132, 138–146. [Google Scholar] [CrossRef]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold nanoparticles in chemical and biological sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ross, R.D.; Roeder, R.K. Preparation of functionalized gold nanoparticles as a targeted x-ray contrast agent for damaged bone tissue. Nanoscale 2010, 2, 582–586. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.M.; Chu, H.L.; Lee, Y.C.; Wang, D.Y.; Chang, C.C.; Chung, K.L.; Yen, H.C.; Hsiao, C.W.; Pan, X.Y.; Kuo, T.R.; et al. Quantitative analysis of glucose metabolic cleavage in glucose transporters overexpressed cancer cells by target-specific fluorescent gold nanoclusters. Anal. Chem. 2018, 90, 3974–3980. [Google Scholar] [CrossRef]
- Kaur, N.; Aditya, R.N.; Singh, A.; Kuo, T.R. Biomedical applications for gold nanoclusters: Recent developments and future perspectives. Nanoscale Res. Lett. 2018, 13, 302. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Jin, R. Atomically precise gold nanoclusters as new model catalysts. Acc. Chem. Res. 2013, 46, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-A.J.; Yang, T.-Y.; Lee, C.-H.; Huang, S.H.; Sperling, R.A.; Zanella, M.; Li, J.K.; Shen, J.-L.; Wang, H.-H.; Yeh, H.-I. Synthesis, characterization, and bioconjugation of fluorescent gold nanoclusters toward biological labeling applications. ACS Nano 2009, 3, 395–401. [Google Scholar] [CrossRef]
- Wen, F.; Dong, Y.; Feng, L.; Wang, S.; Zhang, S.; Zhang, X. Horseradish peroxidase functionalized fluorescent gold nanoclusters for hydrogen peroxide sensing. Anal. Chem. 2011, 83, 1193–1196. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chen, S. Oxygen electroreduction catalyzed by gold nanoclusters: Strong core size effects. Angew. Chem. Int. Ed. 2009, 48, 4386–4389. [Google Scholar] [CrossRef]
- Miyamura, H.; Matsubara, R.; Miyazaki, Y.; Kobayashi, S. Aerobic oxidation of alcohols at room temperature and atmospheric conditions catalyzed by reusable gold nanoclusters stabilized by the benzene rings of polystyrene derivatives. Angew. Chem. Int. Ed. 2007, 46, 4151–4154. [Google Scholar] [CrossRef]
- Tsunoyama, H.; Sakurai, H.; Negishi, Y.; Tsukuda, T. Size-specific catalytic activity of polymer-stabilized gold nanoclusters for aerobic alcohol oxidation in water. J. Am. Chem. Soc. 2005, 127, 9374–9375. [Google Scholar] [CrossRef]
- Xie, J.; Zheng, Y.; Ying, J.Y. Protein-directed synthesis of highly fluorescent gold nanoclusters. J. Am. Chem. Soc. 2009, 131, 888–889. [Google Scholar] [CrossRef] [PubMed]
- Herzing, A.A.; Kiely, C.J.; Carley, A.F.; Landon, P.; Hutchings, G.J. Identification of active gold nanoclusters on iron oxide supports for co oxidation. Science 2008, 321, 1331–1335. [Google Scholar] [CrossRef] [PubMed]
- Garzón, I.; Michaelian, K.; Beltrán, M.; Posada-Amarillas, A.; Ordejón, P.; Artacho, E.; Sánchez-Portal, D.; Soler, J. Lowest energy structures of gold nanoclusters. Phys. Rev. Lett. 1998, 81, 1600. [Google Scholar] [CrossRef]
- Li, C.-H.; Kuo, T.-R.; Su, H.-J.; Lai, W.-Y.; Yang, P.-C.; Chen, J.-S.; Wang, D.-Y.; Wu, Y.-C.; Chen, C.-C. Fluorescence-guided probes of aptamer-targeted gold nanoparticles with computed tomography imaging accesses for in vivo tumor resection. Sci. Rep. 2015, 5, 15675. [Google Scholar] [CrossRef] [PubMed]
- Yahia-Ammar, A.; Sierra, D.; Merola, F.; Hildebrandt, N.; Le Guevel, X. Self-assembled gold nanoclusters for bright fluorescence imaging and enhanced drug delivery. ACS Nano 2016, 10, 2591–2599. [Google Scholar] [CrossRef] [PubMed]
- Bootharaju, M.S.; Joshi, C.P.; Parida, M.R.; Mohammed, O.F.; Bakr, O.M. Templated atom-precise galvanic synthesis and structure elucidation of a [ag24au(sr)18]− nanocluster. Angew. Chem. Int. Ed. 2016, 55, 922–926. [Google Scholar] [CrossRef] [PubMed]
- Higaki, T.; Liu, C.; Zeng, C.J.; Jin, R.X.; Chen, Y.X.; Rosi, N.L.; Jin, R.C. Controlling the atomic structure of au-30 nanoclusters by a ligand-based strategy. Angew. Chem. Int. Ed. 2016, 55, 6694–6697. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Wang, S.X.; Song, Y.B.; Jin, S.; Sun, G.D.; Yu, H.Z.; Zhu, M.Z. Bimetallic au2cu6 nanoclusters: Strong luminescence induced by the aggregation of copper(i) complexes with gold(0) species. Angew. Chem. Int. Ed. 2016, 55, 3611–3614. [Google Scholar] [CrossRef]
- Wang, Y.Z.; De, S.; Yan, N. Rational control of nano-scale metal-catalysts for biomass conversion. Chem. Commun. 2016, 52, 6210–6224. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.X.; Liu, C.; Tang, Q.; Zeng, C.J.; Higaki, T.; Das, A.; Jiang, D.E.; Rosi, N.L.; Jin, R.C. Isomerism in au28(sr)20 nanocluster and stable structures. J. Am. Chem. Soc. 2016, 138, 1482–1485. [Google Scholar] [CrossRef]
- Wang, Y.; Wan, X.K.; Ren, L.T.; Su, H.F.; Li, G.; Malola, S.; Lin, S.C.; Tang, Z.C.; Hakkinen, H.; Teo, B.K.; et al. Atomically precise alkynyl-protected metal nanoclusters as a model catalyst: Observation of promoting effect of surface ligands on catalysis by metal nanoparticles. J. Am. Chem. Soc. 2016, 138, 3278–3281. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.J.; Chen, Y.X.; Iida, K.; Nobusada, K.; Kirschbaum, K.; Lambright, K.J.; Jin, R.C. Gold quantum boxes: On the periodicities and the quantum confinement in the au-28, au-36, au-44, and au-52 magic series. J. Am. Chem. Soc. 2016, 138, 3950–3953. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.F.; Yuan, X.; Fung, V.; Yu, Y.; Leong, D.T.; Jiang, D.E.; Xie, J.P. Understanding seed-mediated growth of gold nanoclusters at molecular level. Nat. Commun. 2017, 8, 927. [Google Scholar] [CrossRef] [PubMed]
- Govindaraju, S.; Ankireddy, S.R.; Viswanath, B.; Kim, J.; Yun, K. Fluorescent gold nanoclusters for selective detection of dopamine in cerebrospinal fluid. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Weng, B.; Lu, K.-Q.; Tang, Z.; Chen, H.M.; Xu, Y.-J. Stabilizing ultrasmall au clusters for enhanced photoredox catalysis. Nat. Commun. 2018, 9, 1543. [Google Scholar] [CrossRef] [PubMed]
- Yamgar, R.S.; Nivid, Y.; Nalawade, S.; Mandewale, M.; Atram, R.; Sawant, S.S. Novel zinc (ii) complexes of heterocyclic ligands as antimicrobial agents: Synthesis, characterisation, and antimicrobial studies. Bioinorg. Chem. Appl. 2014, 2014, 276598. [Google Scholar] [CrossRef] [PubMed]
- Fernando, S.; Fernando, T.; Stefanik, M.; Eyer, L.; Ruzek, D. An approach for zika virus inhibition using homology structure of the envelope protein. Mol. Biotechnol. 2016, 58, 801–806. [Google Scholar] [CrossRef]
- Nwibo, D.D.; Levi, C.A.; Nwibo, M.I. Small molecule drugs; down but not out: A future for medical research and therapeutics. J. Appl. Dent. 2015, 14, 70–77. [Google Scholar]
- Hay, M.; Thomas, D.W.; Craighead, J.L.; Economides, C.; Rosenthal, J. Clinical development success rates for investigational drugs. Nat. Biotechnol. 2014, 32, 40. [Google Scholar] [CrossRef]
- Zheng, K.; Setyawati, M.I.; Leong, D.T.; Xie, J. Antimicrobial gold nanoclusters. ACS Nano 2017, 11, 6904–6910. [Google Scholar] [CrossRef]
- Setyawati, M.I.; Kutty, R.V.; Tay, C.Y.; Yuan, X.; Xie, J.; Leong, D.T. Novel theranostic DNA nanoscaffolds for the simultaneous detection and killing of escherichia coli and staphylococcus aureus. ACS Appl. Mater. Interfaces 2014, 6, 21822–21831. [Google Scholar] [CrossRef] [PubMed]
- Sinha, T.; Ahmaruzzaman, M. A new and facile strategy for the one-pot fabrication of luminescent gold nanoclusters and their prospective application. RSC Adv. 2016, 6, 44–56. [Google Scholar] [CrossRef]
- Tseng, Y.-T.; Chang, H.-T.; Chen, C.-T.; Chen, C.-H.; Huang, C.-C. Preparation of highly luminescent mannose–gold nanodots for detection and inhibition of growth of escherichia coli. Biosens. Bioelectron. 2011, 27, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Liu, Y.; Yang, J.; Liu, Y.; Hu, F.; Zhu, K.; Jiang, X. Gold nanoclusters for targeting methicillin-resistant staphylococcus aureus in vivo. Angew. Chem. Int. Ed. Engl. 2018, 57, 3958–3962. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Liu, W.; Qin, Z.; Chen, Y.; Jiang, H.; Wang, X. Mercaptopyrimidine-conjugated gold nanoclusters as nanoantibiotics for combating multidrug-resistant superbugs. Bioconjug. Chem. 2018, 29, 3094–3103. [Google Scholar] [CrossRef]
- Zheng, K.; Setyawati, M.I.; Leong, D.T.; Xie, J. Surface ligand chemistry of gold nanoclusters determines their antimicrobial ability. Chem. Mater. 2018, 30, 2800–2808. [Google Scholar] [CrossRef]
- Chen, W.Y.; Lin, J.Y.; Chen, W.J.; Luo, L.; Wei-Guang Diau, E.; Chen, Y.C. Functional gold nanoclusters as antimicrobial agents for antibiotic-resistant bacteria. Nanomedicine 2010, 5, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Kalita, S.; Kandimalla, R.; Bhowal, A.C.; Kotoky, J.; Kundu, S. Functionalization of beta-lactam antibiotic on lysozyme capped gold nanoclusters retrogress mrsa and its persisters following awakening. Sci. Rep. 2018, 8, 5778. [Google Scholar] [CrossRef]
- Liang, J.; Xiong, H.; Wang, W.; Wen, W.; Zhang, X.; Wang, S. “Luminescent-off/on” sensing mechanism of antibiotic-capped gold nanoclusters to phosphate-containing metabolites and its antibacterial characteristics. Sensor. Actuat. B-Chem. 2018, 255, 2170–2178. [Google Scholar] [CrossRef]
- Li, Q.; Pan, Y.; Chen, T.; Du, Y.; Ge, H.; Zhang, B.; Xie, J.; Yu, H.; Zhu, M. Design and mechanistic study of a novel gold nanocluster-based drug delivery system. Nanoscale 2018, 10, 10166–10172. [Google Scholar] [CrossRef]
- Liao, F.H.; Wu, T.H.; Huang, Y.T.; Lin, W.J.; Su, C.J.; Jeng, U.S.; Kuo, S.C.; Lin, S.Y. Subnanometer gold clusters adhere to lipid a for protection against endotoxin-induced sepsis. Nano Lett. 2018, 18, 2864–2869. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, Y.; Peng, Y.; Yang, X. Exploring the anti-bacteria performance of multi-color ag, au and cu nanoclusters. ACS Appl. Mater. Interfaces 2019, 11, 8461–8469. [Google Scholar] [CrossRef] [PubMed]
- Grace, A.N.; Pandian, K. Antibacterial efficacy of aminoglycosidic antibiotics protected gold nanoparticles—a brief study. Colloids Surf. A 2007, 297, 63–70. [Google Scholar] [CrossRef]
- Rai, A.; Prabhune, A.; Perry, C.C. Antibiotic mediated synthesis of gold nanoparticles with potent antimicrobial activity and their application in antimicrobial coatings. J. Mater. Chem. 2010, 20, 6789–6798. [Google Scholar] [CrossRef]
- Burygin, G.; Khlebtsov, B.; Shantrokha, A.; Dykman, L.; Bogatyrev, V.; Khlebtsov, N. On the enhanced antibacterial activity of antibiotics mixed with gold nanoparticles. Nanoscale Res. Lett. 2009, 4, 794. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Robinson, S.M.; Gupta, A.; Saha, K.; Jiang, Z.; Moyano, D.F.; Sahar, A.; Riley, M.A.; Rotello, V.M. Functional gold nanoparticles as potent antimicrobial agents against multi-drug-resistant bacteria. ACS Nano 2014, 8, 10682–10686. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.C. Antimicrobial reactive oxygen and nitrogen species: Concepts and controversies. Nat. Rev. Microbiol. 2004, 2, 820–832. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Fan, C.; Pei, H.; Shi, J.; Huang, Q. Smart drug delivery nanocarriers with self-assembled DNA nanostructures. Adv. Mater. 2013, 25, 4386–4396. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, J.; Lykotrafitis, G.; Bao, G.; Suresh, S. Size-dependent endocytosis of nanoparticles. Adv. Mater. 2009, 21, 419–424. [Google Scholar] [CrossRef]
- Dubnau, D. DNA uptake in bacteria. Annu. Rev. Microbiol. 1999, 53, 217–244. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Zhao, Y.; Song, Q. Synthesis, optical properties and applications of ultra-small luminescent gold nanoclusters. TrAC Trends Anal. Chem. 2014, 57, 73–82. [Google Scholar] [CrossRef]
- Lin, Y.; Ren, J.; Qu, X. Catalytically active nanomaterials: A promising candidate for artificial enzymes. Acc. Chem. Res. 2014, 47, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ding, S.; Dietrich, R.; Märtlbauer, E.; Zhu, K. A biosurfactant-inspired heptapeptide with improved specificity to kill mrsa. Angew. Chem. Int. Ed. 2017, 56, 1486–1490. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, X.; Yang, J.; Jia, H.-R.; Li, Y.-H.; Chen, Z.; Wu, F.-G. Quaternized silicon nanoparticles with polarity-sensitive fluorescence for selectively imaging and killing gram-positive bacteria. Adv. Funct. Mater. 2016, 26, 5958–5970. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, W.; Chen, Y.; Jiang, H.; Yan, H.; Kosenko, I.; Chekulaeva, L.; Sivaev, I.; Bregadze, V.; Wang, X. A highly potent antibacterial agent targeting methicillin-resistant staphylococcus aureus based on cobalt bis(1,2-dicarbollide) alkoxy derivative. Organometallics 2017, 36, 3484–3490. [Google Scholar] [CrossRef]
- Shi, M.; Kwon, H.S.; Peng, Z.; Elder, A.; Yang, H. Effects of surface chemistry on the generation of reactive oxygen species by copper nanoparticles. ACS Nano 2012, 6, 2157–2164. [Google Scholar] [CrossRef] [PubMed]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238. [Google Scholar] [CrossRef] [PubMed]
- Yeaman, M.R.; Yount, N.Y. Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. 2003, 55, 27–55. [Google Scholar] [CrossRef]
- Chan, P.H.; Wong, S.Y.; Lin, S.H.; Chen, Y.C. Lysozyme-encapsulated gold nanocluster-based affinity mass spectrometry for pathogenic bacteria. Rapid Commun. Mass. Spectrom. 2013, 27, 2143–2148. [Google Scholar] [CrossRef]
- Kiristi, M.; Singh, V.V.; Esteban-Fernandez de Avila, B.; Uygun, M.; Soto, F.; Aktas Uygun, D.; Wang, J. Lysozyme-based antibacterial nanomotors. ACS Nano 2015, 9, 9252–9259. [Google Scholar] [CrossRef]
- Alsaiari, S.K.; Hammami, M.A.; Croissant, J.G.; Omar, H.W.; Neelakanda, P.; Yapici, T.; Peinemann, K.V.; Khashab, N.M. Colloidal gold nanoclusters spiked silica fillers in mixed matrix coatings: Simultaneous detection and inhibition of healthcare-associated infections. Adv. Healthc. Mater. 2017, 6, 1601135. [Google Scholar] [CrossRef] [PubMed]
- Kohanski, M.A.; Dwyer, D.J.; Collins, J.J. How antibiotics kill bacteria: From targets to networks. Nat. Rev. Microbiol. 2010, 8, 423. [Google Scholar] [CrossRef] [PubMed]
- Py, B.; Barras, F. Building fe–s proteins: Bacterial strategies. Nat. Rev. Microbiol. 2010, 8, 436. [Google Scholar] [CrossRef] [PubMed]
- Turci, F.; Ghibaudi, E.; Colonna, M.; Boscolo, B.; Fenoglio, I.; Fubini, B. An integrated approach to the study of the interaction between proteins and nanoparticles. Langmuir 2010, 26, 8336–8346. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Zhao, Z.; Li, Y.; Zou, J.; Ma, Q.; Zhao, Y.; Ke, Y.; Zhu, Y.; Chen, H.; Baker, M.A. Enhanced efflux activity facilitates drug tolerance in dormant bacterial cells. Mol. Cell 2016, 62, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Park, B.S.; Lee, J.-O. Recognition of lipopolysaccharide pattern by tlr4 complexes. Exp. Mol. Med. 2013, 45, e66. [Google Scholar] [CrossRef]
- Luo, Y.-H.; Wu, Z.W.; Tsai, H.-T.; Lin, S.-Y.; Lin, P. Endotoxin nanovesicles: Hydrophilic gold nanodots control supramolecular lipopolysaccharide assembly for modulating immunological responses. Nano Lett. 2015, 15, 6446–6453. [Google Scholar] [CrossRef] [PubMed]
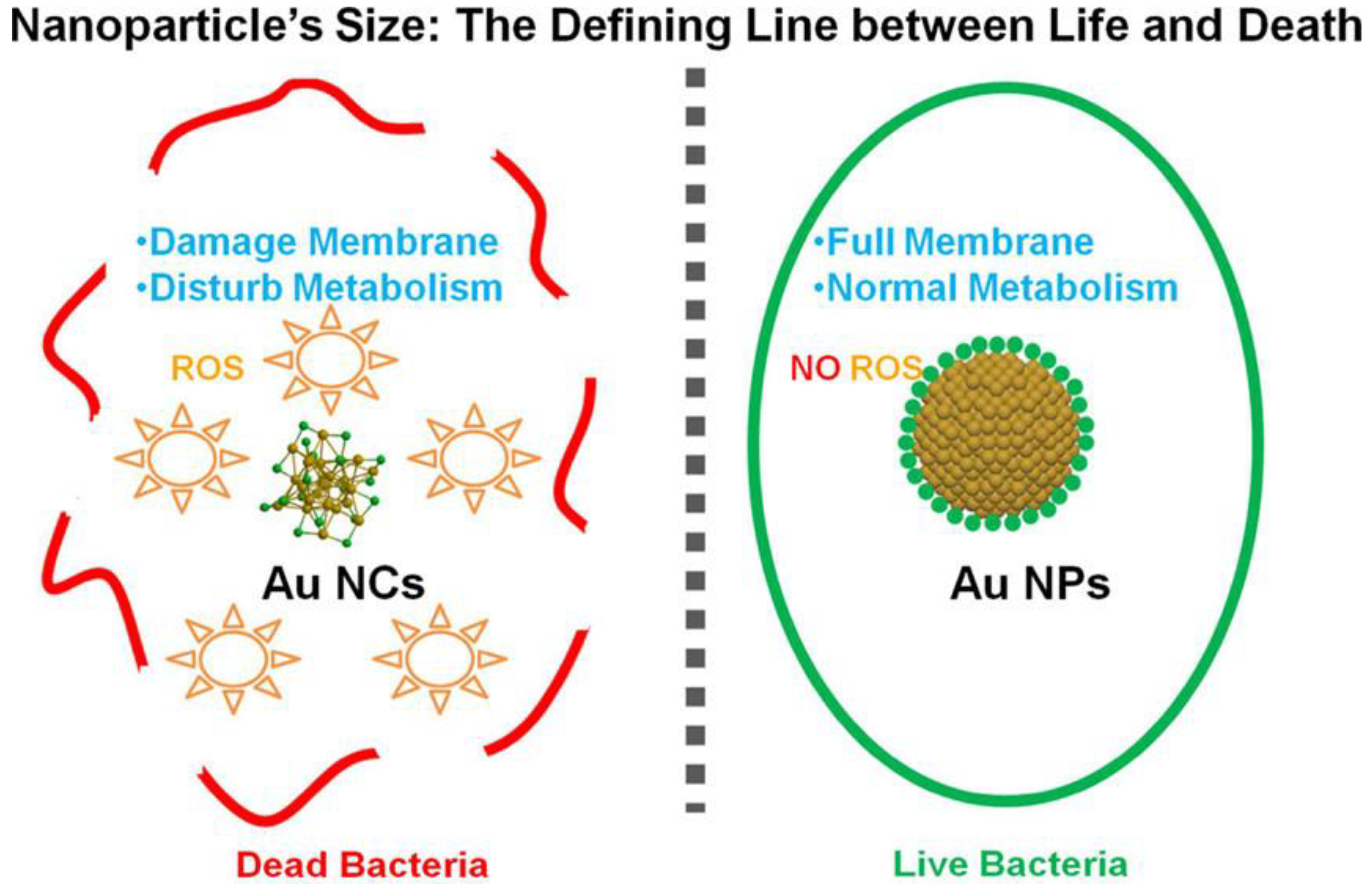


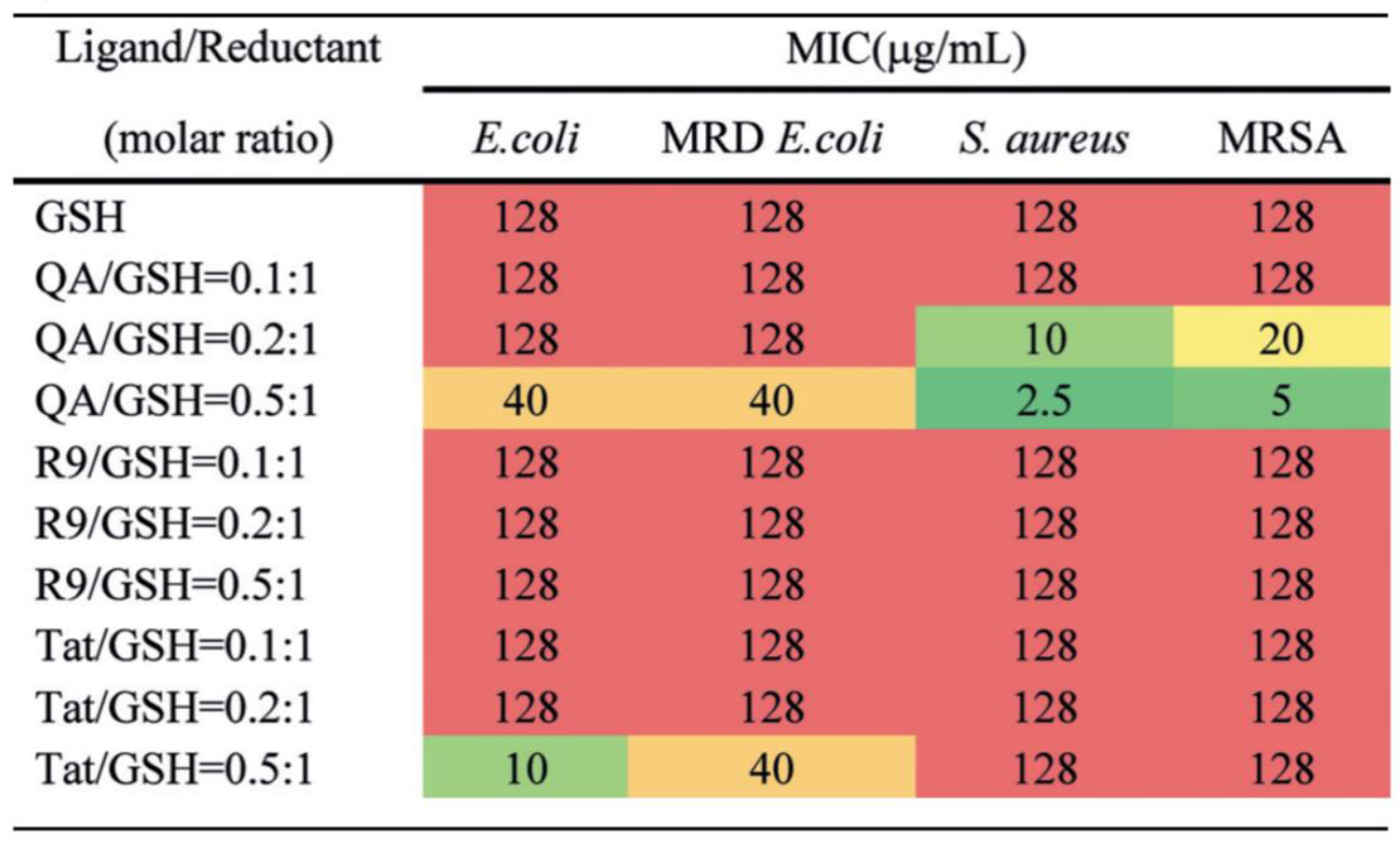

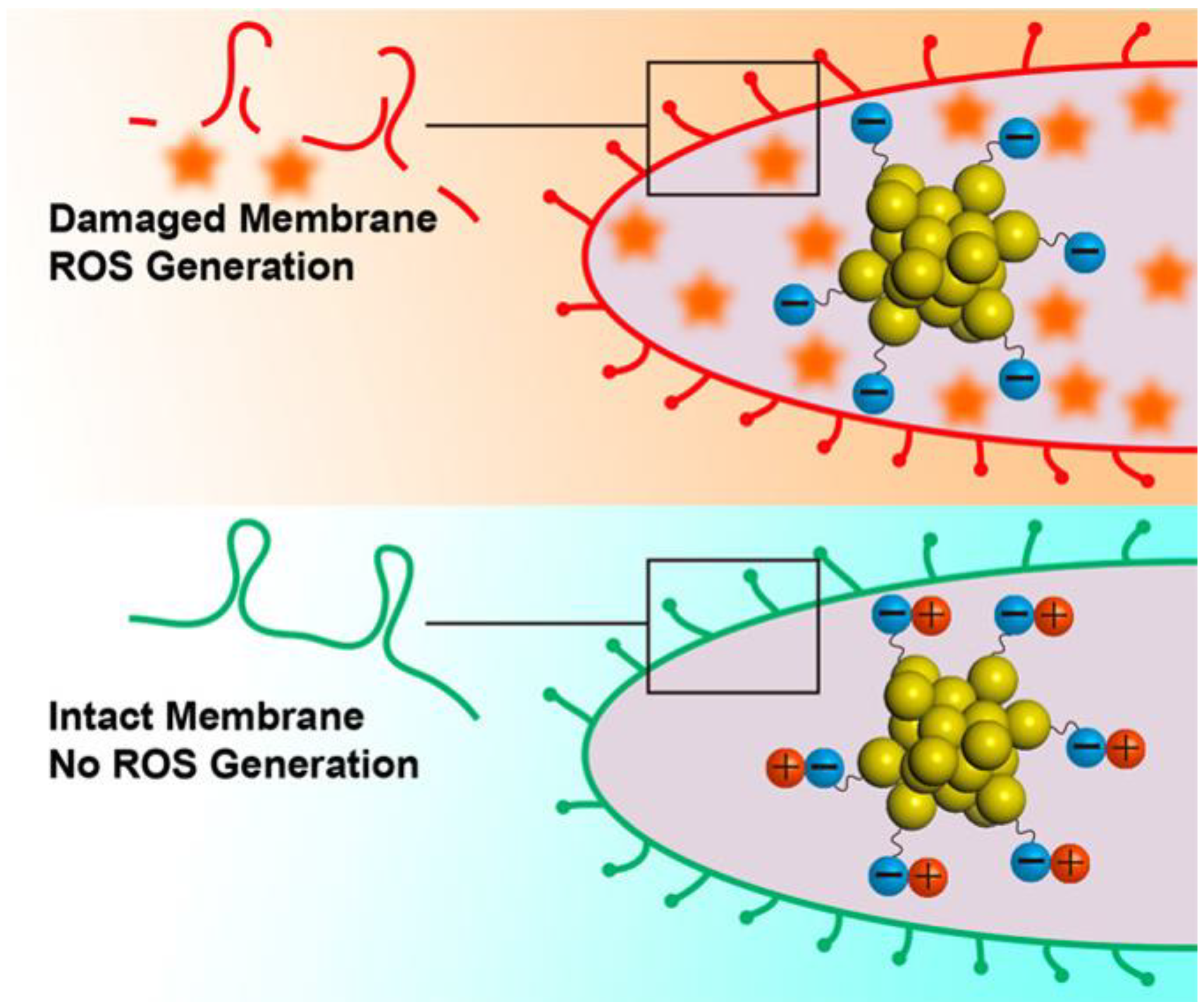


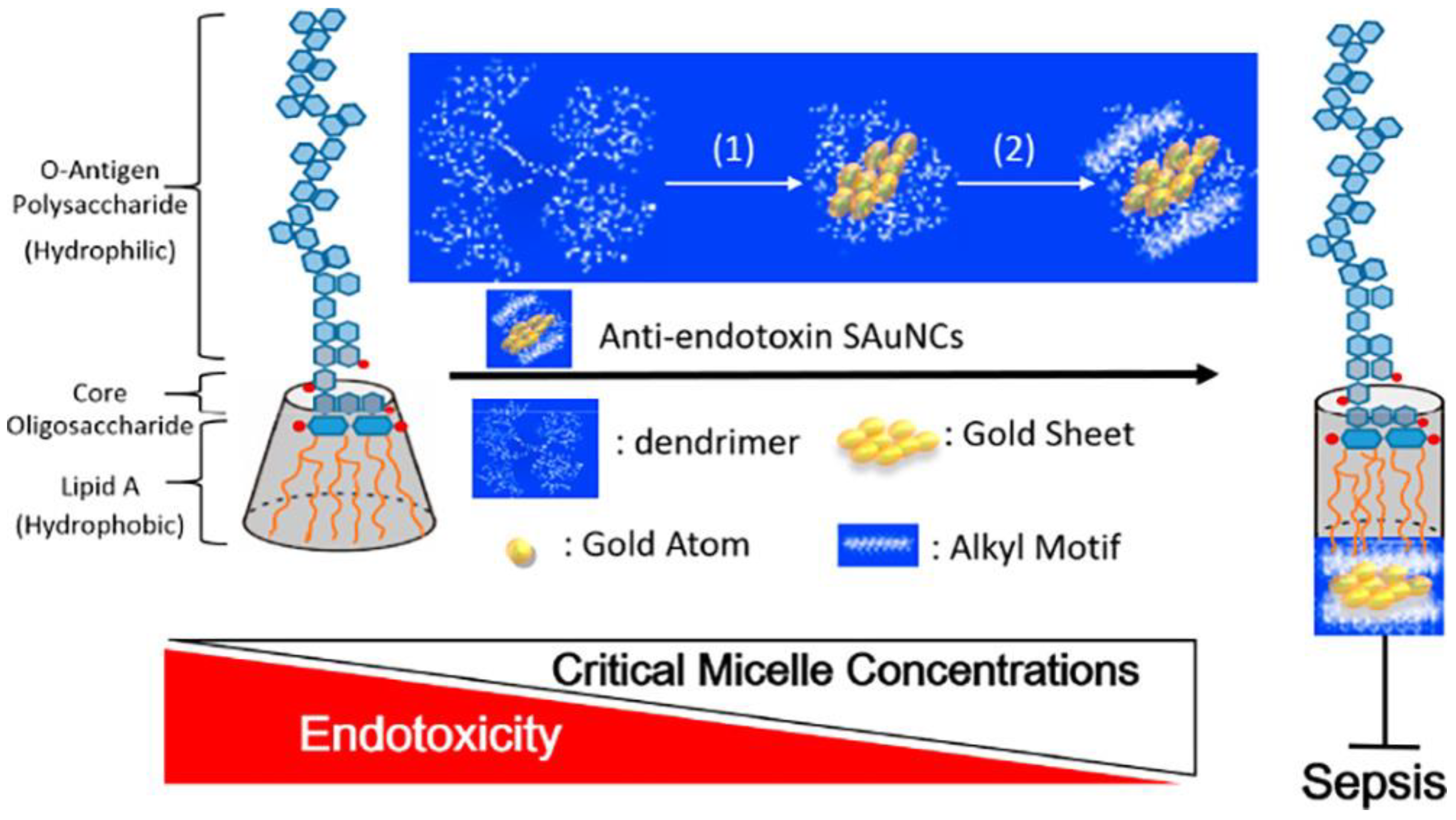
| Types | Ligands (Molecular Weight) | Antibacterial Mechanisms | References |
|---|---|---|---|
| Small molecules | 6-Mercaptohexanoic acid (148 Da) | Increase of ROS generation by MHA-AuNCs to kill bacteria | [71] |
| Glutathione (307 Da) | Optimal radius of DPAu/AMD for the increase of cell uptake | [72] | |
| Allium cepa L. (Mixture) | Increase of the interaction between AuNCs and bacterial membrane | [73] | |
| Mannose (180 Da) | Bacterial aggregations | [74] | |
| Quaternary ammonium (282 Da) | Increase of ROS generation by QA-AuNCs with positive charge | [75] | |
| 4-Amino-2-mercaptopyrimidine (127 Da) | Increase of ROS generation by AuDHMP with positive charge | [76] | |
| 6-Mercaptohexanoic acid (148 Da) | Increase of ROS generation by Au25NCs with negative charge | [77] | |
| Macromolecules | Lysozyme (143000 Da) | Hydrolysis of bacterial cell wall by lysozyme-AuNCs | [78] |
| Lysozyme (143000 Da) | Multivalent interactions between AuNC-L-Amp and bacterial | [79] | |
| Vancomycin (1449 Da) | Delivery of vancomycin into bacteria by AuNC@Van | [80] | |
| Vancomycin (1449 Da) | Delivery of vancomycin into bacteria by Au-SGaa-Van | [81] | |
| G4NH2 & G4OH (14266 & 14277 Da) | Inhibition of LPS aggregation | [82] | |
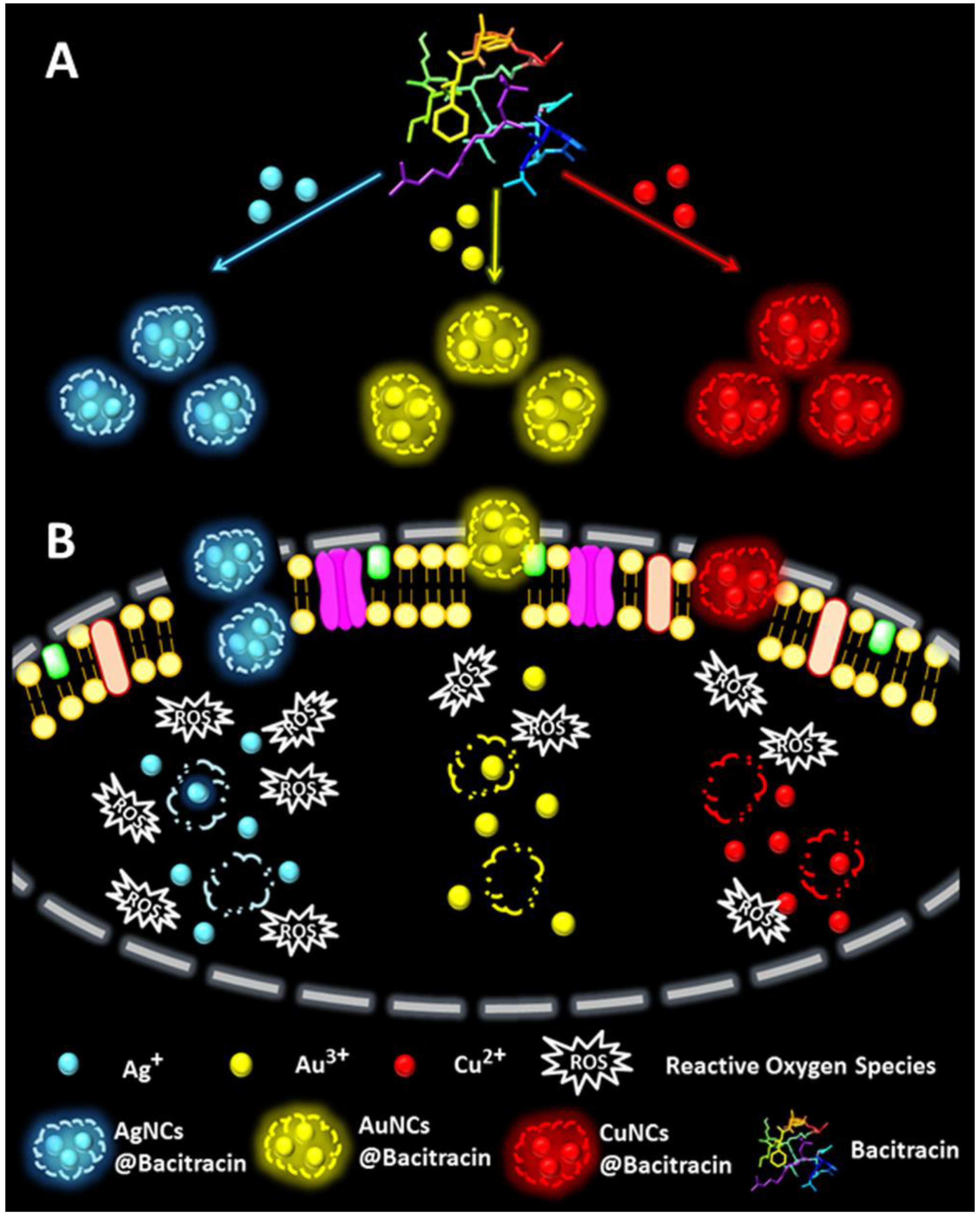
| Bacitracin (1422 Da) | Damage of cell wall and increase of ROS production by AuNCs@Bacitracin | [83] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yougbare, S.; Chang, T.-K.; Tan, S.-H.; Kuo, J.-C.; Hsu, P.-H.; Su, C.-Y.; Kuo, T.-R. Antimicrobial Gold Nanoclusters: Recent Developments and Future Perspectives. Int. J. Mol. Sci. 2019, 20, 2924. https://doi.org/10.3390/ijms20122924
Yougbare S, Chang T-K, Tan S-H, Kuo J-C, Hsu P-H, Su C-Y, Kuo T-R. Antimicrobial Gold Nanoclusters: Recent Developments and Future Perspectives. International Journal of Molecular Sciences. 2019; 20(12):2924. https://doi.org/10.3390/ijms20122924
Chicago/Turabian StyleYougbare, Sibidou, Ting-Kuang Chang, Shih-Hua Tan, Jui-Chi Kuo, Po-Hsuan Hsu, Chen-Yen Su, and Tsung-Rong Kuo. 2019. "Antimicrobial Gold Nanoclusters: Recent Developments and Future Perspectives" International Journal of Molecular Sciences 20, no. 12: 2924. https://doi.org/10.3390/ijms20122924
APA StyleYougbare, S., Chang, T.-K., Tan, S.-H., Kuo, J.-C., Hsu, P.-H., Su, C.-Y., & Kuo, T.-R. (2019). Antimicrobial Gold Nanoclusters: Recent Developments and Future Perspectives. International Journal of Molecular Sciences, 20(12), 2924. https://doi.org/10.3390/ijms20122924






