Purinergic Signaling and Cochlear Injury-Targeting the Immune System?
Abstract
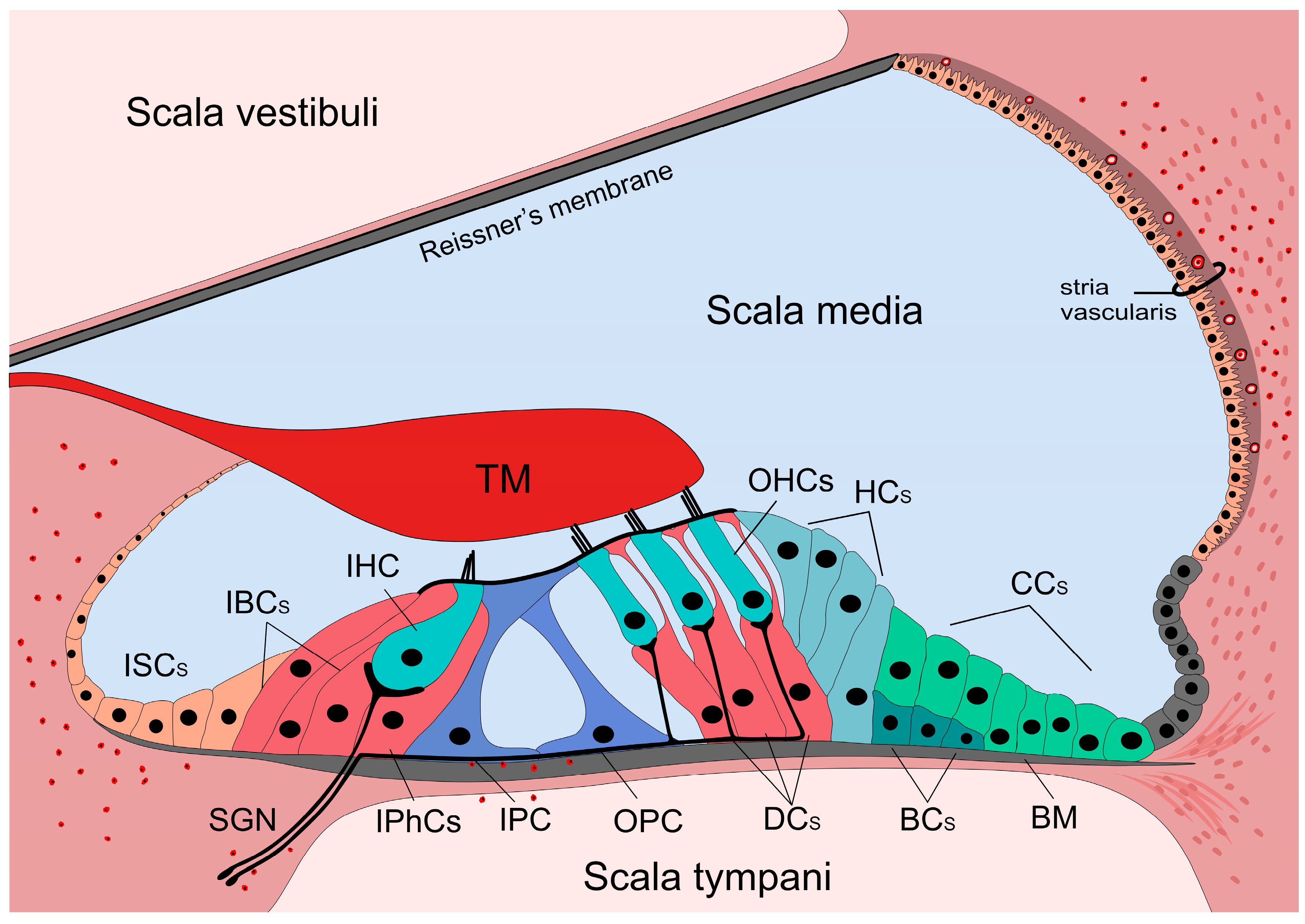
1. Introduction—the Hearing Organ
2. Purinergic Signaling and its Components in the Cochlea
3. P2 Receptor-Mediated Mechanisms Influencing the Cochlear Functions
3.1. K+ Recycling
3.2. Cochlear Amplification and Membrane Rigidity
3.3. Intercellular Ca2+ Waves
3.4. Development of the Organ of Corti
4. Adenosine Receptor-Mediated Mechanisms Influencing the Cochlear Functions
5. Sensorineural Hearing Losses
6. Purinergic Signaling and Sensorineural Hearing Losses
7. Purinergic Signaling and the Immune System—Possible Impact on Sensorineural Hearing Loss
8. Immune Mechanisms in Sensorineural Hearing Losses
8.1. Components of the Cochlear Immune System
8.2. Activation, Resolution and Function of the Cochlear Immune System
8.3. Role of the Cochlear Immune System in SNHLs
8.4. Role of the Cochlear Immune System in NIHLs
8.5. Role of the Cochlear Immune System in Aminoglycoside- and Cisplatin-Induced Hearing Losses
8.6. Role of the Cochlear Immune System in AHLs
8.7. Opposing Harmful Cochlear Inflammation by the Purinergic System–Therapeutic Potential
9. Conclusions and Outlook
Funding
Conflicts of Interest
Abbreviations
| ABR | auditory brainstem response |
| AHL | age-related hearing loss |
| ATP | adenosine triphosphate |
| BLB | blood-labyrinth barrier |
| CBF | cochlear blood flow |
| COX-2 | cyclooxygenase 2 |
| DAMP | damage-associated molecular pattern |
| DC | Deiters’ cell |
| DPOAE | distortion product of otoacoustic emission |
| HC | Hensen’s cell |
| IL-1β | interleukin 1 beta |
| IL-6 | interleukin 6 |
| iNOS | inducible nitric oxide synthetase |
| NFAT | nuclear factor of activated T-cells |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NIHL | noise-induced hearing loss |
| NOX3 | nicotinamide adenine dinucleotide phosphate oxidase 3 |
| OHC | outer hair cell |
| PPADS | pyridoxalphosphate-6-azophenyl-2’,4’-disulfonic acid |
| PRR | pattern recognition receptor |
| RGS | regulators of G protein signaling |
| RGS4 | regulator of G-protein signaling 4 |
| ROS | reactive oxygen species |
| SGN | spiral ganglion neuron |
| SNHL | sensorineural hearing loss |
| STAT-1 | signal transducer and activator of transcription 1 |
| SV | stria vascularis |
| TNFα | tumor necrosis factor alpha |
References
- Patuzzi, R. Ion flow in cochlear hair cells and the regulation of hearing sensitivity. Hear. Res. 2011, 280, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Patuzzi, R. Ion flow in stria vascularis and the production and regulation of cochlear endolymph and the endolymphatic potential. Hear. Res. 2011, 277, 4–19. [Google Scholar] [CrossRef] [PubMed]
- Wan, G.; Corfas, G.; Stone, J.S. Inner ear supporting cells: Rethinking the silent majority. Semin. Cell Dev. Biol. 2013, 24, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Wangemann, P. Supporting sensory transduction: Cochlear fluid homeostasis and the endocochlear potential. J. Physiol. 2006, 576, 11–21. [Google Scholar] [CrossRef]
- Ciuman, R.R. Cochlea—A Physiological Description of a Finely Structured Sense Organ. In book Advancies in Clinical Audiology; Stavros Hatzopoulos, Ed.; IntechOpen: London, England, 2017; pp. 207–230. [Google Scholar]
- Rybak, L.P.; Whitworth, C.; Scott, V. Development of endocochlear potential and compound action potential in the rat. Hear. Res. 1992, 59, 189–194. [Google Scholar] [CrossRef]
- Harris, J.P. Immunology of the inner ear: Response of the inner ear to antigen challenge. Otolaryngol. Neck Surg. 1983, 91, 18–23. [Google Scholar] [CrossRef]
- Harris, J.P. Immunology of the Inner Ear: Evidence of Local Antibody Production. Ann. Otol. Rhinol. Laryngol. 1984, 93, 157–162. [Google Scholar] [CrossRef]
- Dietz, B.; Jovanovic, S.; Wielsch, B.; Nerlich, J.; Rubsamen, R.; Milenkovic, I. Purinergic Modulation of Neuronal Activity in Developing Auditory Brainstem. J. Neurosci. 2012, 32, 10699–10712. [Google Scholar] [CrossRef]
- Wang, J.C.-C.; Raybould, N.P.; Luo, L.; Ryan, A.F.; Cannell, M.B.; Thorne, P.R.; Housley, G.D. Noise induces up-regulation of P2X2 receptor subunit of ATP-gated ion channels in the rat cochlea. Neuroreport 2003, 14, 817–823. [Google Scholar] [CrossRef]
- Liu, C.; Glowatzki, E.; Fuchs, P.A. Unmyelinated type II afferent neurons report cochlear damage. Proc. Natl. Acad. Sci. USA 2015, 112, 14723–14727. [Google Scholar] [CrossRef]
- Zhang, K.D.; Coate, T.M. Recent advances in the development and function of type II spiral ganglion neurons in the mammalian inner ear. Semin. Cell Dev. Biol. 2017, 65, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Frye, M.D.; Yang, W.; Zhang, C.; Xiong, B.; Hu, B.H. Dynamic activation of basilar membrane macrophages in response to chronic sensory cell degeneration in aging mouse cochleae. Hear. Res. 2017, 344, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Kalinec, G.M.; Lomberk, G.; Urrutia, R.A.; Kalinec, F. Resolution of Cochlear Inflammation: Novel Target for Preventing or Ameliorating Drug-, Noise- and Age-related Hearing Loss. Front. Cell. Neurosci. 2017, 11, 192. [Google Scholar] [CrossRef] [PubMed]
- Hirose, K.; Discolo, C.M.; Keasler, J.R.; Ransohoff, R. Mononuclear phagocytes migrate into the murine cochlea after acoustic trauma. J. Comp. Neurol. 2005, 489, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Dai, M.; Fridberger, A.; Hassan, A.; DeGagne, J.; Neng, L.; Zhang, F.; He, W.; Ren, T.; Trune, D.; et al. Perivascular-resident macrophage-like melanocytes in the inner ear are essential for the integrity of the intrastrial fluid-blood barrier. Proc. Natl. Acad. Sci. USA 2012, 109, 10388–10393. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, M.; Okano, H.; Ogawa, K. Inflammatory and immune responses in the cochlea: Potential therapeutic targets for sensorineural hearing loss. Front. Pharmacol. 2014, 5, 287. [Google Scholar] [CrossRef]
- Koo, J.-W.; Quintanilla-Dieck, L.; Jiang, M.; Liu, J.; Urdang, Z.D.; Allensworth, J.J.; Cross, C.P.; Li, H.; Steyger, P.S. Endotoxemia-mediated inflammation potentiates aminoglycoside-induced ototoxicity. Sci. Transl. Med. 2015, 7, 298ra118. [Google Scholar] [CrossRef] [PubMed]
- Shi, X. Pathophysiology of the cochlear intrastrial fluid-blood barrier (review). Hear. Res. 2016, 338, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.H.; Zhang, C.; Frye, M.D. Immune cells and non-immune cells with immune function in mammalian cochleae. Hear. Res. 2018, 362, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Abrashkin, K.A.; Izumikawa, M.; Miyazawa, T.; Wang, C.-H.; Crumling, M.A.; Swiderski, D.L.; Beyer, L.A.; Gong, T.-W.L.; Raphael, Y. The fate of outer hair cells after acoustic or ototoxic insults. Hear. Res. 2006, 218, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Purinergic nerves. Pharmacol. Rev. 1972, 24, 509–581. [Google Scholar] [PubMed]
- Fields, R.D.; Burnstock, G. Purinergic signalling in neuron-glia interactions. Nat. Rev. Neurosci. 2006, 7, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Köles, L.; Leichsenring, A.; Rubini, P.; Illes, P. P2 receptor signaling in neurons and glial cells of the central nervous system. Adv. Pharmacol. 2011, 61, 441–493. [Google Scholar] [PubMed]
- Abbracchio, M.P.; Burnstock, G.; Verkhratsky, A.; Zimmermann, H. Purinergic signalling in the nervous system: An overview. Trends Neurosci. 2009, 32, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Bodin, P.; Burnstock, G. Purinergic signalling: ATP release. Neurochem. Res. 2001, 26, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.P.; McCleskey, E.W. Cell damage excites nociceptors through release of cytosolic ATP. Pain 2002, 95, 41–47. [Google Scholar] [CrossRef]
- Burnstock, G. Physiology and Pathophysiology of Purinergic Neurotransmission. Physiol. Rev. 2007, 87, 659–797. [Google Scholar] [CrossRef] [PubMed]
- Franke, H.; Illes, P. Involvement of P2 receptors in the growth and survival of neurons in the CNS. Pharmacol. Ther. 2006, 109, 297–324. [Google Scholar] [CrossRef] [PubMed]
- Köles, L.; Furst, S.; Illes, P. P2X and P2Y receptors as possible targets of therapeutic manipulations in CNS illnesses. Drug News Perspect. 2005, 18, 85–101. [Google Scholar] [CrossRef]
- Zimmermann, H. Ectonucleotidases in the nervous system. Novartis Found. Symp. 2006, 276, 113–128; discussion 128–130, 233–237, 275–281. [Google Scholar]
- Burnstock, G.; Kennedy, C. Is there a basis for distinguishing two types of P2-purinoceptor? Gen. Pharmacol. 1985, 16, 433–440. [Google Scholar] [CrossRef]
- Abbracchio, M.P.; Burnstock, G.; Boeynaems, J.-M.; Barnard, E.A.; Boyer, J.L.; Kennedy, C.; Knight, G.E.; Fumagalli, M.; Gachet, C.; Jacobson, K.A.; et al. International Union of Pharmacology LVIII: Update on the P2Y G Protein-Coupled Nucleotide Receptors: From Molecular Mechanisms and Pathophysiology to Therapy. Pharmacol. Rev. 2006, 58, 281–341. [Google Scholar] [CrossRef] [PubMed]
- North, R.A. Molecular Physiology of P2X Receptors. Physiol. Rev. 2002, 82, 1013–1067. [Google Scholar] [CrossRef] [PubMed]
- Ralevic, V.; Burnstock, G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998, 50, 413–492. [Google Scholar] [PubMed]
- Fredholm, B.B.; IJzerman, A.P.; Jacobson, K.A.; Klotz, K.N.; Linden, J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 2001, 53, 527–552. [Google Scholar] [PubMed]
- Housley, G.D.; Kanjhan, R.; Raybould, N.P.; Greenwood, D.; Salih, S.G.; Järlebark, L.; Burton, L.D.; Setz, V.C.; Cannell, M.B.; Soeller, C.; et al. Expression of the P2X(2) receptor subunit of the ATP-gated ion channel in the cochlea: Implications for sound transduction and auditory neurotransmission. J. Neurosci. 1999, 19, 8377–8388. [Google Scholar] [CrossRef] [PubMed]
- Housley, G.D.; Bringmann, A.; Reichenbach, A. Purinergic signaling in special senses. Trends Neurosci. 2009, 32, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G.; Krügel, U.; Abbracchio, M.P.; Illes, P. Purinergic signalling: From normal behaviour to pathological brain function. Prog. Neurobiol. 2011, 95, 229–274. [Google Scholar] [CrossRef] [PubMed]
- Szücs, A.; Szappanos, H.; Tóth, A.; Farkas, Z.; Panyi, G.; Csernoch, L.; Sziklai, I. Differential expression of purinergic receptor subtypes in the outer hair cells of the guinea pig. Hear. Res. 2004, 196, 2–7. [Google Scholar] [CrossRef]
- Telang, R.S.; Paramananthasivam, V.; Vlajkovic, S.M.; Munoz, D.J.B.; Housley, G.D.; Thorne, P.R. Reduced P2x(2) receptor-mediated regulation of endocochlear potential in the ageing mouse cochlea. Purinergic Signal. 2010, 6, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Zhu, Y.; Walsh, T.; Xie, D.; Yuan, H.; Sirmaci, A.; Fujikawa, T.; Wong, A.C.Y.; Loh, T.L.; Du, L.; et al. Mutation of the ATP-gated P2X(2) receptor leads to progressive hearing loss and increased susceptibility to noise. Proc. Natl. Acad. Sci. USA 2013, 110, 2228–2233. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.-B.; Yu, N.; Fleming, C.R. Gap junctional hemichannel-mediated ATP release and hearing controls in the inner ear. Proc. Natl. Acad. Sci. USA 2005, 102, 18724–18729. [Google Scholar] [CrossRef] [PubMed]
- Szűcs, A.; Szappanos, H.; Batta, T.J.; Tóth, A.; Szigeti, G.P.; Panyi, G.; Csernoch, L.; Sziklai, I. Changes in Purinoceptor Distribution and Intracellular Calcium Levels following Noise Exposure in the Outer Hair Cells of the Guinea Pig. J. Membr. Biol. 2006, 213, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Harada, N.; Nakazawa, H.; Yamashita, T. Involvement of the nitric oxide-cyclic GMP pathway and neuronal nitric oxide synthase in ATP-induced Ca2+ signalling in cochlear inner hair cells. Eur. J. Neurosci. 2005, 21, 2912–2922. [Google Scholar] [CrossRef] [PubMed]
- Glowatzki, E.; Ruppersberg, J.P.; Zenner, H.-P.; Rüsch, A. Mechanically and ATP-induced currents of mouse outer hair cells are independent and differentially blocked by d-tubocurarine. Neuropharmacology 1997, 36, 1269–1275. [Google Scholar] [CrossRef]
- Housley, G.D.; Luo, L.; Ryan, A.F. Localization of mRNA encoding the P2X2 receptor subunit of the adenosine 5′-triphosphate-gated ion channel in the adult and developing rat inner ear by in situ hybridization. J. Comp. Neurol. 1998, 393, 403–414. [Google Scholar] [CrossRef]
- Housley, G.D.; Morton-Jones, R.; Vlajkovic, S.M.; Telang, R.S.; Paramananthasivam, V.; Tadros, S.F.; Wong, A.C.Y.; Froud, K.E.; Cederholm, J.M.E.; Sivakumaran, Y.; et al. ATP-gated ion channels mediate adaptation to elevated sound levels. Proc. Natl. Acad. Sci. USA 2013, 110, 7494–7499. [Google Scholar] [CrossRef] [PubMed]
- Järlebark, L.E.; Housley, G.D.; Raybould, N.P.; Vlajkovic, S.; Thorne, P.R. ATP-gated ion channels assembled from P2X2 receptor subunits in the mouse cochlea. Neuroreport 2002, 13, 1979–1984. [Google Scholar] [CrossRef] [PubMed]
- Järlebark, L.E.; Housley, G.D.; Thorne, P.R. Immunohistochemical localization of adenosine 5′-triphosphate-gated ion channel P2X(2) receptor subunits in adult and developing rat cochlea. J. Comp. Neurol. 2000, 421, 289–301. [Google Scholar] [CrossRef]
- Parker, M.S.; Larroque, M.L.; Campbell, J.M.; Bobbin, R.P.; Deininger, P.L. Novel variant of the P2X2 ATP receptor from the guinea pig organ of Corti. Hear. Res. 1998, 121, 62–70. [Google Scholar] [CrossRef]
- Salih, S.G.; Housley, G.D.; Raybould, N.P.; Thorne, P.R. ATP-gated ion channel expression in primary auditory neurones. Neuroreport 1999, 10, 2579–2586. [Google Scholar] [CrossRef] [PubMed]
- Sueta, T.; Paki, B.; Everett, A.W.; Robertson, D. Purinergic receptors in auditory neurotransmission. Hear. Res. 2003, 183, 97–108. [Google Scholar] [CrossRef]
- Chen, C.; Bobbin, R.P. P2X receptors in cochlear Deiters’ cells. Br. J. Pharmacol. 1998, 124, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhao, H.-B. ATP-mediated potassium recycling in the cochlear supporting cells. Purinergic Signal. 2010, 6, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Brändle, U.; Zenner, H.P.; Ruppersberg, J.P. Gene expression of P2X-receptors in the developing inner ear of the rat. Neurosci. Lett. 1999, 273, 105–108. [Google Scholar] [CrossRef]
- Greenwood, D.; Jagger, D.J.; Huang, L.C.; Hoya, N.; Thorne, P.R.; Wildman, S.S.; King, B.F.; Pak, K.; Ryan, A.F.; Housley, G.D. P2X receptor signaling inhibits BDNF-mediated spiral ganglion neuron development in the neonatal rat cochlea. Development 2007, 134, 1407–1417. [Google Scholar] [CrossRef]
- Xiang, Z.; Bo, X.; Burnstock, G. P2X receptor immunoreactivity in the rat cochlea, vestibular ganglion and cochlear nucleus. Hear. Res. 1999, 128, 190–196. [Google Scholar] [CrossRef]
- Salih, S.G.; Housley, G.D.; Burton, L.D.; Greenwood, D. P2X2receptor subunit expression in a subpopulation of cochlear type I spiral ganglion neurones. Neuroreport 1998, 9, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.C.; Greenwood, D.; Thorne, P.R.; Housley, G.D. Developmental regulation of neuron-specific P2X3 receptor expression in the rat cochlea. J. Comp. Neurol. 2005, 484, 133–143. [Google Scholar] [CrossRef]
- Nikolic, P.; Housley, G.D.; Luo, L.; Ryan, A.F.; Thorne, P.R. Transient Expression of P2X Receptor Subunits of ATP-Gated Ion 1 Channels in the Developing Rat Cochlea. Brain Res. Dev. Brain Res. 2001, 126, 173–182. [Google Scholar] [CrossRef]
- Nikolic, P.; Housley, G.D.; Thorne, P.R. Expression of the P2X7 receptor subunit of the adenosine 5′-triphosphate-gated ion channel in the developing and adult rat cochlea. Audiol. Neuro-Otol. 2003, 8, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-H.; Streeter, M.; Liu, Y.-P.; Zhao, H.-B. Identification and characterization of pannexin expression in the mammalian cochlea. J. Comp. Neurol. 2009, 512, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Collo, G.; Neidhart, S.; Kawashima, E.; Kosco-Vilbois, M.; North, R.A.; Buell, G. Tissue distribution of the P2X7 receptor. Neuropharmacology 1997, 36, 1277–1283. [Google Scholar] [CrossRef]
- Huang, L.C.; Ryan, A.F.; Cockayne, D.A.; Housley, G.D. Developmentally regulated expression of the P2X3 receptor in the mouse cochlea. Histochem. Cell Biol. 2006, 125, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.C.; Thorne, P.R.; Vlajkovic, S.M.; Housley, G.D. Differential expression of P2Y receptors in the rat cochlea during development. Purinergic Signal. 2010, 6, 231–248. [Google Scholar] [CrossRef] [PubMed]
- Piazza, V.; Ciubotaru, C.D.; Gale, J.E.; Mammano, F. Purinergic signalling and intercellular Ca2+ wave propagation in the organ of Corti. Cell Calcium 2007, 41, 77–86. [Google Scholar] [CrossRef]
- O’Keeffe, M.G.; Thorne, P.R.; Housley, G.D.; Robson, S.C.; Vlajkovic, S.M. Developmentally regulated expression of ectonucleotidases NTPDase5 and NTPDase6 and UDP-responsive P2Y receptors in the rat cochlea. Histochem. Cell Biol. 2010, 133, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Heo, J.-H.; Kim, C.-H.; Chang, S.O.; Kim, C.-S.; Oh, S.-H. Changes in P2Y4 receptor expression in rat cochlear outer sulcus cells during development. Hear. Res. 2007, 228, 201–211. [Google Scholar] [CrossRef]
- Parker, M.S.; Onyenekwu, N.N.; Bobbin, R.P. Localization of the P2Y4 receptor in the guinea pig organ of Corti. J. Am. Acad. Audiol. 2003, 14, 286–295. [Google Scholar]
- Kaur, T.; Borse, V.; Sheth, S.; Sheehan, K.; Ghosh, S.; Tupal, S.; Jajoo, S.; Mukherjea, D.; Rybak, L.P.; Ramkumar, V. Adenosine A1 Receptor Protects Against Cisplatin Ototoxicity by Suppressing the NOX3/STAT1 Inflammatory Pathway in the Cochlea. J. Neurosci. 2016, 36, 3962–3977. [Google Scholar] [CrossRef]
- Vlajkovic, S.; Housley, G.; Thorne, P. Adenosine and the Auditory System. Curr. Neuropharmacol. 2009, 7, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Vlajkovic, S.M.; Abi, S.; Wang, C.J.H.; Housley, G.D.; Thorne, P.R. Differential distribution of adenosine receptors in rat cochlea. Cell Tissue Res. 2007, 328, 461–471. [Google Scholar] [CrossRef]
- Vlajkovic, S.M.; Ambepitiya, K.; Barclay, M.; Boison, D.; Housley, G.D.; Thorne, P.R. Adenosine receptors regulate susceptibility to noise-induced neural injury in the mouse cochlea and hearing loss. Hear. Res. 2017, 345, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Tabuchi, K.; Sakai, S.; Nakayama, M.; Nishimura, B.; Hayashi, K.; Hirose, Y.; Hara, A. The effects of A1 and A2A adenosine receptor agonists on kainic acid excitotoxicity in the guinea pig cochlea. Neurosci. Lett. 2012, 518, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.W. Characterisation of Noise-Induced Cochlear Inflammation. Ph.D. Thesis, The University of Auckland, Research Repository, Auckland, New Zealand, 2015. [Google Scholar]
- Vlajkovic, S.M.; Thorne, P.R.; Sévigny, J.; Robson, S.C.; Housley, G.D. Distribution of ectonucleoside triphosphate diphosphohydrolases 1 and 2 in rat cochlea. Hear. Res. 2002, 170, 127–138. [Google Scholar] [CrossRef]
- Vlajkovic, S.M.; Housley, G.D.; Muñoz, D.J.B.; Robson, S.C.; Sévigny, J.; Wang, C.J.H.; Thorne, P.R. Noise exposure induces up-regulation of ecto-nucleoside triphosphate diphosphohydrolases 1 and 2 in rat cochlea. Neuroscience 2004, 126, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Vlajkovic, S.M.; Housley, G.D.; Thorne, P.R.; Gupta, R.; Enjyoji, K.; Cowan, P.J.; Charles Liberman, M.; Robson, S.C. Preservation of cochlear function in Cd39 deficient mice. Hear. Res. 2009, 253, 77–82. [Google Scholar] [CrossRef]
- Vlajkovic, S.M.; Thorne, P.R.; Sévigny, J.; Robson, S.C.; Housley, G.D. NTPDase1 and NTPDase2 immunolocalization in mouse cochlea: Implications for regulation of P2 receptor signaling. J. Histochem. Cytochem. 2002, 50, 1435–1441. [Google Scholar] [CrossRef]
- Vlajkovic, S.M.; Vinayagamoorthy, A.; Thorne, P.R.; Robson, S.C.; Wang, C.J.H.; Housley, G.D. Noise-induced up-regulation of NTPDase3 expression in the rat cochlea: Implications for auditory transmission and cochlear protection. Brain Res. 2006, 1104, 55–63. [Google Scholar] [CrossRef]
- O’Keeffe, M.G.; Thorne, P.R.; Housley, G.D.; Robson, S.C.; Vlajkovic, S.M. Distribution of NTPDase5 and NTPDase6 and the regulation of P2Y receptor signalling in the rat cochlea. Purinergic Signal. 2010, 6, 249–261. [Google Scholar] [CrossRef]
- Tritsch, N.X.; Yi, E.; Gale, J.E.; Glowatzki, E.; Bergles, D.E. The origin of spontaneous activity in the developing auditory system. Nature 2007, 450, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Tritsch, N.X.; Bergles, D.E. Developmental Regulation of Spontaneous Activity in the Mammalian Cochlea. J. Neurosci. 2010, 30, 1539–1550. [Google Scholar] [CrossRef] [PubMed]
- Tritsch, N.X.; Zhang, Y.-X.; Ellis-Davies, G.; Bergles, D.E. ATP-induced morphological changes in supporting cells of the developing cochlea. Purinergic Signal. 2010, 6, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Housley, G.D.; Marcotti, W.; Navaratnam, D.; Yamoah, E.N. Hair Cells–Beyond the Transducer. J. Membr. Biol. 2006, 209, 89–118. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, D.J.B.; Thorne, P.R.; Housley, G.D.; Billett, T.E. Adenosine 5′-triphosphate (ATP) concentrations in the endolymph and perilymph of the guinea-pig cochlea. Hear. Res. 1995, 90, 119–125. [Google Scholar] [CrossRef]
- Muñoz, D.J.B.; Thorne, P.R.; Housley, G.D.; Billett, T.E.; Battersby, J.M. Extracellular adenosine 5′-triphosphate (ATP) in the endolymphatic compartment influences cochlear function. Hear. Res. 1995, 90, 106–118. [Google Scholar] [CrossRef]
- Thorne, P.R.; Muñoz, D.J.B.; Nikolic, P.; Mander, L.; Jagger, D.J.; Greenwood, D.; Vlajkovic, S.; Housley, G.D. Potential role of purinergic signalling in cochlear pathology. Audiol. Neuro-Otol. 2002, 7, 180–184. [Google Scholar] [CrossRef]
- Eckhard, A.; Gleiser, C.; Rask-Andersen, H.; Arnold, H.; Liu, W.; Mack, A.; Müller, M.; Löwenheim, H.; Hirt, B. Co-localisation of K(ir)4.1 and AQP4 in rat and human cochleae reveals a gap in water channel expression at the transduction sites of endocochlear K(+) recycling routes. Cell Tissue Res. 2012, 350, 27–43. [Google Scholar] [CrossRef]
- Ye, R.; Liu, J.; Jia, Z.; Wang, H.; Wang, Y.; Sun, W.; Wu, X.; Zhao, Z.; Niu, B.; Li, X.; et al. Adenosine Triphosphate (ATP) Inhibits Voltage-Sensitive Potassium Currents in Isolated Hensen’s Cells and Nifedipine Protects Against Noise-Induced Hearing Loss in Guinea Pigs. Med. Sci. Monit. 2016, 22, 2006–2012. [Google Scholar] [CrossRef]
- Yu, N.; Zhao, H.B. Modulation of outer hair cell electromotility by cochlear supporting cells and gap junctions. PLoS ONE 2009, 4, e7923. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, H.B. ATP activates P2X receptors to mediate gap junctional coupling in the cochlea. Biochem. Biophys. Res. Commun. 2012, 426, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Gerevich, Z.; Zadori, Z.S.; Koles, L.; Kopp, L.; Milius, D.; Wirkner, K.; Gyires, K.; Illes, P. Dual Effect of Acid pH on Purinergic P2X3 Receptors Depends on the Histidine 206 Residue. J. Biol. Chem. 2007, 282, 33949–33957. [Google Scholar] [CrossRef] [PubMed]
- Kanjhan, R.; Raybould, N.P.; Jagger, D.J.; Greenwood, D.; Housley, G.D. Allosteric modulation of native cochlear P2X receptors: Insights from comparison with recombinant P2X2 receptors. Audiol. Neuro-Otol. 2003, 8, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Nenov, A.; Bobbin, R.P. Noise exposure alters the response of outer hair cells to ATP. Hear. Res. 1995, 88, 215–221. [Google Scholar] [CrossRef]
- Fukazawa, T. How can the cochlear amplifier be realized by the outer hair cells which have nothing to push against? Hear. Res. 2002, 172, 53–61. [Google Scholar] [CrossRef]
- Nam, J.-H.; Fettiplace, R. Optimal electrical properties of outer hair cells ensure cochlear amplification. PLoS ONE 2012, 7, e50572. [Google Scholar] [CrossRef]
- Zhu, Y.; Liang, C.; Chen, J.; Zong, L.; Chen, G.-D.; Zhao, H.-B. Active cochlear amplification is dependent on supporting cell gap junctions. Nat. Commun. 2013, 4, 1786. [Google Scholar] [CrossRef]
- Mahendrasingam, S.; Beurg, M.; Fettiplace, R.; Hackney, C.M. The ultrastructural distribution of prestin in outer hair cells: A post-embedding immunogold investigation of low-frequency and high-frequency regions of the rat cochlea. Eur. J. Neurosci. 2010, 31, 1595–1605. [Google Scholar] [CrossRef]
- Xia, A.; Song, Y.; Wang, R.; Gao, S.S.; Clifton, W.; Raphael, P.; Chao, S.; Pereira, F.A.; Groves, A.K.; Oghalai, J.S. Prestin regulation and function in residual outer hair cells after noise-induced hearing loss. PLoS ONE 2013, 8, e82602. [Google Scholar] [CrossRef]
- Homma, K.; Niino, Y.; Hotta, K.; Oka, K. Ca(2+) influx through P2X receptors induces actin cytoskeleton reorganization by the formation of cofilin rods in neurites. Mol. Cell. Neurosci. 2008, 37, 261–270. [Google Scholar] [CrossRef]
- Bobbin, R.P. ATP-induced movement of the stalks of isolated cochlear Deiters’ cells. Neuroreport 2001, 12, 2923–2926. [Google Scholar] [CrossRef] [PubMed]
- Horváth, T.; Polony, G.; Fekete, Á.; Aller, M.; Halmos, G.; Lendvai, B.; Heinrich, A.; Sperlágh, B.; Vizi, E.S.; Zelles, T. ATP-Evoked Intracellular Ca2+ Signaling of Different Supporting Cells in the Hearing Mouse Hemicochlea. Neurochem. Res. 2016, 41, 364–375. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gale, J.E.; Piazza, V.; Ciubotaru, C.D.; Mammano, F. A Mechanism for Sensing Noise Damage in the Inner Ear. Curr. Biol. 2004, 14, 526–529. [Google Scholar] [CrossRef] [PubMed]
- Lahne, M.; Gale, J.E. Damage-induced cell-cell communication in different cochlear cell types via two distinct ATP-dependent Ca waves. Purinergic Signal. 2010, 6, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.C.Y.; Ryan, A.F. Mechanisms of sensorineural cell damage, death and survival in the cochlea. Front. Aging Neurosci. 2015, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Berekméri, E.; Deák, O.; Téglás, T.; Sághy, É.; Horváth, T.; Aller, M.; Fekete, Á.; Köles, L.; Zelles, T. Targeted single-cell electroporation loading of Ca2+ indicators in the mature hemicochlea preparation. Hear. Res. 2019, 371, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Anselmi, F.; Hernandez, V.H.; Crispino, G.; Seydel, A.; Ortolano, S.; Roper, S.D.; Kessaris, N.; Richardson, W.; Rickheit, G.; Filippov, M.A.; et al. ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear. Proc. Natl. Acad. Sci. USA 2008, 105, 18770–18775. [Google Scholar] [CrossRef] [PubMed]
- Gossman, D.G.; Zhao, H.-B. Hemichannel-Mediated Inositol 1,4,5-Trisphosphate (IP3) Release in the Cochlea: A Novel Mechanism of IP3 Intercellular Signaling. Cell Commun. Adhes. 2008, 15, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Lahne, M.; Gale, J.E. Damage-induced activation of ERK1/2 in cochlear supporting cells is a hair cell death-promoting signal that depends on extracellular ATP and calcium. J. Neurosci. 2008, 28, 4918–4928. [Google Scholar] [CrossRef]
- Majumder, P.; Crispino, G.; Rodriguez, L.; Ciubotaru, C.D.; Anselmi, F.; Piazza, V.; Bortolozzi, M.; Mammano, F. ATP-mediated cell-cell signaling in the organ of Corti: The role of connexin channels. Purinergic Signal. 2010, 6, 167–187. [Google Scholar] [CrossRef]
- Mistrík, P.; Ashmore, J.F. Reduced electromotility of outer hair cells associated with connexin-related forms of deafness: An in silico study of a cochlear network mechanism. J. Assoc. Res. Otolaryngol. 2010, 11, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Schacht, J. Receptor-mediated release of inositol phosphates in the cochlear and vestibular sensory epithelia of the rat. Hear. Res. 1993, 69, 207–214. [Google Scholar] [CrossRef][Green Version]
- Sirko, P.; Gale, J.E.; Ashmore, J.F. Intercellular Ca2+ signalling in the adult mouse cochlea. J. Physiol. 2019, 597, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.K.; Rouse, S.L. Sound-Induced Intracellular Ca2+ Dynamics in the Adult Hearing Cochlea. PLoS ONE 2016, 11, e0167850. [Google Scholar] [CrossRef] [PubMed]
- De Bock, M.; Decrock, E.; Wang, N.; Bol, M.; Vinken, M.; Bultynck, G.; Leybaert, L. The dual face of connexin-based astroglial Ca(2+) communication: A key player in brain physiology and a prime target in pathology. Biochim. Biophys. Acta 2014, 1843, 2211–2232. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Parker, M.S.; Barnes, A.P.; Deininger, P.; Bobbin, R.P. Functional expression of three P2X(2) receptor splice variants from guinea pig cochlea. J. Neurophysiol. 2000, 83, 1502–1509. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dayaratne, M.W.N.; Vlajkovic, S.M.; Lipski, J.; Thorne, P.R. Kölliker’s organ and the development of spontaneous activity in the auditory system: Implications for hearing dysfunction. BioMed Res. Int. 2014, 2014, 367939. [Google Scholar] [CrossRef]
- Kreinest, M.; Müller, B.; Winkelhoff, J.; Friauf, E.; Löhrke, S. Miniature EPSCs in the lateral superior olive before hearing onset: Regional and cell-type-specific differences and heterogeneous neuromodulatory effects of ATP. Brain Res. 2009, 1295, 21–36. [Google Scholar] [CrossRef]
- Searchfield, G.D.; Muñoz, D.J.B.; Thorne, P.R. Ensemble spontaneous activity in the guinea-pig cochlear nerve. Hear. Res. 2004, 192, 23–35. [Google Scholar] [CrossRef]
- Wang, H.C.; Lin, C.-C.; Cheung, R.; Zhang-Hooks, Y.; Agarwal, A.; Ellis-Davies, G.; Rock, J.; Bergles, D.E. Spontaneous Activity of Cochlear Hair Cells Triggered by Fluid Secretion Mechanism in Adjacent Support Cells. Cell 2015, 163, 1348–1359. [Google Scholar] [CrossRef]
- Dayaratne, M.W.N.; Vlajkovic, S.M.; Lipski, J.; Thorne, P.R. Putative role of border cells in generating spontaneous morphological activity within Kölliker’s organ. Hear. Res. 2015, 330, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Forge, A.; Jagger, D.J.; Kelly, J.J.; Taylor, R.R. Connexin30-mediated intercellular communication plays an essential role in epithelial repair in the cochlea. J. Cell Sci. 2013, 126, 1703–1712. [Google Scholar] [CrossRef] [PubMed]
- Forge, A.; Becker, D.; Casalotti, S.; Edwards, J.; Marziano, N.; Nickel, R. Connexins and Gap Junctions in the Inner Ear. Audiol. Neurotol. 2002, 7, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Lazarowski, E.R. Vesicular and conductive mechanisms of nucleotide release. Purinergic Signal. 2012, 8, 359–373. [Google Scholar] [CrossRef] [PubMed]
- Dale, N. Dynamic ATP signalling and neural development. J. Physiol. 2008, 586, 2429–2436. [Google Scholar] [CrossRef] [PubMed]
- Delacroix, L.; Malgrange, B. Cochlear afferent innervation development. Hear. Res. 2015, 330, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Housley, G.D.; Jagger, D.J.; Greenwood, D.; Raybould, N.P.; Salih, S.G.; Järlebark, L.E.; Vlajkovic, S.M.; Kanjhan, R.; Nikolic, P.; Muñoz, D.J.M.; et al. Purinergic regulation of sound transduction and auditory neurotransmission. Audiol. Neuro-Otol. 2002, 7, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, S.; Radulovic, T.; Coddou, C.; Dietz, B.; Nerlich, J.; Stojilkovic, S.S.; Rübsamen, R.; Milenkovic, I. Tonotopic action potential tuning of maturing auditory neurons through endogenous ATP. J. Physiol. 2017, 595, 1315–1337. [Google Scholar] [CrossRef] [PubMed]
- Robertson, D.; Paki, B. A Role for Purinergic Receptors at the Inner Hair Cell-Afferent Synapse? Audiol. Neurotol. 2002, 7, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Dulon, D.; Jagger, D.J.; Lin, X.; Davis, R.L. Neuromodulation in the spiral ganglion: Shaping signals from the organ of corti to the CNS. J. Membr. Biol. 2006, 209, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Johnson Chacko, L.; Blumer, M.J.F.; Pechriggl, E.; Rask-Andersen, H.; Dietl, W.; Haim, A.; Fritsch, H.; Glueckert, R.; Dudas, J.; Schrott-Fischer, A. Role of BDNF and neurotrophic receptors in human inner ear development. Cell Tissue Res. 2017, 370, 347–363. [Google Scholar] [CrossRef] [PubMed]
- Pirvola, U.; Ylikoski, J. Neurotrophic factors during inner ear development. Curr. Top. Dev. Biol. 2003, 57, 207–223. [Google Scholar] [PubMed]
- Cunha, R.A. Adenosine as a neuromodulator and as a homeostatic regulator in the nervous system: Different roles, different sources and different receptors. Neurochem. Int. 2001, 38, 107–125. [Google Scholar] [CrossRef]
- Muñoz, D.J.B.; McFie, C.; Thorne, P.R. Modulation of cochlear blood flow by extracellular purines. Hear. Res. 1999, 127, 55–61. [Google Scholar] [CrossRef]
- Sheffield, A.M.; Smith, R.J.H. The Epidemiology of Deafness. Cold Spring Harb. Perspect. Med. 2018, a033258. [Google Scholar] [CrossRef]
- Radigan, E.A.; Gilchrist, N.A.; Miller, M.A. Management of Aminoglycosides in the Intensive Care Unit. J. Intensive Care Med. 2010, 25, 327–342. [Google Scholar] [CrossRef]
- O’Sullivan, M.E.; Perez, A.; Lin, R.; Sajjadi, A.; Ricci, A.J.; Cheng, A.G. Towards the Prevention of Aminoglycoside-Related Hearing Loss. Front. Cell. Neurosci. 2017, 11, 325. [Google Scholar] [CrossRef]
- Guthrie, O.W. Aminoglycoside induced ototoxicity. Toxicology 2008, 249, 91–96. [Google Scholar] [CrossRef]
- Jiang, M.; Taghizadeh, F.; Steyger, P.S. Potential Mechanisms Underlying Inflammation-Enhanced Aminoglycoside-Induced Cochleotoxicity. Front. Cell. Neurosci. 2017, 11, 362. [Google Scholar] [CrossRef]
- Schacht, J.; Talaska, A.E.; Rybak, L.P. Cisplatin and aminoglycoside antibiotics: Hearing loss and its prevention. Anat. Rec. 2012, 295, 1837–1850. [Google Scholar] [CrossRef]
- Duan, M.; Agerman, K.; Ernfors, P.; Canlon, B. Complementary roles of neurotrophin 3 and a N-methyl-d-aspartate antagonist in the protection of noise and aminoglycoside-induced ototoxicity. Proc. Natl. Acad. Sci. USA 2000, 97, 7597–7602. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Park, S.K.; Cho, Y.-S.; Lee, H.-S.; Kim, K.R.; Kim, M.G.; Chung, W.-H. Gentamicin induced nitric oxide-related oxidative damages on vestibular afferents in the guinea pig. Hear. Res. 2006, 211, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Sha, S.-H.; Forge, A.; Schacht, J. Caspase-independent pathways of hair cell death induced by kanamycin in vivo. Cell Death Differ. 2006, 13, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Hume, R.I.; Nuttall, A.L. Voltage-dependent block by neomycin of the ATP-induced whole cell current of guinea-pig outer hair cells. J. Neurophysiol. 1993, 70, 1593–1605. [Google Scholar] [CrossRef]
- Ruel, J.; Wang, J.; Rebillard, G.; Eybalin, M.; Lloyd, R.; Pujol, R.; Puel, J.-L. Physiology, pharmacology and plasticity at the inner hair cell synaptic complex. Hear. Res. 2007, 227, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Lipton, P. Ischemic Cell Death in Brain Neurons. Physiol. Rev. 1999, 79, 1431–1568. [Google Scholar] [CrossRef]
- Ohlemiller, K.K.; Wright, J.S.; Dugan, L.L. Early elevation of cochlear reactive oxygen species following noise exposure. Audiol. Neurootol. 1999, 4, 229–236. [Google Scholar] [CrossRef]
- Fekete, A.; Vizi, E.S.; Kovács, K.J.; Lendvai, B.; Zelles, T. Layer-specific differences in reactive oxygen species levels after oxygen-glucose deprivation in acute hippocampal slices. Free Radic. Biol. Med. 2008, 44, 1010–1022. [Google Scholar] [CrossRef]
- Fridberger, A.; Flock, A.; Ulfendahl, M.; Flock, B. Acoustic overstimulation increases outer hair cell Ca2+ concentrations and causes dynamic contractions of the hearing organ. Proc. Natl. Acad. Sci. USA 1998, 95, 7127–7132. [Google Scholar] [CrossRef]
- Hackney, C.M.; Mahendrasingam, S.; Penn, A.; Fettiplace, R. The concentrations of calcium buffering proteins in mammalian cochlear hair cells. J. Neurosci. 2005, 25, 7867–7875. [Google Scholar] [CrossRef]
- Hirose, K.; Liberman, M.C. Lateral wall histopathology and endocochlear potential in the noise-damaged mouse cochlea. J. Assoc. Res. Otolaryngol. 2003, 4, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Robertson, D. Functional significance of dendritic swelling after loud sounds in the guinea pig cochlea. Hear. Res. 1983, 9, 263–278. [Google Scholar] [CrossRef]
- Tan, W.J.; Thorne, P.R.; Vlajkovic, S.M. Noise-induced cochlear inflammation. World J. Otorhinolaryngol. 2013, 3, 89. [Google Scholar] [CrossRef]
- Fujioka, M.; Kanzaki, S.; Okano, H.J.; Masuda, M.; Ogawa, K.; Okano, H. Proinflammatory cytokines expression in noise-induced damaged cochlea. J. Neurosci. Res. 2006, 83, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Tornabene, S.V.; Sato, K.; Pham, L.; Billings, P.; Keithley, E.M. Immune cell recruitment following acoustic trauma. Hear. Res. 2006, 222, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Kurabi, A.; Keithley, E.M.; Housley, G.D.; Ryan, A.F.; Wong, A.C.-Y. Cellular mechanisms of noise-induced hearing loss. Hear. Res. 2017, 349, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Henderson, D.; Hu, B.; Bielefeld, E. Patterns and Mechanisms of Noise-Induced Cochlear Pathology. In Auditory Trauma, Protection, and Repair; Springer: Boston, MA, USA, 2008; pp. 195–217. [Google Scholar]
- Wang, Y.; Hirose, K.; Liberman, M.C. Dynamics of noise-induced cellular injury and repair in the mouse cochlea. J. Assoc. Res. Otolaryngol. 2002, 3, 248–268. [Google Scholar] [CrossRef] [PubMed]
- Thorne, P.R.; Duncan, C.E.; Gavin, J.B. The pathogenesis of stereocilia abnormalities in acoustic trauma. Hear. Res. 1986, 21, 41–49. [Google Scholar] [CrossRef]
- Hu, B.H.; Henderson, D.; Nicotera, T.M. Involvement of apoptosis in progression of cochlear lesion following exposure to intense noise. Hear. Res. 2002, 166, 62–71. [Google Scholar] [CrossRef]
- Henderson, D.; Bielefeld, E.C.; Harris, K.C.; Hu, B.H. The Role of Oxidative Stress in Noise-Induced Hearing Loss. Ear Hear. 2006, 27, 1–19. [Google Scholar] [CrossRef]
- Yamasoba, T.; Lin, F.R.; Someya, S.; Kashio, A.; Sakamoto, T.; Kondo, K. Current concepts in age-related hearing loss: Epidemiology and mechanistic pathways. Hear. Res. 2013, 303, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Verschuur, C.; Agyemang-Prempeh, A.; Newman, T.A. Inflammation is associated with a worsening of presbycusis: Evidence from the MRC national study of hearing. Int. J. Audiol. 2014, 53, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Morton-Jones, R.T.; Vlajkovic, S.M.; Thorne, P.R.; Cockayne, D.A.; Ryan, A.F.; Housley, G.D. Properties of ATP-gated ion channels assembled from P2X2 subunits in mouse cochlear Reissner’s membrane epithelial cells. Purinergic Signal. 2015, 11, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Thorne, P.R.; Muñoz, D.J.B.; Housley, G.D. Purinergic Modulation of Cochlear Partition Resistance and Its Effect on the Endocochlear Potential in the Guinea Pig. J. Assoc. Res. Otolaryngol. 2004, 5, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Kujawa, S.G.; Erostegui, C.; Fallon, M.; Crist, J.; Bobbin, R.P. Effects of adenosine 5′-triphosphate and related agonists on cochlear function. Hear. Res. 1994, 76, 87–100. [Google Scholar] [CrossRef]
- Sugahara, K.; Shimogori, H.; Okuda, T.; Takemoto, T.; Hashimoto, M.; Yamashita, H. Cochlear administration of adenosine triphosphate facilitates recovery from acoustic trauma (temporary threshold shift). ORL. J. Otorhinolaryngol. Relat. Spec. 2004, 66, 80–84. [Google Scholar] [CrossRef]
- Chen, C.; Skellett, R.A.; Fallon, M.; Bobbin, R.P. Additional pharmacological evidence that endogenous ATP modulates cochlear mechanics. Hear. Res. 1998, 118, 47–61. [Google Scholar] [CrossRef]
- Kujawa, S.G.; Fallon, M.; Bobbin, R.P. ATP antagonists cibacron blue, basilen blue and suramin alter sound-evoked responses of the cochlea and auditory nerve. Hear. Res. 1994, 78, 181–188. [Google Scholar] [CrossRef]
- LeBlanc, C.; Bobbin, R.P. An interaction between PPADS, an ATP antagonist, and a moderately intense sound in the cochlea. Hear. Res. 1999, 138, 192–200. [Google Scholar] [CrossRef]
- Bobbin, R.P. PPADS, an ATP antagonist, attenuates the effects of a moderately intense sound on cochlear mechanics. Hear. Res. 2001, 156, 10–16. [Google Scholar] [CrossRef]
- Ford, M.S.; Nie, Z.; Whitworth, C.; Rybak, L.P.; Ramkumar, V. Up-regulation of adenosine receptors in the cochlea by cisplatin. Hear. Res. 1997, 111, 143–152. [Google Scholar] [CrossRef]
- Ramkumar, V.; Whitworth, C.A.; Pingle, S.C.; Hughes, L.F.; Rybak, L.P. Noise induces A1 adenosine receptor expression in the chinchilla cochlea. Hear. Res. 2004, 188, 47–56. [Google Scholar] [CrossRef]
- Whitworth, C.A.; Ramkumar, V.; Jones, B.; Tsukasaki, N.; Rybak, L.P. Protection against cisplatin ototoxicity by adenosine agonists. Biochem. Pharmacol. 2004, 67, 1801–1807. [Google Scholar] [CrossRef] [PubMed]
- Thorne, P.R.; Nuttall, A.L. Alterations in oxygenation of cochlear endolymph during loud sound exposure. Acta Otolaryngol. 1989, 107, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Thorne, P.R.; Nuttall, A.L. Laser Doppler measurements of cochlear blood flow during loud sound exposure in the guinea pig. Hear. Res. 1987, 27, 1–10. [Google Scholar] [CrossRef]
- Okamoto, A.; Tamura, T.; Yokoyama, K.; Kobayashi, N.; Hasegawa, M. Effect of loud sound exposure on the cochlear blood flow. Acta Otolaryngol. 1990, 109, 378–382. [Google Scholar] [CrossRef]
- Ciccone, M.M.; Cortese, F.; Pinto, M.; Di Teo, C.; Fornarelli, F.; Gesualdo, M.; Mezzina, A.; Sabatelli, E.; Scicchitano, P.; Quaranta, N. Endothelial function and cardiovascular risk in patients with idiopathic sudden sensorineural hearing loss. Atherosclerosis 2012, 225, 511–516. [Google Scholar] [CrossRef]
- Tanigawa, T.; Shibata, R.; Ouchi, N.; Kondo, K.; Ishii, M.; Katahira, N.; Kambara, T.; Inoue, Y.; Takahashi, R.; Ikeda, N.; et al. Adiponectin deficiency exacerbates age-related hearing impairment. Cell Death Dis. 2014, 5, e1189. [Google Scholar] [CrossRef]
- Tabuchi, K.; Ito, Z.; Wada, T.; Takahashi, K.; Hara, A.; Kusakari, J. Effect of A1 adenosine receptor agonist upon cochlear dysfunction induced by transient ischemia. Hear. Res. 1999, 136, 86–90. [Google Scholar] [CrossRef]
- Mujica-Mota, M.A.; Gasbarrino, K.; Rappaport, J.M.; Shapiro, R.S.; Daniel, S.J. The effect of caffeine on hearing in a guinea pig model of acoustic trauma. Am. J. Otolaryngol. Head Neck Med. Surg. 2014, 35, 99–105. [Google Scholar] [CrossRef]
- Vlajkovic, S.M.; Lee, K.-H.; Wong, A.C.Y.; Guo, C.X.; Gupta, R.; Housley, G.D.; Thorne, P.R. Adenosine amine congener mitigates noise-induced cochlear injury. Purinergic Signal. 2010, 6, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Vlajkovic, S.M.; Chang, H.; Paek, S.Y.; Chi, H.H.T.; Sreebhavan, S.; Telang, R.S.; Tingle, M.; Housley, G.D.; Thorne, P.R. Adenosine Amine Congener as a Cochlear Rescue Agent. BioMed Res. Int. 2014, 2014, 841489. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Telang, R.S.; Sreebhavan, S.; Tingle, M.; Thorne, P.R.; Vlajkovic, S.M. Pharmacokinetic properties of adenosine amine congener in cochlear perilymph after systemic administration. BioMed Res. Int. 2017, 2017, 8091462. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Y.; Cottingham, C.; McMahon, L.; Jiao, K.; Greengard, P.; Wang, Q. Neurabin scaffolding of adenosine receptor and RGS4 regulates anti-seizure effect of endogenous adenosine. J. Neurosci. 2012, 32, 2683–2695. [Google Scholar] [CrossRef] [PubMed]
- Idzko, M.; Ferrari, D.; Eltzschig, H.K. Nucleotide signalling during inflammation. Nature 2014, 509, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G.; Boeynaems, J.-M. Purinergic signalling and immune cells. Purinergic Signal. 2014, 10, 529–564. [Google Scholar] [CrossRef]
- Cekic, C.; Linden, J. Purinergic regulation of the immune system. Nat. Rev. Immunol. 2016, 16, 177–192. [Google Scholar] [CrossRef]
- Haskó, G.; Cronstein, B. Regulation of Inflammation by Adenosine. Front. Immunol. 2013, 4, 85. [Google Scholar] [CrossRef]
- Linden, J.; Koch-Nolte, F.; Dahl, G. Purine Release, Metabolism, and Signaling in the Inflammatory Response. Annu. Rev. Immunol. 2019, 37, 325–347. [Google Scholar] [CrossRef]
- Jacob, F.; Pérez Novo, C.; Bachert, C.; Van Crombruggen, K. Purinergic signaling in inflammatory cells: P2 receptor expression, functional effects, and modulation of inflammatory responses. Purinergic Signal. 2013, 9, 285–306. [Google Scholar] [CrossRef]
- Beamer, E.; Gölöncsér, F.; Horváth, G.; Bekő, K.; Otrokocsi, L.; Koványi, B.; Sperlágh, B. Purinergic mechanisms in neuroinflammation: An update from molecules to behavior. Neuropharmacology 2016, 104, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Di Virgilio, F.; Vuerich, M. Purinergic signaling in the immune system. Auton. Neurosci. 2015, 191, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Calovi, S.; Mut-Arbona, P.; Sperlágh, B. Microglia and the Purinergic Signaling System. Neuroscience 2018, 405, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Antonioli, L.; Fornai, M.; Blandizzi, C.; Pacher, P.; Haskó, G. Adenosine signaling and the immune system: When a lot could be too much. Immunol. Lett. 2018, 205, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Haskó, G.; Pacher, P. Regulation of Macrophage Function by Adenosine. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Sperlágh, B.; Illes, P. P2X7 receptor: An emerging target in central nervous system diseases. Trends Pharmacol. Sci. 2014, 35, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Surprenant, A.; Rassendren, F.; Kawashima, E.; North, R.A.; Buell, G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science 1996, 272, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, D.; Wesselborg, S.; Bauer, M.K.; Schulze-Osthoff, K. Extracellular ATP activates transcription factor NF-kappaB through the P2Z purinoreceptor by selectively targeting NF-kappaB p65. J. Cell Biol. 1997, 139, 1635–1643. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, D.; Stroh, C.; Schulze-Osthoff, K. P2X7/P2Z purinoreceptor-mediated activation of transcription factor NFAT in microglial cells. J. Biol. Chem. 1999, 274, 13205–13210. [Google Scholar] [CrossRef] [PubMed]
- Adinolfi, E.; Giuliani, A.L.; De Marchi, E.; Pegoraro, A.; Orioli, E.; Di Virgilio, F. The P2X7 receptor: A main player in inflammation. Biochem. Pharmacol. 2018, 151, 234–244. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Chiozzi, P.; Falzoni, S.; Ferrari, D.; Sanz, J.M.; Venketaraman, V.; Baricordi, O.R. Cytolytic P2X purinoceptors. Cell Death Differ. 1998, 5, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Corriden, R.; Inoue, Y.; Yip, L.; Hashiguchi, N.; Zinkernagel, A.; Nizet, V.; Insel, P.A.; Junger, W.G. ATP Release Guides Neutrophil Chemotaxis via P2Y2 and A3 Receptors. Science 2006, 314, 1792–1795. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M.R.; Chekeni, F.B.; Trampont, P.C.; Lazarowski, E.R.; Kadl, A.; Walk, S.F.; Park, D.; Woodson, R.I.; Ostankovich, M.; Sharma, P.; et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 2009, 461, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Oshima, T.; Fukui, H.; Watari, J.; Miwa, H. Adenosine triphosphate induces P2Y2 activation and interleukin-8 release in human esophageal epithelial cells. J. Gastroenterol. Hepatol. 2017, 32, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Amadio, S.; Parisi, C.; Montilli, C.; Carrubba, A.S.; Apolloni, S.; Volonté, C. P2Y(12) receptor on the verge of a neuroinflammatory breakdown. Mediators Inflamm. 2014, 2014, 975849. [Google Scholar] [CrossRef] [PubMed]
- Haynes, S.E.; Hollopeter, G.; Yang, G.; Kurpius, D.; Dailey, M.E.; Gan, W.-B.; Julius, D. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat. Neurosci. 2006, 9, 1512–1519. [Google Scholar] [CrossRef] [PubMed]
- Eyo, U.B.; Peng, J.; Swiatkowski, P.; Mukherjee, A.; Bispo, A.; Wu, L.-J. Neuronal hyperactivity recruits microglial processes via neuronal NMDA receptors and microglial P2Y12 receptors after status epilepticus. J. Neurosci. 2014, 34, 10528–10540. [Google Scholar] [CrossRef]
- Ben Addi, A.; Cammarata, D.; Conley, P.B.; Boeynaems, J.-M.; Robaye, B. Role of the P2Y12 Receptor in the Modulation of Murine Dendritic Cell Function by ADP. J. Immunol. 2010, 185, 5900–5906. [Google Scholar] [CrossRef]
- Liverani, E.; Rico, M.C.; Yaratha, L.; Tsygankov, A.Y.; Kilpatrick, L.E.; Kunapuli, S.P. LPS-induced systemic inflammation is more severe in P2Y12 null mice. J. Leukoc. Biol. 2014, 95, 313–323. [Google Scholar] [CrossRef]
- Webster, C.M.; Hokari, M.; McManus, A.; Tang, X.N.; Ma, H.; Kacimi, R.; Yenari, M.A. Microglial P2Y12 deficiency/inhibition protects against brain ischemia. PLoS ONE 2013, 8, e70927. [Google Scholar] [CrossRef]
- Fischer, W.; Krügel, U. P2Y receptors: Focus on structural, pharmacological and functional aspects in the brain. Curr. Med. Chem. 2007, 14, 2429–2455. [Google Scholar] [CrossRef] [PubMed]
- Guzman, S.J.; Schmidt, H.; Franke, H.; Krügel, U.; Eilers, J.; Illes, P.; Gerevich, Z. P2Y1 receptors inhibit long-term depression in the prefrontal cortex. Neuropharmacology 2010, 59, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Wirkner, K.; Köles, L.; Thümmler, S.; Luthardt, J.; Poelchen, W.; Franke, H.; Fürst, S.; Illes, P. Interaction between P2Y and NMDA receptors in layer V pyramidal neurons of the rat prefrontal cortex. Neuropharmacology 2002, 42, 476–488. [Google Scholar] [CrossRef]
- Krügel, U.; Köles, L.; Illés, P. Integration of neuronal and glial signalling by pyramidal cells of the rat prefrontal cortex; control of cognitive functions and addictive behaviour by purinergic mechanisms. Neuropsychopharmacol. Hung. 2013, 15, 206–213. [Google Scholar] [PubMed]
- Fujita, T.; Tozaki-Saitoh, H.; Inoue, K. P2Y1 receptor signaling enhances neuroprotection by astrocytes against oxidative stress via IL-6 release in hippocampal cultures. Glia 2009, 57, 244–257. [Google Scholar] [CrossRef] [PubMed]
- De Simone, R.; Niturad, C.E.; De Nuccio, C.; Ajmone-Cat, M.A.; Visentin, S.; Minghetti, L. TGF-β and LPS modulate ADP-induced migration of microglial cells through P2Y1 and P2Y12 receptor expression. J. Neurochem. 2010, 115, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Cronstein, B.N.; Kramer, S.B.; Weissmann, G.; Hirschhorn, R. Adenosine: A physiological modulator of superoxide anion generation by human neutrophils. J. Exp. Med. 1983, 158, 1160–1177. [Google Scholar] [CrossRef]
- Schrier, D.J.; Imre, K.M. The effects of adenosine agonists on human neutrophil function. J. Immunol. 1986, 137, 3284–3289. [Google Scholar]
- Bao, Y.; Chen, Y.; Ledderose, C.; Li, L.; Junger, W.G. Pannexin 1 channels link chemoattractant receptor signaling to local excitation and global inhibition responses at the front and back of polarized neutrophils. J. Biol. Chem. 2013, 288, 22650–22657. [Google Scholar] [CrossRef]
- Peachell, P.T.; Lichtenstein, L.M.; Schleimer, R.P. Differential regulation of human basophil and lung mast cell function by adenosine. J. Pharmacol. Exp. Ther. 1991, 256, 717–726. [Google Scholar]
- Haskó, G.; Pacher, P.; Deitch, E.A.; Vizi, E.S. Shaping of monocyte and macrophage function by adenosine receptors. Pharmacol. Ther. 2007, 113, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Orr, A.G.; Orr, A.L.; Li, X.-J.; Gross, R.E.; Traynelis, S.F. Adenosine A(2A) receptor mediates microglial process retraction. Nat. Neurosci. 2009, 12, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Haskó, G.; Linden, J.; Cronstein, B.; Pacher, P. Adenosine receptors: Therapeutic aspects for inflammatory and immune diseases. Nat. Rev. Drug Discov. 2008, 7, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Zuo, A.; Shao, H.; Chen, M.; Kaplan, H.J.; Sun, D. Anti-Inflammatory or Proinflammatory Effect of an Adenosine Receptor Agonist on the Th17 Autoimmune Response Is Inflammatory Environment–Dependent. J. Immunol. 2014, 193, 5498–5505. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.-S.; Zhou, Y.-G. Adenosine 2A receptor: A crucial neuromodulator with bidirectional effect in neuroinflammation and brain injury. Rev. Neurosci. 2011, 22, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Hindley, S.; Herman, M.A.R.; Rathbone, M.P. Stimulation of reactive astrogliosis in vivo by extracellular adenosine diphosphate or an adenosine A2 receptor agonist. J. Neurosci. Res. 1994, 38, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Chiu, G.S.; Darmody, P.T.; Walsh, J.P.; Moon, M.L.; Kwakwa, K.A.; Bray, J.K.; McCusker, R.H.; Freund, G.G. Adenosine through the A2A adenosine receptor increases IL-1β in the brain contributing to anxiety. Brain Behav. Immun. 2014, 41, 218–231. [Google Scholar] [CrossRef]
- Rebola, N.; Simões, A.P.; Canas, P.M.; Tomé, A.R.; Andrade, G.M.; Barry, C.E.; Agostinho, P.M.; Lynch, M.A.; Cunha, R.A. Adenosine A2A receptors control neuroinflammation and consequent hippocampal neuronal dysfunction. J. Neurochem. 2011, 117, 100–111. [Google Scholar] [CrossRef]
- Rock, K.L.; Latz, E.; Ontiveros, F.; Kono, H. The sterile inflammatory response. Annu. Rev. Immunol. 2010, 28, 321–342. [Google Scholar] [CrossRef]
- Kono, H.; Rock, K.L. How dying cells alert the immune system to danger. Nat. Rev. Immunol. 2008, 8, 279–289. [Google Scholar] [CrossRef]
- Zitvogel, L.; Kepp, O.; Kroemer, G. Decoding Cell Death Signals in Inflammation and Immunity. Cell 2010, 140, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Lowthian, J.A.; Britt, C.J.; Rance, G.; Lin, F.R.; Woods, R.L.; Wolfe, R.; Nelson, M.R.; Dillon, H.A.; Ward, S.; Reid, C.M.; et al. Slowing the progression of age-related hearing loss: Rationale and study design of the ASPIRIN in HEARING, retinal vessels imaging and neurocognition in older generations (ASPREE-HEARING) trial. Contemp. Clin. Trials 2016, 46, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.C. Clinical implications of inflammatory cytokines in the cochlea: A technical note. Otol. Neurotol. 2002, 23, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.J.T.; Thorne, P.R.; Vlajkovic, S.M. Characterisation of cochlear inflammation in mice following acute and chronic noise exposure. Histochem. Cell Biol. 2016, 146, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Kaur, T.; Zamani, D.; Tong, L.; Rubel, E.W.; Ohlemiller, K.K.; Hirose, K.; Warchol, M.E. Fractalkine Signaling Regulates Macrophage Recruitment into the Cochlea and Promotes the Survival of Spiral Ganglion Neurons after Selective Hair Cell Lesion. J. Neurosci. 2015, 35, 15050–15061. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, J.T.; Nadol, J.B.; McKenna, M.J.; McKenna, M.J. Anti CD163+, Iba1+, and CD68+ Cells in the Adult Human Inner Ear: Normal Distribution of an Unappreciated Class of Macrophages/Microglia and Implications for Inflammatory Otopathology in Humans. Otol. Neurotol. 2016, 37, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Molnar, M.; Garnham, C.; Benav, H.; Rask-Andersen, H. Macrophages in the Human Cochlea: Saviors or Predators-A Study Using Super-Resolution Immunohistochemistry. Front. Immunol. 2018, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Shi, X. Resident macrophages in the cochlear blood-labyrinth barrier and their renewal via migration of bone-marrow-derived cells. Cell Tissue Res. 2010, 342, 21–30. [Google Scholar] [CrossRef]
- Okano, T.; Nakagawa, T.; Kita, T.; Kada, S.; Yoshimoto, M.; Nakahata, T.; Ito, J. Bone marrow-derived cells expressing Iba1 are constitutively present as resident tissue macrophages in the mouse cochlea. J. Neurosci. Res. 2008, 86, 1758–1767. [Google Scholar] [CrossRef]
- Matern, M.; Vijayakumar, S.; Margulies, Z.; Milon, B.; Song, Y.; Elkon, R.; Zhang, X.; Jones, S.M.; Hertzano, R. Gfi1Cre mice have early onset progressive hearing loss and induce recombination in numerous inner ear non-hair cells. Sci. Rep. 2017, 7, 42079. [Google Scholar] [CrossRef]
- Adams, J.C.; Seed, B.; Lu, N.; Landry, A.; Xavier, R.J. Selective activation of nuclear factor kappa B in the cochlea by sensory and inflammatory stress. Neuroscience 2009, 160, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Ichimiya, I.; Yoshida, K.; Hirano, T.; Suzuki, M.; Mogi, G. Significance of spiral ligament fibrocytes with cochlear inflammation. Int. J. Pediatr. Otorhinolaryngol. 2000, 56, 45–51. [Google Scholar] [CrossRef]
- Yoshida, K.; Ichimiya, I.; Suzuki, M.; Mogi, G. Effect of proinflammatory cytokines on cultured spiral ligament fibrocytes. Hear. Res. 1999, 137, 155–159. [Google Scholar] [CrossRef]
- Takaashi, M.; Harris, J.P. Analysis of immunocompetent cells following inner ear immunostimulation. Laryngoscope 1988, 98, 1133–1138. [Google Scholar]
- Yang, W.; Vethanayagam, R.R.; Dong, Y.; Cai, Q.; Hu, B.H. Activation of the antigen presentation function of mononuclear phagocyte populations associated with the basilar membrane of the cochlea after acoustic overstimulation. Neuroscience 2015, 303, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Fleming, J.C.; Norenberg, M.D.; Ramsay, D.A.; Dekaban, G.A.; Marcillo, A.E.; Saenz, A.D.; Pasquale-Styles, M.; Dietrich, W.D.; Weaver, L.C. The cellular inflammatory response in human spinal cords after injury. Brain 2006, 129, 3249–3269. [Google Scholar] [CrossRef]
- Kochanek, P.M.; Hallenbeck, J.M. Polymorphonuclear leukocytes and monocytes/macrophages in the pathogenesis of cerebral ischemia and stroke. Stroke 1992, 23, 1367–1379. [Google Scholar] [CrossRef]
- Warchol, M.E. Interactions between Macrophages and the Sensory Cells of the Inner Ear. Cold Spring Harb. Perspect. Med. 2018, 9, a033555. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Fujioka, M.; Kanzaki, S.; Okano, H.J.; Shibata, S.; Yamashita, D.; Masuda, M.; Mihara, M.; Ohsugi, Y.; Ogawa, K.; et al. Blockade of interleukin-6 signaling suppressed cochlear inflammatory response and improved hearing impairment in noise-damaged mice cochlea. Neurosci. Res. 2010, 66, 345–352. [Google Scholar] [CrossRef]
- Wood, M.B.; Zuo, J. The Contribution of Immune Infiltrates to Ototoxicity and Cochlear Hair Cell Loss. Front. Cell. Neurosci. 2017, 11, 106. [Google Scholar] [CrossRef]
- Satoh, H.; Firestein, G.S.; Billings, P.B.; Harris, J.P.; Keithley, E.M. Tumor Necrosis Factor-α, an Initiator, and Etanercept, an Inhibitor of Cochlear Inflammation. Laryngoscope 2002, 112, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Satoh, H.; Firestein, G.S.; Billings, P.B.; Harris, J.P.; Keithley, E.M. Proinflammatory cytokine expression in the endolymphatic sac during inner ear inflammation. J. Assoc. Res. Otolaryngol. 2003, 4, 139–147. [Google Scholar] [CrossRef] [PubMed]
- So, H.; Kim, H.; Lee, J.-H.; Park, C.; Kim, Y.; Kim, E.; Kim, J.-K.; Yun, K.-J.; Lee, K.-M.; Lee, H.-Y.; et al. Cisplatin cytotoxicity of auditory cells requires secretions of proinflammatory cytokines via activation of ERK and NF-kappaB. J. Assoc. Res. Otolaryngol. 2007, 8, 338–355. [Google Scholar] [CrossRef] [PubMed]
- D’Acquisto, F.; Perretti, M.; Flower, R.J. Annexin-A1: A pivotal regulator of the innate and adaptive immune systems. Br. J. Pharmacol. 2008, 155, 152–169. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.H.K.; Pervaiz, S. Annexin 1: The new face of an old molecule. FASEB J. 2007, 21, 968–975. [Google Scholar] [CrossRef]
- Perretti, M.; D’Acquisto, F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat. Rev. Immunol. 2009, 9, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Kalinec, F.; Webster, P.; Maricle, A.; Guerrero, D.; Chakravarti, D.N.; Chakravarti, B.; Gellibolian, R.; Kalinec, G. Glucocorticoid-stimulated, transcription-independent release of annexin A1 by cochlear Hensen cells. Br. J. Pharmacol. 2009, 158, 1820–1834. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.A.; Vago, J.P.; Teixeira, M.M.; Sousa, L.P. Annexin A1 and the Resolution of Inflammation: Modulation of Neutrophil Recruitment, Apoptosis, and Clearance. J. Immunol. Res. 2016, 2016, 8239258. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zheng, G.; Zheng, H.; Zhou, R.; Zhu, X.; Zhang, Q. Primary Observation of Early Transtympanic Steroid Injection in Patients with Delayed Treatment of Noise-Induced Hearing Loss. Audiol. Neurotol. 2013, 18, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Harrop-Jones, A.; Wang, X.; Fernandez, R.; Dellamary, L.; Ryan, A.F.; LeBel, C.; Piu, F. The Sustained-Exposure Dexamethasone Formulation OTO-104 Offers Effective Protection against Noise-Induced Hearing Loss. Audiol. Neurotol. 2016, 21, 12–21. [Google Scholar] [CrossRef]
- Urrutia, R.A.; Kalinec, F. Biology and pathobiology of lipid droplets and their potential role in the protection of the organ of Corti. Hear. Res. 2015, 330, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Masuda, M.; Nagashima, R.; Kanzaki, S.; Fujioka, M.; Ogita, K.; Ogawa, K. Nuclear factor-kappa B nuclear translocation in the cochlea of mice following acoustic overstimulation. Brain Res. 2006, 1068, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Vethanayagam, R.R.; Yang, S.; Bard, J.; Jamison, J.; Cartwright, D.; Dong, Y.; Hu, B.H. Molecular profile of cochlear immunity in the resident cells of the organ of Corti. J. Neuroinflamm. 2014, 11, 173. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Omelchenko, I.; Shi, X.; Nuttall, A.L. The influence of NF-kappaB signal-transduction pathways on the murine inner ear by acoustic overstimulation. J. Neurosci. Res. 2009, 87, 1832–1840. [Google Scholar] [CrossRef] [PubMed]
- Bas Infante, E.; Channer, G.A.; Telischi, F.F.; Gupta, C.; Dinh, J.T.; Vu, L.; Eshraghi, A.A.; Van De Water, T.R. Mannitol Protects Hair Cells Against Tumor Necrosis Factor α–Induced Loss. Otol. Neurotol. 2012, 33, 1656–1663. [Google Scholar] [CrossRef] [PubMed]
- Sato, E.; Shick, H.E.; Ransohoff, R.M.; Hirose, K. Expression of Fractalkine Receptor CX3CR1 on Cochlear Macrophages Influences Survival of Hair Cells Following Ototoxic Injury. J. Assoc. Res. Otolaryngol. 2010, 11, 223–234. [Google Scholar] [CrossRef]
- Francis, S.P.; Cunningham, L.L. Non-autonomous Cellular Responses to Ototoxic Drug-Induced Stress and Death. Front. Cell. Neurosci. 2017, 11, 252. [Google Scholar] [CrossRef]
- Kanzaki, J.; Ouchi, T. Steroid-responsive bilateral sensorineural hearing loss and immune complexes. Arch. Otorhinolaryngol. 1981, 230, 5–9. [Google Scholar] [CrossRef]
- Nakamoto, T.; Mikuriya, T.; Sugahara, K.; Hirose, Y.; Hashimoto, T.; Shimogori, H.; Takii, R.; Nakai, A.; Yamashita, H. Geranylgeranylacetone suppresses noise-induced expression of proinflammatory cytokines in the cochlea. Auris. Nasus. Larynx 2012, 39, 270–274. [Google Scholar] [CrossRef]
- Hirose, K.; Li, S.-Z.; Ohlemiller, K.K.; Ransohoff, R.M. Systemic lipopolysaccharide induces cochlear inflammation and exacerbates the synergistic ototoxicity of kanamycin and furosemide. J. Assoc. Res. Otolaryngol. 2014, 15, 555–570. [Google Scholar] [CrossRef]
- Warchol, M.E.; Schwendener, R.A.; Hirose, K. Depletion of resident macrophages does not alter sensory regeneration in the avian cochlea. PLoS ONE 2012, 7, e51574. [Google Scholar] [CrossRef] [PubMed]
- Kaur, T.; Mukherjea, D.; Sheehan, K.; Jajoo, S.; Rybak, L.P.; Ramkumar, V. Short interfering RNA against STAT1 attenuates cisplatin-induced ototoxicity in the rat by suppressing inflammation. Cell Death Dis. 2011, 2, e180. [Google Scholar] [CrossRef] [PubMed]
- Oh, G.-S.; Kim, H.-J.; Choi, J.-H.; Shen, A.; Kim, C.-H.; Kim, S.-J.; Shin, S.-R.; Hong, S.-H.; Kim, Y.; Park, C.; et al. Activation of Lipopolysaccharide-TLR4 Signaling Accelerates the Ototoxic Potential of Cisplatin in Mice. J. Immunol. 2011, 186, 1140–1150. [Google Scholar] [CrossRef] [PubMed]
- Fetoni, A.R.; Picciotti, P.M.; Paludetti, G.; Troiani, D. Pathogenesis of presbycusis in animal models: A review. Exp. Gerontol. 2011, 46, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Iwai, H.; Lee, S.; Inaba, M.; Sugiura, K.; Baba, S.; Tomoda, K.; Yamashita, T.; Ikehara, S. Correlation between accelerated presbycusis and decreased immune functions. Exp. Gerontol. 2003, 38, 319–325. [Google Scholar] [CrossRef]
- Iwai, H.; Baba, S.; Omae, M.; Lee, S.; Yamashita, T.; Ikehara, S. Maintenance of systemic immune functions prevents accelerated presbycusis. Brain Res. 2008, 1208, 8–16. [Google Scholar] [CrossRef]
- Backhouse, S.; Coleman, B.; Shepherd, R. Surgical access to the mammalian cochlea for cell-based therapies. Exp. Neurol. 2008, 214, 193–200. [Google Scholar] [CrossRef]
- Verschuur, C.; Causon, A.; Green, K.; Bruce, I.; Agyemang-Prempeh, A.; Newman, T. The role of the immune system in hearing preservation after cochlear implantation. Cochlear Implants Int. 2015, 16 (Suppl. 1), S40–S42. [Google Scholar] [CrossRef]
- Prasad, K.N.; Bondy, S.C. MicroRNAs in Hearing Disorders: Their Regulation by Oxidative Stress, Inflammation and Antioxidants. Front. Cell. Neurosci. 2017, 11, 276. [Google Scholar] [CrossRef]
- Sekiya, T.; Tanaka, M.; Shimamura, N.; Suzuki, S. Macrophage invasion into injured cochlear nerve and its modification by methylprednisolone. Brain Res. 2001, 905, 152–160. [Google Scholar] [CrossRef]
- Lang, H.; Nishimoto, E.; Xing, Y.; Brown, L.N.; Noble, K.V.; Barth, J.L.; LaRue, A.C.; Ando, K.; Schulte, B.A. Contributions of Mouse and Human Hematopoietic Cells to Remodeling of the Adult Auditory Nerve After Neuron Loss. Mol. Ther. 2016, 24, 2000–2011. [Google Scholar] [CrossRef] [PubMed]
- Di Virgilio, F.; Dal Ben, D.; Sarti, A.C.; Giuliani, A.L.; Falzoni, S. The P2X7 Receptor in Infection and Inflammation. Immunity 2017, 47, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.T.G.; Lee, M.M.G.; Ruan, R. Bone marrow-derived cells that home to acoustic deafened cochlea preserved their hematopoietic identity. J. Comp. Neurol. 2008, 509, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Riva, C.; Donadieu, E.; Magnan, J.; Lavieille, J.-P. Age-related hearing loss in CD/1 mice is associated to ROS formation and HIF target proteins up-regulation in the cochlea. Exp. Gerontol. 2007, 42, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Sautter, N.B.; Shick, E.H.; Ransohoff, R.M.; Charo, I.F.; Hirose, K. CC Chemokine Receptor 2 is Protective Against Noise-Induced Hair Cell Death: Studies in CX3CR1+/GFP Mice. J. Assoc. Res. Otolaryngol. 2006, 7, 361–372. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Du, X.; Choi, C.-H.; Chen, K.; Cheng, W.; Floyd, R.A.; Kopke, R.D. Reduced formation of oxidative stress biomarkers and migration of mononuclear phagocytes in the cochleae of chinchilla after antioxidant treatment in acute acoustic trauma. Int. J. Otolaryngol. 2011, 2011, 612690. [Google Scholar] [CrossRef]
- Ladrech, S.; Wang, J.; Simonneau, L.; Puel, J.-L.; Lenoir, M. Macrophage contribution to the response of the rat organ of Corti to amikacin. J. Neurosci. Res. 2007, 85, 1970–1979. [Google Scholar] [CrossRef] [PubMed]
- Hirose, K.; Sato, E. Comparative analysis of combination kanamycin-furosemide versus kanamycin alone in the mouse cochlea. Hear. Res. 2011, 272, 108–116. [Google Scholar] [CrossRef]
- Kaur, T.; Ohlemiller, K.K.; Warchol, M.E. Genetic disruption of fractalkine signaling leads to enhanced loss of cochlear afferents following ototoxic or acoustic injury. J. Comp. Neurol. 2018, 526, 824–835. [Google Scholar] [CrossRef]
- Koo, J.-W.; Wang, Q.; Steyger, P.S. Infection-mediated vasoactive peptides modulate cochlear uptake of fluorescent gentamicin. Audiol. Neurootol. 2011, 16, 347–358. [Google Scholar] [CrossRef]
- Köröskényi, K.; Duró, E.; Pallai, A.; Sarang, Z.; Kloor, D.; Ucker, D.S.; Beceiro, S.; Castrillo, A.; Chawla, A.; Ledent, C.A.; et al. Involvement of adenosine A2A receptors in engulfment-dependent apoptotic cell suppression of inflammation. J. Immunol. 2011, 186, 7144–7155. [Google Scholar] [CrossRef] [PubMed]
- Csóka, B.; Selmeczy, Z.; Koscsó, B.; Németh, Z.H.; Pacher, P.; Murray, P.J.; Kepka-Lenhart, D.; Morris, S.M.; Gause, W.C.; Leibovich, S.J.; et al. Adenosine promotes alternative macrophage activation via A2A and A2B receptors. FASEB J. 2012, 26, 376–386. [Google Scholar] [CrossRef] [PubMed]
| P2 Receptor Subtypes | Presence in the Cochlea (P < 15) | |||||||||||
| Species | Detected | OHC | IHC | SGN | IBC & IPhC | PC | DC | HC | OSC | SV | RM | |
| P2X1 | rat | protein | [61] | [61] | ||||||||
| P2X2 | rat | mRNA | [47,52] | [47,52] | [47,52,56,57] | [47] | [47] | [47,52] | [47] | [47] | [47] | [47] |
| mouse | protein | [42,49] | [49] | [49] | [49] | |||||||
| function | [46] | |||||||||||
| P2X3 | rat | mRNA | [56,57] | |||||||||
| protein | [60] | |||||||||||
| mouse | protein | [65] | [65] | [65] | ||||||||
| P2X4 | rat | mRNA | [56,57] | |||||||||
| P2X7 | rat | mRNA | [56] | |||||||||
| protein | [62] | |||||||||||
| P2Y1 | rat | protein | [66] | |||||||||
| P2Y2 | rat | protein | [66] | [66] | [66] | [66] | [66] | [66] | ||||
| function | [67] | [67] | ||||||||||
| P2Y4 | rat | protein | [66,68] | [66,68] | [66,68] | [68] | [66] | |||||
| function | [67] | [67] | ||||||||||
| P2Y6 | rat | protein | [66,68] | [66,68] | [66] | [68] | [68] | [66] | ||||
| P2Y12 | rat | protein | [66] | [66] | [66] | [66] | ||||||
| P2Y14 | rat | protein | [68] | [68] | [68] | [68] | ||||||
| P2 receptor subtypes | Presence in the cochlea (P > 15) | |||||||||||
| Species | Detected | OHC | IHC | SGN | IBC & IPhC | PC | DC | HC | OSC | SV | RM | |
| P2X1 | rat | protein | [58] | |||||||||
| guinea pig | protein | [40,44] | ||||||||||
| P2X2 | rat | mRNA | [10,47] | [10,47] | [10,47,56] | [47] | [10,47] | [10,47] | [47] | [47] | [10] | [10,47] |
| protein | [50] | [10,58,59] | [10] | [10,50,59] | [50] | [50] | [58] | [10] | ||||
| mouse | protein | [41,48] | [48] | [41] | [48] | [41,48] | [41,48] | [48] | [48] | [41,48] | ||
| guinea pig | mRNA | [51] | [51] | [51] | [51] | [51] | [51] | |||||
| protein | [37,40,43,44] | [37,45] | [37] | [37] | [37] | |||||||
| function | [53] | [45,53] | [53] | [54] | ||||||||
| P2X3 | rat | mRNA | [56] | |||||||||
| protein | [58] | |||||||||||
| P2X4 | rat | mRNA | [56] | |||||||||
| protein | [58] | |||||||||||
| guinea pig | protein | [40,44] | ||||||||||
| P2X7 | rat | mRNA | [56] | |||||||||
| protein | [62] | [62] | [62] | [62] | ||||||||
| guinea pig | protein | [40,43,44] | ||||||||||
| function | [55] | [55] | [55] | [55] | ||||||||
| P2Y1 | rat | protein | [66] | [66] | [66] | |||||||
| guinea pig | protein | [40,44] | ||||||||||
| P2Y2 | rat | protein | [66] | [66] | ||||||||
| guinea pig | protein | [40,44] | ||||||||||
| P2Y4 | rat | protein | [66,68] | [66,68] | [66,68] | [66] | [66] | |||||
| function | [69] | |||||||||||
| guinea pig | protein | [40,44] | [70] | [70] | ||||||||
| P2Y6 | rat | protein | [68] | [66,68] | [66,68] | [66] | [66] | |||||
| P2Y12 | rat | protein | [66] | [66] | ||||||||
| P2Y14 | rat | protein | [68] | [68] | ||||||||
| Receptor Subtypes | Presence in the Cochlea (P > 15) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Detected | OHC | IHC | SGN | IBC & IPhC | PC | DC | HC | OSC | SV | RM | |
| A1 | rat | mRNA | [71] | [71] | ||||||||
| protein | [71] | [71,72,73] | [72,73] | [72,73] | ||||||||
| function | [71] | [71] | ||||||||||
| mouse | function | [74] | [74] | |||||||||
| guinea pig | function | [75] | [75] | [75] | ||||||||
| A2A | rat | protein | [72,73] | [72,73] | [72,73] | |||||||
| mouse | protein | [76] | [76] | |||||||||
| function | [74] | [74] | ||||||||||
| A3 | rat | protein | [72,73] | [72,73] | [72,73] | [73] | [72,73] | [72,73] | [72,73] | |||
| Ectonucleotidases | Presence in the Cochlea (P < 15) | |||||||||||
| Species | Detected | OHC | IHC | SGN | IBC & IPhC | PC | DC | HC | OSC | SV | RM | |
| NTPDase5 | rat | protein | [68] | [68] | [68] | [68] | [68] | [68] | [68] | [68] | [68] | |
| NTPDase6 | rat | protein | [68] | [68] | ||||||||
| Ectonucleotidases | Presence in the cochlea (P > 15) | |||||||||||
| NTPDase1 | rat | protein | [77] | [77] | [77] | [77] | [77] | [77] | [78] | |||
| mouse | protein | [78,79,80] | [79,80] | |||||||||
| NTPDase2 | rat | protein | [77] | [77] | [77] | [77,78] | ||||||
| mouse | protein | [80] | [80] | [78] | [80] | |||||||
| NTPDase3 | rat | protein | [81] | [81] | [81] | [81] | [81] | |||||
| NTPDase5 | rat | protein | [68,82] | [82] | [68] | [68,82] | ||||||
| NTPDase6 | rat | protein | [68,82] | |||||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Köles, L.; Szepesy, J.; Berekméri, E.; Zelles, T. Purinergic Signaling and Cochlear Injury-Targeting the Immune System? Int. J. Mol. Sci. 2019, 20, 2979. https://doi.org/10.3390/ijms20122979
Köles L, Szepesy J, Berekméri E, Zelles T. Purinergic Signaling and Cochlear Injury-Targeting the Immune System? International Journal of Molecular Sciences. 2019; 20(12):2979. https://doi.org/10.3390/ijms20122979
Chicago/Turabian StyleKöles, László, Judit Szepesy, Eszter Berekméri, and Tibor Zelles. 2019. "Purinergic Signaling and Cochlear Injury-Targeting the Immune System?" International Journal of Molecular Sciences 20, no. 12: 2979. https://doi.org/10.3390/ijms20122979
APA StyleKöles, L., Szepesy, J., Berekméri, E., & Zelles, T. (2019). Purinergic Signaling and Cochlear Injury-Targeting the Immune System? International Journal of Molecular Sciences, 20(12), 2979. https://doi.org/10.3390/ijms20122979






