Biphasic Effect of Sildenafil on Energy Sensing is Mediated by Phosphodiesterases 2 and 3 in Adipocytes and Hepatocytes
Abstract
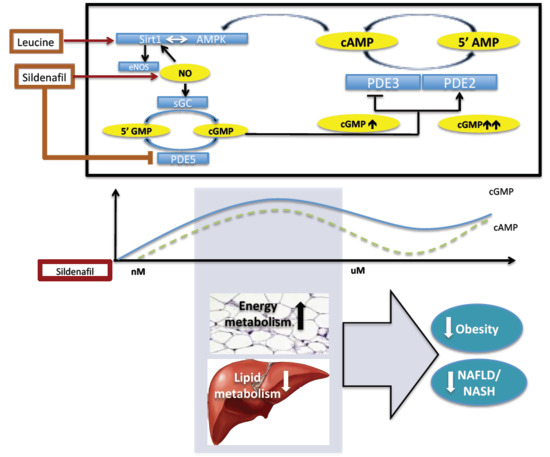
:1. Introduction
2. Results
2.1. The Biphasic Response of cAMP to Increasing Sildenafil Concentrations is Mediated by PDE2
2.2. The Biphasic cAMP Response to Sildenafil is Reflected in Downstream Effects
2.3. Knockdown of PDE2 Prevents the Biphasic Sildenafil Response of cAMP and Downstream Targets
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. siRNA Transfection
4.3. Gene Expression
4.4. cAMP and cGMP Assay
4.5. Western Blot
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PDE | phosphodiesterase |
| NASH | Nonalcoholic steatohepatitis |
| NAFLD | Nonalcoholic fatty liver disease |
| eNOS | Endothelial nitric oxide synthase |
| NO | Nitric oxide |
| cGMP | Guanosine 3′,5′ cyclic monophosphate |
| cAMP | adenosine 3′,5′ cyclic monophosphate |
| EPAC | cAMP activated guanine-nucleotide exchange protein |
| CamKKβ | calcium/calmodulin-dependent kinase kinase β |
References
- Ekstedt, M.; Hagström, H.; Nasr, P.; Fredrikson, M.; Stål, P.; Kechagias, S.; Hultcrantz, R. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 2015, 61, 1547–1554. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P.; Kleiner, D.E.; Dam-LArsen, S.; Adams, L.A.; Bjornsson, E.S.; Charatcharoenwitthaya, P.; Mills, P.R.; Keach, J.C.; Lafferty, H.D.; Stahler, A.; et al. Liver Fibrosis, but no Other Histologic Features, Associates with Long-Term Outcomes of Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2015, 149, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Cantó, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef] [PubMed]
- Cantó, C.; Auwerx, J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. 2009, 20, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Xu, S.; Maitland-Toolan, K.A.; Sato, K.; Jiang, B.; Ido, Y.; Lan, F.; Walsh, K.; Wierzbicki, M.; Verbeuren, T.J.; et al. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J. Biol. Chem. 2008, 283, 20015–20026. [Google Scholar] [CrossRef] [PubMed]
- Nisoli, E.; Tonello, C.; Cardile, A.; Cozzi, V.; Bracale, R.; Tedesco, L.; Falcone, S.; Valerio, A.; Cantoni, O.; Clementi, E.; et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science 2005, 310, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Shinmura, K.; Tamaki, K.; Bolli, R. Impact of 6-mo caloric restriction on myocardial ischemic tolerance: Possible involvement of nitric oxide-dependent increase in nuclear Sirt1. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H2348–H2355. [Google Scholar] [CrossRef]
- Fu, L.; Bruckbauer, A.; Li, F.; Cao, Q.; Cui, X.; Wu, R.; Shi, H.; Zemel, M.B.; Xue, B. Leucine amplifies the effects of metformin on insulin sensitivity and glycemic control in diet-induced obese mice. Metabolism 2015, 64, 845–856. [Google Scholar] [CrossRef]
- Bruckbauer, A.; Banerjee, J.; Fu, L.; Li, F.; Cao, Q.; Cui, X.; Wu, R.; Shi, H.; Xue, B.; Zemel, M.B. A Combination of Leucine, Metformin, and Sildenafil Treats Nonalcoholic Fatty Liver Disease and Steatohepatitis in Mice. Int. J. Hepatol. 2016, 2016, 9185987. [Google Scholar] [CrossRef]
- Fu, L.; Bruckbauer, A.; Li, F.; Cao, Q.; Cui, X.; Wu, R.; Shi, H.; Zemel, M.B.; Xue, B. Interaction between metformin and leucine in reducing hyperlipidemia and hepatic lipid accumulation in diet-induced obese mice. Metabolism 2015, 64, 1426–1434. [Google Scholar] [CrossRef]
- Fu, L.; Li, F.; Bruckbauer, A.; Cao, Q.; Cui, X.; Wu, R.; Shi, H.; Xue, B.; Zemel, M.B. Interaction between leucine and phosphodiesterase 5 inhibition in modulating insulin sensitivity and lipid metabolism. Diabetes Metab. Syndr. Obes. 2015, 8, 227–239. [Google Scholar] [PubMed]
- Hiramoto, K.; Murata, T.; Shimizu, K.; Morita, H.; Inui, M.; Manganiello, V.C.; Tagawa, T.; Arai, N. Role of Phosphodiesterase 2 in Growth and Invasion of Human Malignant Melanoma Cells. Cell Signal. 2015, 26, 1807–1817. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, E. Potency, selectivity, and consequences of nonselectivity of PDE inhibition. Int. J. Impot. Res. 2004, 16 (Suppl. 1), S11–S14. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Murata, T.; Simizu, K.; Degerman, E.; Maurice, D.; Manganiello, V. Cyclic nucleotide phosphodiesterases: Important signaling modulators and therapeutic targets. Oral Dis. 2015, 21, e25–e50. [Google Scholar] [CrossRef] [PubMed]
- Vettel, C.; Lämmle, S.; Ewens, S.; Cervirgen, C.; Emons, J.; Ongherth, A.; Dewenter, M.; Lindner, D.; Westermann, D.; Nikolaev, V.O.; et al. PDE2-mediated cAMP hydrolysis accelerates cardiac fibroblast to myofibroblast conversion and is antagonized by exogenous activation of cGMP signaling pathways. Am. J. Physiol. Hear. Circ. Physiol. 2014, 306, H1246–H1252. [Google Scholar] [CrossRef] [PubMed]
- Francis, S.H.; Busch, J.L.; Corbin, J.D.; Sibley, D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol. Rev. 2010, 62, 525–563. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Kovar, A.; Meibohm, B. The clinical pharmacokinetics of phosphodiesterase-5 inhibitors for erectile dysfunction. J. Clin. Pharmacol. 2005, 45, 987–1003. [Google Scholar] [CrossRef]
- Das, A.; Durrant, D.; Salloum, F.N.; Xi, L.; Kukreja, R.C. PDE5 inhibitors as therapeutics for heart disease, diabetes and cancer. Pharmacol. Ther. 2014, 147, 12–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satoh, T.; Saji, T.; Watanabe, H.; Ogawa, S.; Takehara, K.; Tanabe, N.; Yamada, N.; Yao, A.; Miyaji, K.; Nakanishi, N.; et al. A phase III, multicenter, collaborative, open-label clinical trial of sildenafil in Japanese patients with pulmonary arterial hypertension. Circ. J. 2011, 75, 677–682. [Google Scholar] [CrossRef]
- Shalwala, M.; Zhu, S.-G.; Das, A.; Salloum, F.N.; Xi, L.; Kukreja, R.C. Sirtuin 1 (SIRT1) activation mediates sildenafil induced delayed cardioprotection against ischemia-reperfusion injury in mice. PLoS ONE 2014, 9, e86977. [Google Scholar] [CrossRef] [PubMed]
- Aversa, A. Systemic and metabolic effects of PDE5-inhibitor drugs. World J. Diabetes 2010, 1, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Ahmad, F.; Philp, A.; Baar, K.; Williams, T.; Ke, H.; Rehmann, H.; Taussig, R.; Brown, A.L.; Kim, M.K.; et al. Resveratrol Ameliorates Aging-Related Metabolic Phenotypes by inhibiting cAMP Phosphodiesterases. Cell 2012, 148, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Bender, A.T. Cyclic Nucleotide Phosphodiesterases: Molecular Regulation to Clinical Use. Pharmacol. Rev. 2006, 58, 488–520. [Google Scholar] [CrossRef] [PubMed]
- Francis, S.H.; Blount, M.A.; Corbin, J.D. Mammalian Cyclic Nucleotide Phosphodiesterases: Molecular Mechanisms and Physiological Functions. Physiol. Rev. 2011, 91, 651–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, E.J.; Kass, D.A. Cyclic GMP signaling in cardiovascular pathophysiology and therapeutics. Pharmacol. Ther. 2009, 122, 216–238. [Google Scholar] [CrossRef] [Green Version]
- Roscioni, S.S.; Elzinga, C.R.S. Epac: Effectors and biological functions. Naunyn Schmiedebergs Arch. Pharmacol. 2008, 377, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Omar, B.; Zmuda-Trzebiatowska, E.; Manganiello, V.; Göransson, O.; Degerman, E. Regulation of AMP-activated protein kinase by cAMP in adipocytes: Roles for Phosphodiesterases, Protein kinase B, Protein kinase A, Epac and lipolysis. Cell Signal. 2009, 21, 760–766. [Google Scholar] [CrossRef]
- Woods, A.; Dickerson, K.; Heath, R.; Hong, S.; Momcilovic, M.; Johnstone, S.R.; Carlson, M.; Carling, D. Ca2+/calmodulin-dependent protein kinase kinase-β acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005, 2, 21–33. [Google Scholar] [CrossRef]
- Hawley, S.A.; Pan, D.A.; Mustard, K.J.; Ross, L.; Bain, J.; Edelman, A.M.; Frenguelli, B.G.; Hardie, D.G. Calmodulin-dependent protein kinase kinase-β is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005, 2, 9–19. [Google Scholar] [CrossRef]
- Laurent, A.; Bisserier, M.; Lucas, A.; Tortosa, F.; Swiader, A.; Sainte-marie, Y.; Heymes, C.; Vindis, C.; Lezoualc’h, F. Exchange protein directly activated by cAMP 1 promotes autophagy during cardiomyocyte hypertrophy. Cardiovasc. Res. 2015, 105, 55–64. [Google Scholar] [CrossRef]
- Banerjee, J.; Bruckbauer, A.; Zemel, M.B. Activation of the AMPK/Sirt1 pathway by a leucine-metformin combination increases insulin sensitivity in skeletal muscle, and stimulates glucose and lipid metabolism and increases life span in Caenorhabditis elegans. Metabolism 2016, 65, 1679–1691. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Vuppalanchi, R.; Rinella, M.; Middleton, M.S.; Siddiqui, M.; Barritt, A.S.; Kolterman, O.; Flores, O.; Alonso, C.; Iruarrizaga-Lejarreta, M.; et al. Randomised clinical trial: A leucine-metformin-sildenafil combination (NS-0200) vs. placebo in patients with non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2018, 47, 1639–1651. [Google Scholar] [CrossRef] [PubMed]
- Zemel, M.B.; Kolterman, O.; Rinella, M.; Vuppalanchi, R.; Omar, F.; Barritt, S.; Siddiqui, M.; Chalasani, N. Randomized Controlled Trial of a Leucine-Metformin-Sildenafil Combination (NS-0200) on Weight and Metabolic Parameters. Obesity 2019, 27, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Dunkern, T.R.; Hatzelmann, A. The effect of Sildenafil on human platelet secretory function is controlled by a complex interplay between phosphodiesterases 2, 3 and 5. Cell Signal. 2005, 17, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.S.; Guo, M.; Umana, M.B.; Maurice, D.H. Distinct phosphodiesterase 5A-containing compartments allow selective regulation of cGMP-dependent signalling in human arterial smooth muscle cells. Cell Signal. 2017, 36, 204–211. [Google Scholar] [CrossRef]
- Maurice, D.H.; Haslam, R.J. Molecular basis of the synergistic inhibition of platelet function by nitrovasodilators and activators of adenylate cyclase: Inhibition of cyclic AMP breakdown by cyclic GMP. Mol. Pharmacol. 1990, 37, 671–681. [Google Scholar] [PubMed]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Musicki, B.; Bivalacqua, T.J.; Champion, H.C.; Burnett, A.L. Sildenafil promotes eNOS activation and inhibits NADPH oxidase in the transgenic sickle cell mouse penis. J. Sex. Med. 2014, 11, 424–430. [Google Scholar] [CrossRef]
- Isidori, A.M.; Cornacchione, M.; Barbagallo, F.; Grazia, A.; Di Barrios, F.; Fassina, L.; Monaco, L.; Giannetta, E.; Gianfrilli, D.; Garofalo, S.; et al. Inhibition of type 5 phosphodiesterase counteracts b 2-adrenergic signalling in beating cardiomyocytes. Cardiovasc. Res. 2015, 106, 408–420. [Google Scholar] [CrossRef]
- Kawasaki, H.; Springett, G.M.; Mochizuki, N.; Toki, S.; Nakaya, M.; Matsuda, M.; Housman, D.E.; Graybiel, A.M. A family of cAMP-binding proteins that directly activate Rap1. Science 1998, 282, 2275–2279. [Google Scholar] [CrossRef]
- García-morales, V.; Luaces-regueira, M.; Campos-toimil, M. The cAMP effectors PKA and Epac activate endothelial NO synthase through PI3K/Akt pathway in human endothelial cells. Biochem. Pharmacol. 2017, 145, 94–101. [Google Scholar] [CrossRef] [PubMed]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banerjee, J.; Bruckbauer, A.; Thorpe, T.; Zemel, M.B. Biphasic Effect of Sildenafil on Energy Sensing is Mediated by Phosphodiesterases 2 and 3 in Adipocytes and Hepatocytes. Int. J. Mol. Sci. 2019, 20, 2992. https://doi.org/10.3390/ijms20122992
Banerjee J, Bruckbauer A, Thorpe T, Zemel MB. Biphasic Effect of Sildenafil on Energy Sensing is Mediated by Phosphodiesterases 2 and 3 in Adipocytes and Hepatocytes. International Journal of Molecular Sciences. 2019; 20(12):2992. https://doi.org/10.3390/ijms20122992
Chicago/Turabian StyleBanerjee, Jheelam, Antje Bruckbauer, Teresa Thorpe, and Michael B. Zemel. 2019. "Biphasic Effect of Sildenafil on Energy Sensing is Mediated by Phosphodiesterases 2 and 3 in Adipocytes and Hepatocytes" International Journal of Molecular Sciences 20, no. 12: 2992. https://doi.org/10.3390/ijms20122992
APA StyleBanerjee, J., Bruckbauer, A., Thorpe, T., & Zemel, M. B. (2019). Biphasic Effect of Sildenafil on Energy Sensing is Mediated by Phosphodiesterases 2 and 3 in Adipocytes and Hepatocytes. International Journal of Molecular Sciences, 20(12), 2992. https://doi.org/10.3390/ijms20122992