Production, Signaling, and Scavenging Mechanisms of Reactive Oxygen Species in Fruit–Pathogen Interactions
Abstract
:1. Introduction
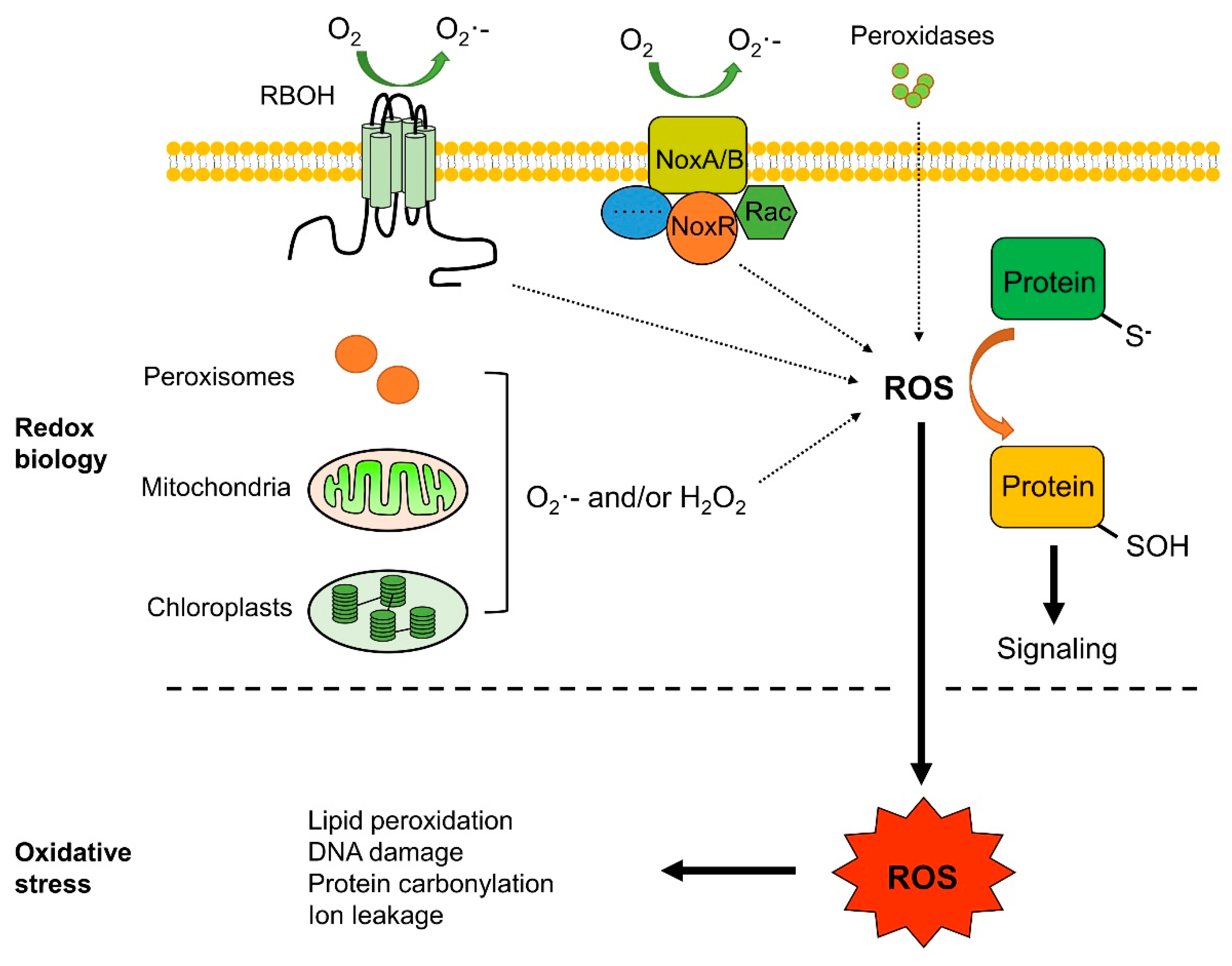
2. ROS Production Sites and Scavenging Systems
3. Roles of ROS in Regulating Fruit Defense Responses
3.1. Antioxidants Participate in Fruit Defense Responses
3.2. ROS–Phytohormone Crosstalk
3.3. ROS–NO Reactions
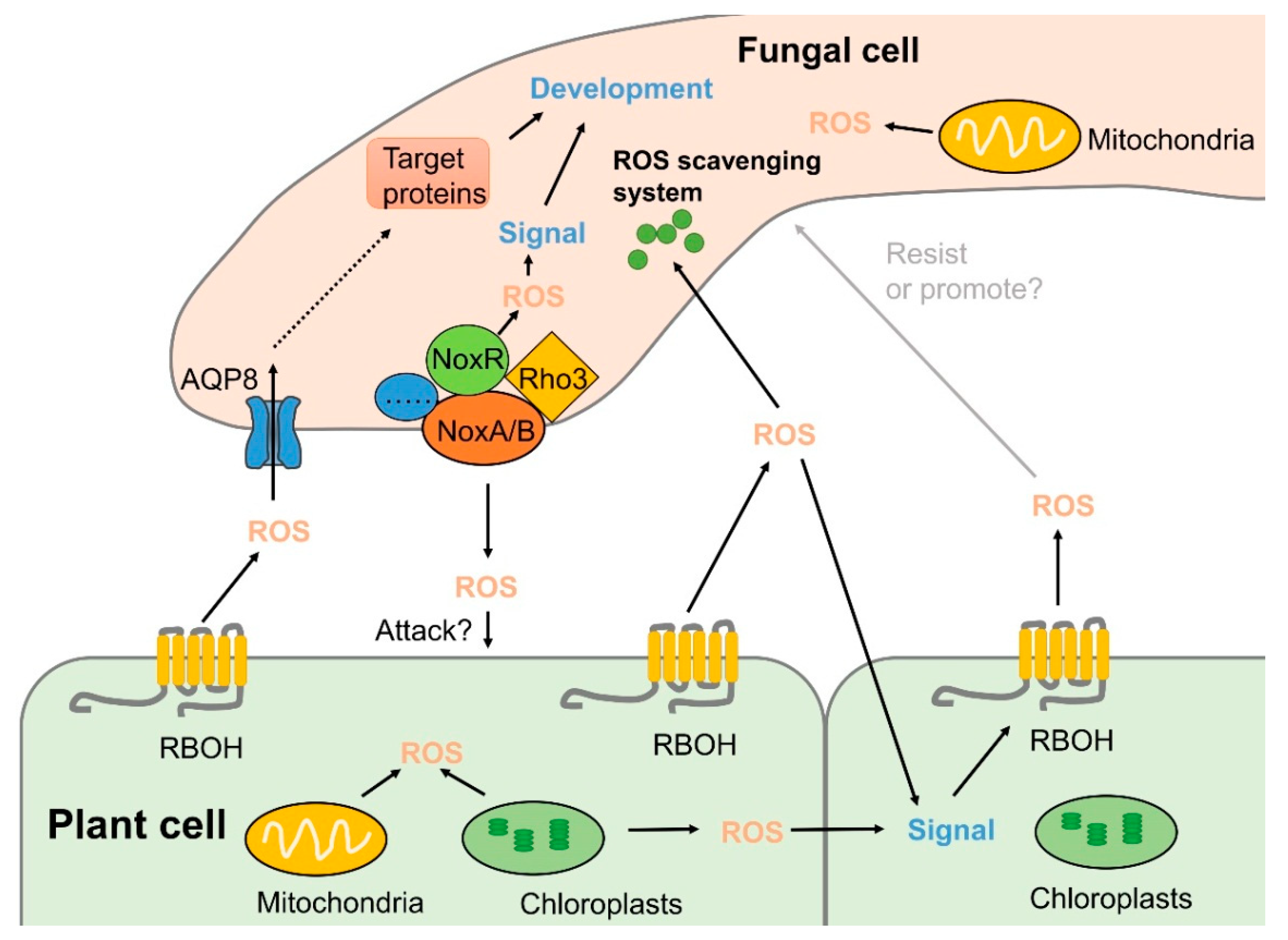
4. Roles of ROS in Fungal Development and Pathogenicity
4.1. Roles of NADPH Oxidases in Pathogens
4.2. Effects of Antioxidants on Fungal Pathogenicity
4.3. ROS Transport Affects Fungal Pathogenicity
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tian, S.P.; Torres, R.; Ballester, A.R.; Li, B.Q.; Vilanova, L.; González-Candelas, L. Molecular aspects in pathogen-fruit interactions: Virulence and resistance. Postharvest Biol. Technol. 2016, 122, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Buron-Moles, G.; Torres, R.; Teixidó, N.; Usall, J.; Vilanova, L.; Viñas, I. Characterisation of H2O2 production to study compatible and non-host pathogen interactions in orange and apple fruit at different maturity stages. Postharvest Biol. Technol. 2015, 99, 27–36. [Google Scholar] [CrossRef]
- Heller, J.; Tudzynski, P. Reactive oxygen species in phytopathogenic fungi: Signaling, development, and disease. Annu. Rev. Phytopathol. 2011, 49, 369–390. [Google Scholar] [CrossRef] [PubMed]
- Camejo, D.; Guzmán-Cedeño, A.; Moreno, A. Reactive oxygen species, essential molecules, during plant-pathogen interactions. Plant Physiol. Bioch. 2016, 103, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Overmyer, K.; Brosché, M.; Kangasjärvi, J. Reactive oxygen species and hormonal control of cell death. Trends Plant Sci. 2003, 8, 335–342. [Google Scholar] [CrossRef]
- Balaban, R.; Nemoto, S.; Finkel, T. Mitochondria, oxidants, and aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef]
- Aken, O.V.; Breusegem, F.V. Licensed to kill: Mitochondria, chloroplasts, and cell death. Trends Plant Sci. 2015, 20, 754–766. [Google Scholar] [CrossRef]
- Tian, S.P.; Qin, G.Z.; Li, B.Q. Reactive oxygen species involved in regulating fruit senescence and fungal pathogenicity. Plant Mol. Biol. 2013, 82, 593–602. [Google Scholar] [CrossRef]
- Kayano, Y.; Tanaka, A.; Akano, F.; Scott, B.; Takemoto, D. Differential roles of NADPH oxidases and associated regulators in polarized growth, conidiation and hyphal fusion in the symbiotic fungus Epichloë festucae. Fungal Genet. Biol. 2013, 56, 87–97. [Google Scholar] [CrossRef]
- An, B.; Li, B.Q.; Qin, G.Z.; Tian, S.P. Function of small GTPase Rho3 in regulating growth, conidiation and virulence of Botrytis cinerea. Fungal Genet. Biol. 2015, 75, 46–55. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Z.Q.; He, C.; Qin, G.Z.; Tian, S.P. Comparative proteomics reveals the potential targets of BcNoxR, a putative regulatory subunit of NADPH oxidase of Botrytis cinerea. Mol. Plant Microbe Interact. 2016, 29, 990–1003. [Google Scholar] [CrossRef] [PubMed]
- Pilati, S.; Brazzale, D.; Guella, G.; Milli, A.; Ruberti, C.; Biasioli, F. The onset of grapevine berry ripening is characterized by ROS accumulation and lipoxygenase-mediated membrane peroxidation in the skin. BMC Plant Biol. 2014, 14, 87. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, J.; Ríos-Momberg, M.; Hewitt, D.; Hansberg, W. Reactive oxygen species and development in microbial eukaryotes. Trends Microbiol. 2005, 13, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Fridovich, I. Superoxide radical and superoxide dismutases. Annu. Rev. Biochem. 1995, 64, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Lara-Ortiz, T.; Riveros-Rosas, H.; Aguirre, J. Reactive oxygen species generated by microbial NADPH oxidase NoxA regulate sexual development in Aspergillus nidulans. Mol. Microbiol. 2003, 50, 1241–1255. [Google Scholar] [CrossRef] [PubMed]
- Giesbert, S.; Schürg, T.; Scheele, S.; Tudzunski, P. The NADPH oxidase Cpnox1 is required for full pathogenicity of the ergot fungus Claviceps purpurea. Mol. Plant Pathol. 2008, 9, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Marino, D.; Dunand, C.; Puppo, A.; Pauly, N. A burst of plant NADPH oxidases. Trends Plant Sci. 2012, 17, 1360–1385. [Google Scholar] [CrossRef] [PubMed]
- Kar, R.K. ROS Signaling: Relevance with Site of Production and Metabolism of ROS. In Reactive Oxygen Species and Oxidative Damage in Plants under Stress; Gupta, D.K., Palma, J.M., Corpas, F.J., Eds.; Springer International Publishing: Berlin, Germany, 2015; pp. 117–118. [Google Scholar] [CrossRef]
- Qin, G.Z.; Liu, J.; Cao, B.H.; Li, B.Q.; Tian, S.P. Hydrogen peroxide acts on sensitive mitochondrial proteins to induce death of a fungal pathogen revealed by proteomic analysis. PLoS ONE 2011, 6, e21945. [Google Scholar] [CrossRef] [PubMed]
- Waszczak, C.; Carmody, M.; Kangasjärvi, J. Reactive oxygen species in plant signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.G.; Dang, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lester, G.E.; Hodges, D.M. Antioxidants associated with fruit senescence and human health: Novel orange-fleshed non-netted honey dew melon genotype comparisons following different seasonal productions and cold storage durations. Postharvest Biol. Technol. 2008, 48, 347–354. [Google Scholar] [CrossRef]
- Xia, Y.X.; Chen, T.; Qin, G.Z.; Li, B.Q.; Tian, S.P. Synergistic action of antioxidative systems contributes to the alleviation of senescence in kiwifruit. Postharvest Biol. Technol. 2016, 111, 15–24. [Google Scholar] [CrossRef]
- Qin, G.Z.; Meng, X.H.; Wang, Q.; Tian, S.P. Oxidative damage of mitochondrial proteins contributes to fruit senescence: A redox proteomics analysis. J. Proteome Res. 2009, 8, 2449–2462. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.Z.; Wang, Q.; Liu, J.; Li, B.Q.; Tian, S.P. Proteomic analysis of changes in mitochondrial protein expression during fruit senescence. Proteomics 2009, 9, 4241–4253. [Google Scholar] [CrossRef]
- Ballester, A.R.; Lafuente, M.T.; González-Candelas, L. Spatial study of antioxidant enzymes, peroxidase and phenylalanine ammonia-lyase in the citrus fruit-Penicillium digitatum interaction. Postharvest Biol. Technol. 2006, 39, 115–124. [Google Scholar] [CrossRef]
- Vilanova, L.; Wisniewski, M.; Norelli, J.; Vinas, I.; Torres, R.; Usall, J. Transcriptomic profiling of apple in response to inoculation with a pathogen (Penicillium expansum) and a non-pathogen (Penicillium digitatum). Plant Mol. Biol. Rep. 2014, 32, 566–583. [Google Scholar] [CrossRef]
- Wang, Q.; Lai, T.F.; Qin, G.Z.; Tian, S.P. Response of Jujube fruits to exogenous oxalic acid treatment based on proteomic analysis. Plant Cell Physiol. 2009, 50, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.H.; Chen, J.; Lu, G.; Zhao, Y.; Tian, S.P.; Qin, G.Z. Control of brown rot on jujube and peach fruit by trisodium phosphate. Postharvest Biol. Technol. 2015, 99, 93–98. [Google Scholar] [CrossRef]
- Yan, F.J.; Hu, H.; Lu, L.F.; Zheng, X.D. Rhamnolipids induce oxidative stress responses in cherry tomato fruit to Alternaria alternate. Pest Manag. Sci. 2015, 72, 1500–1507. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.C.; Chen, T.; Ma, D.Y.; Liu, J.L.; Xu, Y.; Tian, S.P. Inhibitory effects of methyl thujate on mycelial growth of Botrytis cinerea and possible mechanisms. Postharvest Biol. Technol. 2018, 142, 46–54. [Google Scholar] [CrossRef]
- Liu, J.; Tian, S.P.; Meng, X.H.; Xu, Y. Effects of chitosan on control of postharvest diseases and physiological responses of tomato fruit. Postharvest Biol. Technol. 2007, 44, 300–306. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Chen, J.; Li, B.Q.; He, C.; Chen, Y.; Tian, S.P. Influence of oxidative stress on biocontrol activity of Cryptococcus laurentii against blue mold on peach fruit. Front Microbiol. 2017, 8, 151. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Huang, W.; Xiong, F.J.; Xian, Z.Q.; Su, D.D.; Ren, M.Z. Silencing of SlPL, which encodes a pectate lyase in tomato, confers enhanced fruit firmness, prolonged shelf-life and reduced susceptibility to grey mould. Plant Biotechnol. J. 2017, 15, 1544–1555. [Google Scholar] [CrossRef] [PubMed]
- Lima-Silva, V.; Rosado, A.; Amorim-Silva, V.; Muñoz-Mérida, A.; Pons, C.; Bombarely, A.; Trelles, O.; Fernández-Muñoz, R.; Granell, A.; Valpuesta, V.; et al. Genetic and genome-wide transcriptomic analyses identify co-regulation of oxidative response and hormone transcript abundance with vitamin C content in tomato fruit. BMC Genom. 2012, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Alkan, N.; Fortes, A.M. Insights in to molecular and metabolic events associated with fruit response to post-harvest fungal pathogens. Front. Plant Sci. 2015, 6, 889. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.J.; Tian, S.P. Effects of pre-and post-harvest application of salicylic acid or methyl jasmonate on inducing disease resistance of sweet cherry fruit in storage. Postharvest Biol. Technol. 2005, 35, 253–262. [Google Scholar] [CrossRef]
- Chan, Z.L.; Tian, S.P. Induction of H2O2-metabolizing enzymes and total protein synthesis by antagonistic yeast and salicylic acid in harvested sweet cherry fruit. Postharvest Biol. Technol. 2006, 39, 314–320. [Google Scholar] [CrossRef]
- Chan, Z.L.; Wang, Q.; Xu, X.B.; Meng, X.H.; Qin, G.Z.; Li, B.Q. Functions of defense-related proteins and dehydrogenases in resistance response induced by salicylic acid in sweet cherry fruits at different maturity stages. Proteomics 2008, 8, 4791–4807. [Google Scholar] [CrossRef]
- Xu, X.B.; Tian, S.P. Salicylic acid alleviated pathogen-induced oxidative stress in harvested sweet cherry fruit. Postharvest Biol. Technol. 2008, 49, 379–385. [Google Scholar] [CrossRef]
- Tian, S.P.; Wan, Y.K.; Qin, G.Z.; Xu, Y. Induction of defense responses against Alternaria rot by different elicitors in harvested pear fruit. Appl. Microbiol. Biotechnol. 2006, 70, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Chen, J.J.; Xiao, X.; Zhang, M.F.; Yun, Z.; Zeng, Y.L. Salicylic acid treatment reduces the rot of postharvest citrus fruit by inducing the accumulation of H2O2, primary metabolites and lipophilic polymethoxylated flavones. Food Chem. 2016, 207, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Asghari, M.; Aghdam, M.S. Impact of salicylic acid on post-harvest physiology of horticultural crops. Trends Food. Sci. Technol. 2010, 21, 502–509. [Google Scholar] [CrossRef]
- Ge, Y.H.; Deng, H.W.; Bi, Y.; Li, C.Y.; Liu, Y.Y.; Dong, B.Y. Postharvest ASM dipping and DPI pre-treatment regulated reactive oxygen species metabolism in muskmelon (Cucumis melo L.) fruit. Postharvest Biol. Technol. 2015, 99, 160–167. [Google Scholar] [CrossRef]
- Gfeller, A.; Dubugnon, L.; Liechti, R.; Farmer, E.E. Jasmonate biochemical pathway. Sci. Signal. 2010, 3, 109. [Google Scholar] [CrossRef]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef]
- Zhu, Z.; Tian, S.P. Resistant responses of tomato fruit treated with exogenous methyl jasmonate to Botrytis cinerea infection. Sci. Hortic. Amst. 2012, 142, 38–43. [Google Scholar] [CrossRef]
- Yao, H.J.; Tian, S.P. Effects of a biocontrol agent and methyl jasmonate on postharvest diseases of peach fruit and the possible mechanisms involved. J. Appl. Microbiol. 2005, 98, 941–950. [Google Scholar] [CrossRef]
- Frenkel, C.; Eskin, M. Ethylene evolution as related to changes in hydroperoxides in ripening tomato fruit. HortScience 1977, 12, 552–553. [Google Scholar]
- Zhang, Z.Q.; Tian, S.P.; Zhu, Z.; Xu, Y.; Qin, G.Z. Effects of 1-methylcyclopropene(1-MCP) on ripening and resistance of jujube (Zizyphus jujuba cv. Huping) fruit against postharvest disease. LWT-Food Sci. Technol. 2012, 45, 13–19. [Google Scholar] [CrossRef]
- Liu, R.L.; Wang, Y.Y.; Qin, G.Z.; Tian, S.P. Molecular basis of 1-methylcyclopropene regulating organic acid metabolism in apple fruit during storage. Postharvest Biol. Technol. 2016, 117, 57–63. [Google Scholar] [CrossRef]
- Park, Y.S.; Im, M.H.; Gorinstein, S. Shelf life extension and antioxidant activity of ‘Hayward’ kiwi fruit as a result of prestorage conditioning and 1-methylcyclopropene treatment. J. Food Sci. Technol. 2015, 52, 2711–2720. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Feng, J.Z.; Zhang, P.Y.; Jia, L.Y.; Chen, K.S. Postharvest treatment with trans-2-hexenal induced resistance against Botrytis cinerea in tomato fruit. Australas. Plant Pathol. 2014, 44, 121–128. [Google Scholar] [CrossRef]
- Roth, U.; Friebe, A.; Schnabl, H. Resistance Induction in plants by a brassinosteroid-containing extract of Lychnis viscaria L. Z. Naturforsch. C 2000, 55, 552–559. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, Z.Q.; Qin, G.Z.; Tian, S.P. Effects of brassinosteroids on postharvest disease and senescence of jujube fruit in storage. Postharvest Biol. Technol. 2010, 56, 50–55. [Google Scholar] [CrossRef]
- Romero-Puertas, M.C.; Perazzolli, M.; Zago, E.D.; Delledonne, M. Nitric oxide signaling functions in plant-pathogen interactions. Cell. Microbiol. 2004, 6, 795–803. [Google Scholar] [CrossRef]
- Arasimowicz, M.; Floryszak-Wieczorek, J. Nitric oxide as a bioactive signalling molecule in plant stress responses. Plant Sci. 2007, 172, 876–887. [Google Scholar] [CrossRef]
- Lai, T.F.; Wang, Y.Y.; Li, B.Q.; Qin, G.Z.; Tian, S.P. Defense responses of tomato fruit to exogenous nitric oxide during postharvest storage. Postharvest Biol. Technol. 2011, 62, 127–132. [Google Scholar] [CrossRef]
- Vandelle, E.; Poinssot, B.; Wendehenne, D.; Bentéjac, M.; Pugin, A. Integrated signaling network involving calcium, nitric oxide, and active oxygen species but not mitogen-activated protein kinases in BcPG1-elicited grapevine defenses. Mol. Plant Microbe Interact. 2006, 19, 429–440. [Google Scholar] [CrossRef]
- Shi, J.Y.; Liu, N.; Gu, R.X.; Zhu, L.Q.; Zhang, C.; Wang, Q.G. Signals induced by exogenous nitric oxide and their role in controlling brown rot disease caused by Monilinia fructicola in postharvest peach fruit. J. Gen. Plant Pathol. 2015, 81, 68–76. [Google Scholar] [CrossRef]
- Kulik, A.; Noirot, E.; Grandperret, V.; Bourque, S.; Fromentin, J.; Salloignon, P. Interplays between nitric oxide and reactive oxygen species in cryptogein signalling. Plant Cell Environ. 2015, 38, 331–348. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.F.; Chen, Y.; Li, B.Q.; Qin, G.Z.; Tian, S.P. Mechanism of Penicillium expansum in response to exogenous nitric oxide based on proteomics analysis. J. Proteomics 2014, 103, 47–56. [Google Scholar] [CrossRef]
- Freschi, L. Nitric oxide and phytohormone interactions: Current status and perspectives. Front. Plant Sci. 2013, 4, 398. [Google Scholar] [CrossRef]
- Sivakumaran, A.; Akinyemi, A.; Mandon, J.; Cristescu, S.M.; Hall, M.A.; Harren, F.J.M. ABA suppresses Botrytis cinerea elicited NO production in tomato to influence H2O2 generation and increase host susceptibility. Front. Plant Sci. 2016, 7, 709. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.R. Reactive oxygen species in the citrus fungal pathogen Alternaria alternata: The roles of NADPH-dependent oxidase. Physiol. Mol. Plant P. 2014, 88, 10–17. [Google Scholar] [CrossRef]
- Siegmund, U.; Marschall, R.; Tudzynski, P. BcNoxD, a putative ER protein, is a new component of the NADPH oxidase complex in Botrytis cinerea. Mol. Microbiol. 2015, 95, 988–1005. [Google Scholar] [CrossRef] [PubMed]
- Segmüller, N.; Kokkelink, L.; Giesbert, S.; Odinius, D.; Kan, J.V.; Tudzynski, P. NADPH oxidases are involved in differentiation and pathogenicity in Botrytis cinerea. Mol. Plant Microbe Interact. 2008, 21, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Chen, C.B.; Kabbage, M.; Dickman, M.B. Identification and characterization of Sclerotinia sclerotiorum NADPH oxidases. Appl. Environ. Microb. 2011, 77, 7721–7729. [Google Scholar] [CrossRef]
- Semighini, G.P.; Harris, S.D. Regulation of apical dominance in Aspergillus nidulans hyphae by reactive oxygen species. Genetics 2008, 179, 1919–1932. [Google Scholar] [CrossRef]
- Yang, S.L.; Chung, K.R. The NADPH oxidase-mediated production of hydrogen peroxide (H2O2) and resistance to oxidative stress in the necrotrophic pathogen Alternaria alternata of citrus. Mol. Plant Pathol. 2012, 13, 900–914. [Google Scholar] [CrossRef]
- Scott, B. Conservation of fungal and animal nicotinamide adenine dinucleotide phosphate oxidase complexes. Mol. Microbiol. 2015, 95, 910–913. [Google Scholar] [CrossRef] [PubMed]
- Galhano, R.; Illana, A.; Ryder, L.S.; Rodríguez-Romero, J.; Demuez, M.; Badaruddin, M.; Martinez-Rocha, A.L.; Soanes, D.M.; Studholme, D.J.; Talbot, N.J.; et al. Tpc1 is an important Zn(II)2Cys6 transcriptional regulator required for polarized growth and virulence in the rice blast fungus. PLoS Pathog. 2017, 13, e1006516. [Google Scholar] [CrossRef] [PubMed]
- Lacaze, I.; Lalucque, H.; Siegmund, U.; Silar, P.; Brun, S. Identification of NoxD/Pro41 as the homologue of the p22phox NADPH oxidase subunit in fungi. Mol. Microbiol. 2015, 95, 1006–1024. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, D.; Kamakura, S.; Saikia, S.; Becker, Y.; Wrenn, R.; Tanaka, A. Polarity proteins Bem1 and Cdc24 are components of the filamentous fungal NADPH oxidase complex. Proc. Natl. Acad. Sci. USA 2011, 108, 2861–2866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takemoto, D.; Tanaka, A.; Scott, B. A p67Phox-like regulator is recruited to control hyphal branching in a fungal-grass mutualistic symbiosis. Plant Cell 2006, 18, 2807–2821. [Google Scholar] [CrossRef] [PubMed]
- Boyce, K.J.; Hynes, M.J.; Andrianopoulos, A. Control of morphogenesis and actin localization by the Penicillium marneffei RAC homolog. J. Cell Sci. 2003, 116, 1249–1260. [Google Scholar] [CrossRef]
- Chen, C.B.; Dickman, M.B. Dominant active Rac and dominant negative Rac revert the dominant active Ras phenotype in Colletotrichum trifolii by distinct signalling pathways. Mol. Microbiol. 2004, 51, 1493–1507. [Google Scholar] [CrossRef]
- Takemoto, D.; Tanaka, A.; Scott, B. NADPH oxidases in fungi: Diverse roles of reactive oxygen species in fungal cellular differentiation. Fungal Genet. Biol. 2007, 44, 1065–1076. [Google Scholar] [CrossRef]
- Gessler, N.N.; Aver’yanov, A.A.; Belozerskaya, T.A. Reactive oxygen species in regulation of fungal development. Biochemistry 2007, 72, 1091–1109. [Google Scholar] [CrossRef]
- Qin, G.Z.; Tian, S.P.; Chan, Z.L.; Li, B.Q. Crucial role of antioxidant proteins and hydrolytic enzymes in pathogenicity of Penicillium expansum: Analysis based on proteomic approach. Mole. Cell. Proteomics 2007, 6, 425–438. [Google Scholar] [CrossRef]
- Kim, K.H.; Willger, S.D.; Park, S.W.; Puttikamonkul, S.; Grahl, N.; Cho, Y. TmpL, a transmembrane protein required for intracellular redox homeostasis and virulence in a plant and an animal fungal pathogen. PLoS Pathog. 2009, 5, e1000653. [Google Scholar] [CrossRef] [PubMed]
- Segal, L.M.; Wilson, R.A. Reactive oxygen species metabolism and plant-fungal interactions. Fungal Genet. Biol. 2018, 110, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warris, A.; Ballou, E.R. Oxidative responses and fungal infection biology. Semin. Cell Dev. Biol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, M.K.; Hu, K.D.; Sun, K.K.; Li, Y.H.; Hu, L.Y. Deletion of Cu/Zn superoxide dismutase gene sodC reduces Aspergillus niger virulence on chinese white pear. J. Am. Soc. Hort. Sci. 2017, 142, 385–392. [Google Scholar] [CrossRef]
- Tondo, M.L.; Petrocelli, S.; Ottado, J.; Orellano, E.G. The monofunctional catalase KatE of Xanthomonas axonopodis pv. citri is required for full virulence in citrus plants. PLoS ONE 2010, 5, e10803. [Google Scholar] [CrossRef]
- Yu, C.; Wang, N.; Wu, M.; Tian, F.; Chen, H.; Yang, F.H. OxyR-regulated catalase CatB promotes the virulence in rice via detoxifying hydrogen peroxide in Xanthomonas oryzae pv. Oryzae. BMC Microbiol. 2016, 16, 229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.K.; Tang, J.; Huang, Z.Q.; Hu, K.D.; Li, Y.H.; Han, Z. Deletion of the catalase gene cpeB reduces Aspergillus niger virulence in apple fruits. J. Agric. Food Chem. 2018, 66, 5401–5409. [Google Scholar] [CrossRef]
- Li, H.; Chen, Y.; Zhang, Z.Q.; Li, B.Q.; Qin, G.Z.; Tian, S.P. Pathogenic mechanisms and control strategies of Botrytis cinerea causing post-harvest decay in fruits and vegetables. Food Qual. Saf. 2018, 3, 111–119. [Google Scholar] [CrossRef]
- Bienert, G.P.; Møller, A.L.B.; Kristiansen, K.A.; Schulz, A.; Møller, I.M.; Schjoerring, J.K. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J. Biol. Chem. 2007, 282, 1183–1192. [Google Scholar] [CrossRef]
- Miller, E.W.; Dickinson, B.C.; Chang, C.J. Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc. Natl. Acad. Sci. USA 2010, 107, 15681–15686. [Google Scholar] [CrossRef] [Green Version]
- Bienert, G.P.; Chaumont, F. Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. BBA Biomembr. 2014, 1840, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Moniaga, C.S.; Nielsen, S.; Hara-Chikuma, M. Aquaporin-9 facilitates membrane transport of hydrogen peroxide in mammalian cells. Biochem. Bioph. Res. Commun. 2016, 471, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Sadhukhan, A.; Kobayashi, Y.; Nakano, Y.; Iuchi, S.; Kobayashi, M.; Sahoo, L. Genome-wide association study reveals that the aquaporin NIP1; 1 contributes to variation in hydrogen peroxide sensitivity in Arabidopsis thaliana. Mol. Plant 2017, 10, 1082–1094. [Google Scholar] [CrossRef]
- Danielson, J.Å.; Johanson, U. Unexpected complexity of the Aquaporin gene family in the moss Physcomitrella patens. BMC Plant Biol. 2008, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, O.; Reshetnyak, G.; Grondin, A.; Saijo, Y.; Leonhardt, N.; Maurel, C. Aquaporins facilitate hydrogen peroxide entry into guard cells to mediate ABA- and pathogen-triggered stomatal closure. Proc. Natl. Acad. Sci. USA 2017, 114, 9200–9205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyers, G.L.; Jung, K.W.; Bang, S.; Kim, J.; Kim, S.; Hong, J. The water channel protein aquaporin 1 regulates cellular metabolism and competitive fitness in a global fungal pathogen Cryptococcus neoformans. Environ. Microbiol. Rep. 2017, 9, 268–278. [Google Scholar] [CrossRef]
- Pettersson, N.; Filipsson, C.; Becit, E.; Brive, L.; Hohmann, S. Aquaporins in yeasts and filamentous fungi. Biol. Cell 2005, 97, 487–500. [Google Scholar] [CrossRef]
- An, B.; Li, B.Q.; Li, H.; Zhang, Z.Q.; Qin, G.Z.; Tian, S.P. Aquaporin8 regulates cellular development and reactive oxygen species production, a critical component of virulence in Botrytis cinerea. New Phytol. 2016, 209, 1668–1680. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Ji, D.; Chen, T.; Li, B.; Zhang, Z.; Qin, G.; Tian, S. Production, Signaling, and Scavenging Mechanisms of Reactive Oxygen Species in Fruit–Pathogen Interactions. Int. J. Mol. Sci. 2019, 20, 2994. https://doi.org/10.3390/ijms20122994
Wang Y, Ji D, Chen T, Li B, Zhang Z, Qin G, Tian S. Production, Signaling, and Scavenging Mechanisms of Reactive Oxygen Species in Fruit–Pathogen Interactions. International Journal of Molecular Sciences. 2019; 20(12):2994. https://doi.org/10.3390/ijms20122994
Chicago/Turabian StyleWang, Ying, Dongchao Ji, Tong Chen, Boqiang Li, Zhanquan Zhang, Guozheng Qin, and Shiping Tian. 2019. "Production, Signaling, and Scavenging Mechanisms of Reactive Oxygen Species in Fruit–Pathogen Interactions" International Journal of Molecular Sciences 20, no. 12: 2994. https://doi.org/10.3390/ijms20122994
APA StyleWang, Y., Ji, D., Chen, T., Li, B., Zhang, Z., Qin, G., & Tian, S. (2019). Production, Signaling, and Scavenging Mechanisms of Reactive Oxygen Species in Fruit–Pathogen Interactions. International Journal of Molecular Sciences, 20(12), 2994. https://doi.org/10.3390/ijms20122994







