Oligomerization of Silicic Acids in Neutral Aqueous Solution: A First-Principles Investigation
Abstract
:1. Introduction
2. Results and Discussions
3. Theoretical Methods
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liang, J.; Liang, Z.B.; Zou, R.Q.; Zhao, Y.L. Heterogeneous catalysis in zeolites, mesoporous silica, and metal-organic frameworks. Adv. Mater. 2017, 29, 21. [Google Scholar] [CrossRef]
- Kulkarni, A.R.; Zhao, Z.J.; Siahrostami, S.; Norskov, J.K.; Studt, F. Cation-exchanged zeolites for the selective oxidation of methane to methanol. Catal. Sci. Technol. 2018, 8, 114–123. [Google Scholar] [CrossRef]
- Dusselier, M.; Davis, M.E. Small-pore zeolites: Synthesis and catalysis. Chem. Rev. 2018, 118, 5265–5329. [Google Scholar] [CrossRef]
- Ennaert, T.; Van Aelst, J.; Dijkmans, J.; De Clercq, R.; Schutyser, W.; Dusselier, M.; Verboekend, D.; Sels, B.F. Potential and challenges of zeolite chemistry in the catalytic conversion of biomass. Chem. Soc. Rev. 2016, 45, 584–611. [Google Scholar] [CrossRef]
- Mansir, N.; Taufiq-Yap, Y.H.; Rashid, U.; Lokman, I.M. Investigation of heterogeneous solid acid catalyst performance on low grade feedstocks for biodiesel production: A review. Energy Conv. Manag. 2017, 141, 171–182. [Google Scholar] [CrossRef]
- Goh, P.S.; Ismail, A.F. A review on inorganic membranes for desalination and wastewater treatment. Desalination 2018, 434, 60–80. [Google Scholar] [CrossRef]
- Burakov, A.E.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.E.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review. Ecotox. Environ. Safe. 2018, 148, 702–712. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpaa, M. Removal of natural organic matter (nom) and its constituents from water by adsorption - a review. Chemosphere 2017, 166, 497–510. [Google Scholar] [CrossRef]
- Goh, P.S.; Ismail, A.F.; Sanip, S.M.; Ng, B.C.; Aziz, M. Recent advances of inorganic fillers in mixed matrix membrane for gas separation. Sep. Purif. Technol. 2011, 81, 243–264. [Google Scholar] [CrossRef]
- Vermeiren, W.; Gilson, J.P. Impact of zeolites on the petroleum and petrochemical industry. Top. Catal. 2009, 52, 1131–1161. [Google Scholar] [CrossRef]
- Smit, B.; Maesen, T.L.M. Towards a molecular understanding of shape selectivity. Nature 2008, 451, 671–678. [Google Scholar] [CrossRef] [Green Version]
- Cejka, J.; Centi, G.; Perez-Pariente, J.; Roth, W.J. Zeolite-based materials for novel catalytic applications: Opportunities, perspectives and open problems. Catal. Today 2012, 179, 2–15. [Google Scholar] [CrossRef]
- Kabalan, I.; Lebeau, B.; Nouali, H.; Toufaily, J.; Hamieh, T.; Koubaissy, B.; Bellat, J.-P.; Daou, T.J. New generation of zeolite materials for environmental applications. J. Phys. Chem. C 2016, 120, 2688–2697. [Google Scholar] [CrossRef]
- Li, S.Y.; Li, J.F.; Dong, M.; Fan, S.B.; Zhao, T.S.; Wang, J.G.; Fan, W.B. Strategies to control zeolite particle morphology. Chem. Soc. Rev. 2019, 48, 885–907. [Google Scholar] [CrossRef]
- Verboekend, D.; Nuttens, N.; Locus, R.; Van Aelst, J.; Verolme, P.; Groen, J.C.; Perez-Ramirez, J.; Sels, B.F. Synthesis, characterisation, and catalytic evaluation of hierarchical faujasite zeolites: Milestones, challenges, and future directions. Chem. Soc. Rev. 2016, 45, 3331–3352. [Google Scholar] [CrossRef]
- Verboekend, D.; Perez-Ramirez, J. Design of hierarchical zeolite catalysts by desilication. Catal. Sci. Technol. 2011, 1, 879–890. [Google Scholar] [CrossRef] [Green Version]
- Fan, F.T.; Feng, Z.C.; Li, C. Uv raman spectroscopic study on the synthesis mechanism and assembly of molecular sieves. Chem. Soc. Rev. 2010, 39, 4794–4801. [Google Scholar] [CrossRef]
- Li, J.Y.; Corma, A.; Yu, J.H. Synthesis of new zeolite structures. Chem. Soc. Rev. 2015, 44, 7112–7127. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Cao, H.; Yu, J. Toward a new era of designed synthesis of nanoporous zeolitic materials. Acs Nano 2018, 12, 4096–4104. [Google Scholar] [CrossRef]
- Li, Y.; Yu, J.H. New stories of zeolite structures: Their descriptions, determinations, predictions, and evaluations. Chem. Rev. 2014, 114, 7268–7316. [Google Scholar] [CrossRef]
- Grand, J.; Awala, H.; Mintova, S. Mechanism of zeolites crystal growth: New findings and open questions. Crystengcomm 2016, 18, 650–664. [Google Scholar] [CrossRef]
- Quesne, M.G.; Silveri, F.; de Leeuw, N.H.; Catlow, C.R.A. Advances in sustainable catalysis: A computational perspective. Front. Chem. 2019, 7, 23. [Google Scholar] [CrossRef]
- Coronas, J. Present and future synthesis challenges for zeolites. Chem. Eng. J. 2010, 156, 236–242. [Google Scholar] [CrossRef]
- Gascon, J.; Kapteijn, F.; Zornoza, B.; Sebastian, V.; Casado, C.; Coronas, J. Practical approach to zeolitic membranes and coatings: State of the art, opportunities, barriers, and future perspectives. Chem. Mater. 2012, 24, 2829–2844. [Google Scholar] [CrossRef]
- Van Speybroeck, V.; Hemelsoet, K.; Joos, L.; Waroquier, M.; Bell, R.G.; Catlow, C.R.A. Advances in theory and their application within the field of zeolite chemistry. Chem. Soc. Rev. 2015, 44, 7044–7111. [Google Scholar] [CrossRef] [Green Version]
- Catlow, C.R.A.; Coombes, D.S.; Pereira, J.C.G. Computer modeling of nucleation, growth, and templating in hydrothermal synthesis. Chem. Mater. 1998, 10, 3249–3265. [Google Scholar] [CrossRef]
- Pereira, J.C.G.; Catlow, C.R.A.; Price, G.D. Ab initio studies of silica-based clusters. Part i. Energies and conformations of simple clusters. J. Phys. Chem. A 1999, 103, 3252–3267. [Google Scholar] [CrossRef]
- Pereira, J.C.G.; Catlow, C.R.A.; Price, G.D. Ab initio studies of silica-based clusters. Part ii. Structures and energies of complex clusters. J. Phys. Chem. A 1999, 103, 3268–3284. [Google Scholar] [CrossRef]
- Pereira, J.C.G.; Catlow, C.R.A.; Price, G.D. Silica condensation reaction: An ab initio study. Chem. Commun. 1998, 1387–1388. [Google Scholar] [CrossRef]
- Trinh, T.T.; Jansen, A.P.J.; van Santen, R.A. Mechanism of oligomerization reactions of silica. J. Phys. Chem. B 2006, 110, 23099–23106. [Google Scholar] [CrossRef]
- Henschel, H.; Schneider, A.M.; Prosenc, M.H. Initial steps of the sol-gel process: Modeling silicate condensation in basic medium. Chem. Mater. 2010, 22, 5105–5111. [Google Scholar] [CrossRef]
- Hu, H.; Hou, H.; He, Z.; Wang, B. Theoretical characterizations of the mechanism for the dimerization of monosilicic acid in basic solution. Phys. Chem. Chem. Phys. 2013, 15, 15027–15032. [Google Scholar] [CrossRef]
- Schaffer, C.L.; Thomson, K.T. Density functional theory investigation into structure and reactivity of prenucleation silica species. J. Phys. Chem. C 2008, 112, 12653–12662. [Google Scholar] [CrossRef]
- Tossell, J.A. Theoretical study on the dimerization of si(oh)(4) in aqueous solution and its dependence on temperature and dielectric constant. Geochim. Cosmochim. Acta 2005, 69, 283–291. [Google Scholar] [CrossRef]
- Mora-Fonz, M.J.; Catlow, C.R.A.; Lewis, D.W. Oligomerization and cyclization processes in the nucleation of microporous silicas. Angew. Chem.-Int. Edit. 2005, 44, 3082–3086. [Google Scholar] [CrossRef]
- Mora-Fonz, M.J.; Catlow, C.R.A.; Lewis, D.W. Modeling aqueous silica chemistry in alkali media. J. Phys. Chem. C 2007, 111, 18155–18158. [Google Scholar] [CrossRef]
- White, C.E.; Provis, J.L.; Kearley, G.J.; Riley, D.P.; van Deventer, J.S.J. Density functional modelling of silicate and aluminosilicate dimerisation solution chemistry. Dalton Trans. 2011, 40, 1348–1355. [Google Scholar] [CrossRef]
- Xiao, Y.T.; Lasaga, A.C. Ab initio quantum mechanical studies of the kinetics and mechanisms of quartz dissolution: Oh- catalysis. Geochim. Cosmochim. Acta 1996, 60, 2283–2295. [Google Scholar] [CrossRef]
- Pelmenschikov, A.; Leszczynski, J.; Pettersson, L.G.M. Mechanism of dissolution of neutral silica surfaces: Including effect of self-healing. J. Phys. Chem. A 2001, 105, 9528–9532. [Google Scholar] [CrossRef]
- Criscenti, L.J.; Kubicki, J.D.; Brantley, S.L. Silicate glass and mineral dissolution: Calculated reaction paths and activation energies for hydrolysis of a q(3) si by h3o+ using ab initio methods. J. Phys. Chem. A 2006, 110, 198–206. [Google Scholar] [CrossRef]
- Zhang, X.-Q.; Trinh, T.T.; van Santen, R.A.; Jansen, A.P.J. Mechanism of the initial stage of silicate oligomerization. J Am Chem Soc 2011, 133, 6613–6625. [Google Scholar] [CrossRef]
- Zhang, X.-Q.; van Santen, R.A.; Jansen, A.P.J. Kinetic monte carlo modeling of silicate oligomerization and early gelation. Phys. Chem. Chem. Phys. 2012, 14, 11969–11973. [Google Scholar] [CrossRef]
- McIntosh, G.J. Theoretical investigations into the nucleation of silica growth in basic solution part ii - derivation and benchmarking of a first principles kinetic model of solution chemistry. Phys. Chem. Chem. Phys. 2013, 15, 17496–17509. [Google Scholar] [CrossRef]
- McIntosh, G.J. Theoretical investigations into the nucleation of silica growth in basic solution part i - ab initio studies of the formation of trimers and tetramers. Phys. Chem. Chem. Phys. 2013, 15, 3155–3172. [Google Scholar] [CrossRef]
- Catlow, C.R.A.; Bromley, S.T.; Hamad, S.; Mora-Fonz, M.; Sokol, A.A.; Woodley, S.M. Modelling nano-clusters and nucleation. Phys. Chem. Chem. Phys. 2010, 12, 786–811. [Google Scholar] [CrossRef]
- Sefcik, J.; McCormick, A.V. Thermochemistry of aqueous silicate solution precursors to ceramics. Aiche J. 1997, 43, 2773–2784. [Google Scholar] [CrossRef]
- Mora-Fonz, M.J.; Catlow, C.R.A.; Lewis, D.W. H-bond interactions between silicates and water during zeolite pre-nucleation. Phys. Chem. Chem. Phys. 2008, 10, 6571–6578. [Google Scholar] [CrossRef]
- Putz, M.V.; Russo, N.; Sicilia, E. On the applicability of the hsab principle through the use of improved computational schemes for chemical hardness evaluation. J. Comput. Chem. 2004, 25, 994–1003. [Google Scholar] [CrossRef]
- Putz, M.V. Density functionals of chemical bonding. Int. J. Mol. Sci. 2008, 9, 1050–1095. [Google Scholar] [CrossRef]
- Putz, M.V. Maximum hardness index of quantum acid-base bonding. Match-Commun. Math. Comput. Chem. 2008, 60, 845–868. [Google Scholar]
- Putz, M.V. Chemical action concept and principle. Match-Commun. Math. Comput. Chem. 2011, 66, 35–63. [Google Scholar]
- Becke, A.D. A multicenter numerical-integration scheme for polyatomic-molecules. J. Chem. Phys. 1988, 88, 2547–2553. [Google Scholar] [CrossRef]
- Lee, C.T.; Yang, W.T.; Parr, R.G. Development of the colle-salvetti correlation-energy formula into a functional of the electron-density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Delley, B. An all-electron numerical-method for solving the local density functional for polyatomic-molecules. J. Chem. Phys. 1990, 92, 508–517. [Google Scholar] [CrossRef]
- Delley, B. From molecules to solids with the dmol(3) approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Klamt, A.; Schuurmann, G. Cosmo–A new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J. Chem. Soc. Perkin Trans. 1993, 2, 799–805. [Google Scholar] [CrossRef]
- Baldridge, K.; Klamt, A. First principles implementation of solvent effects without outlying charge error. J. Chem. Phys. 1997, 106, 6622–6633. [Google Scholar] [CrossRef]
- Andzelm, J.; Kolmel, C.; Klamt, A. Incorporation of solvent effects into density-functional calculations of molecular-energies and geometries. J. Chem. Phys. 1995, 103, 9312–9320. [Google Scholar] [CrossRef]
- Govind, N.; Petersen, M.; Fitzgerald, G.; King-Smith, D.; Andzelm, J. A generalized synchronous transit method for transition state location. Comput. Mater. Sci. 2003, 28, 250–258. [Google Scholar] [CrossRef]
- Kelly, C.P.; Cramer, C.J.; Truhlar, D.G. Sm6: A density functional theory continuum solvation model for calculating aqueous solvation free energies of neutrals, ions, and solute-water clusters. J. Chem. Theory Comput. 2005, 1, 1133–1152. [Google Scholar] [CrossRef]
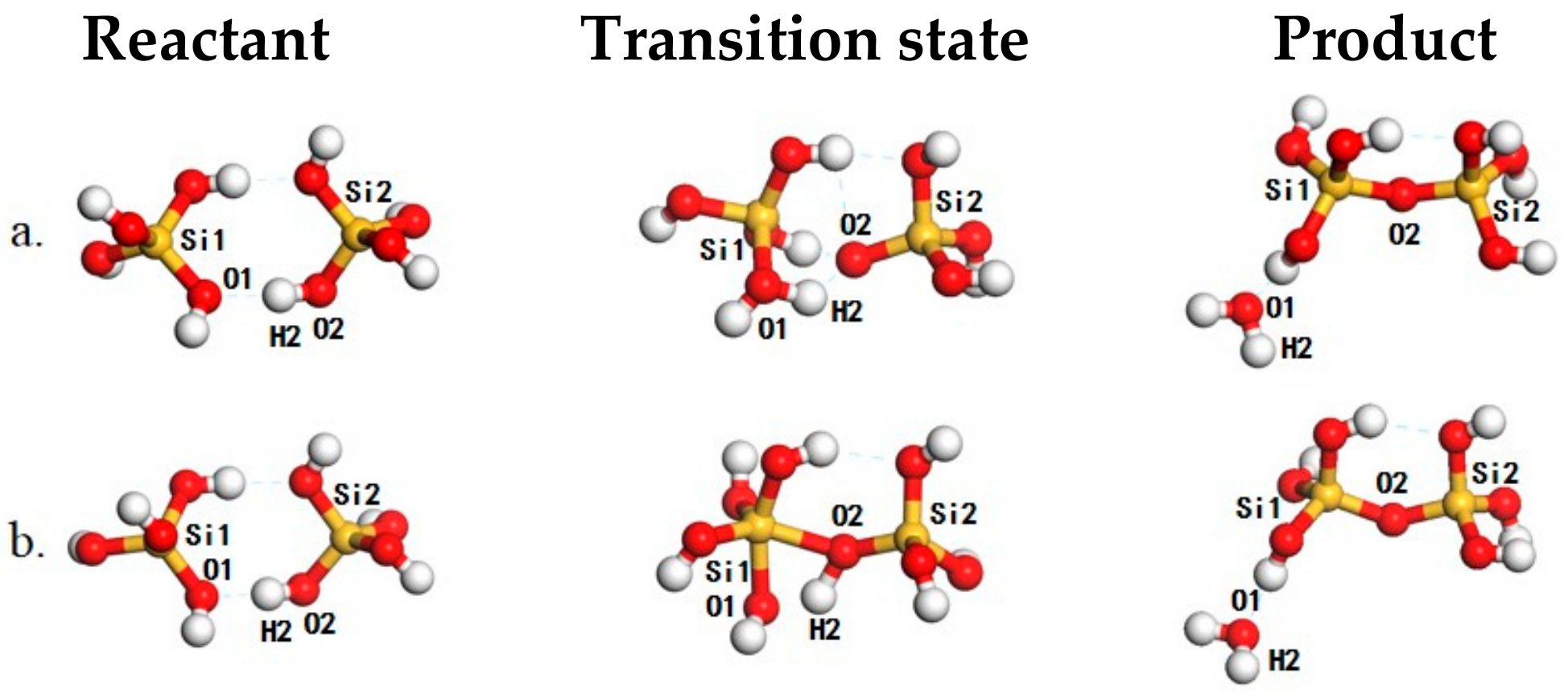
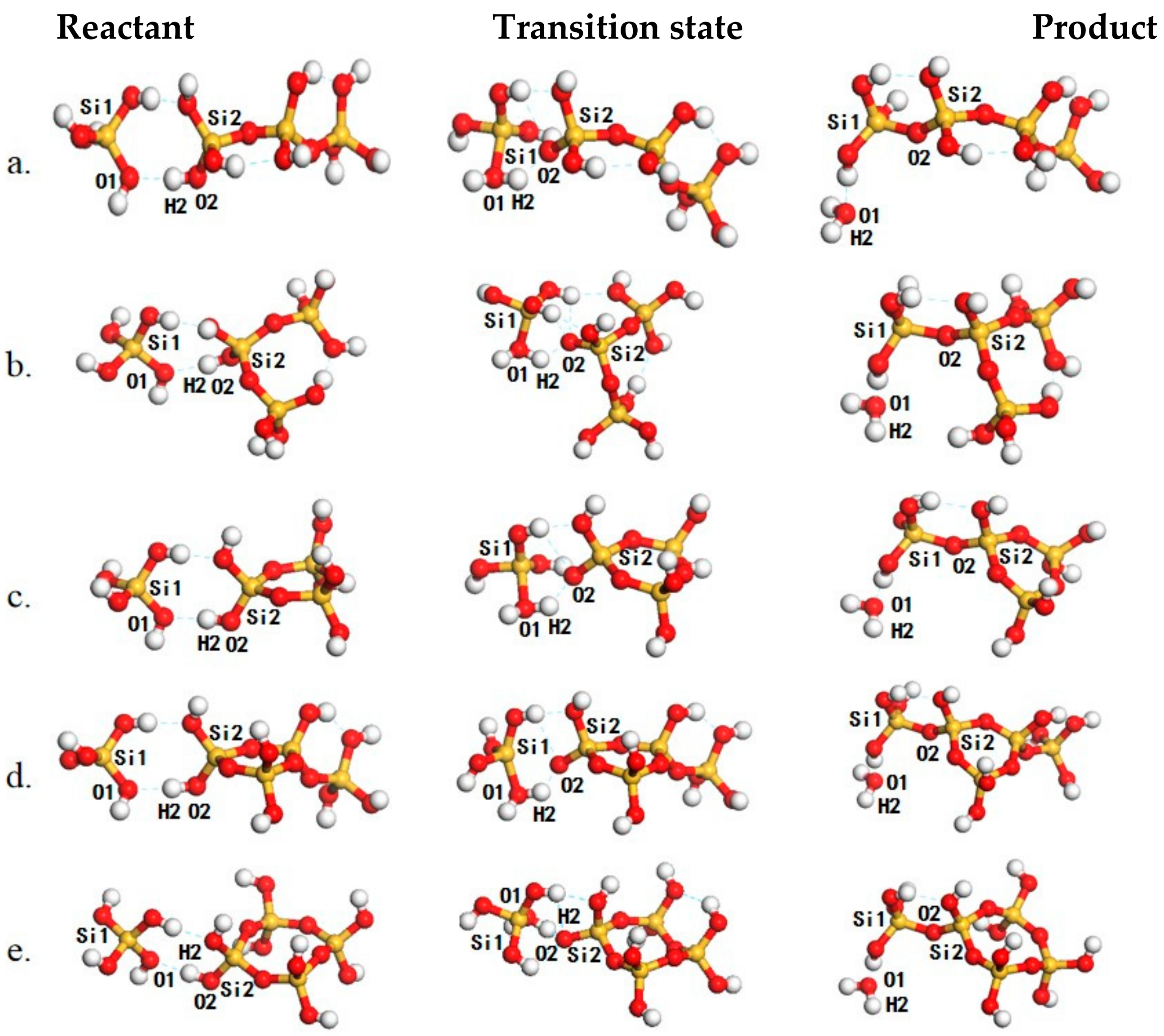

| Entry | Reactions | ΔEact a | ΔE b | ΔG298K c | ΔG450K c | ΔH298K d | ΔH450K d | If e |
|---|---|---|---|---|---|---|---|---|
| 1 | + → + | 133.0 | −3.0 | −8.5 | −11.4 | −3.0 | −2.3 | −294.4 |
| 2 | + → + | 131.9 | −6.3 | −31.3 | −42.0 | −11.1 | −9.5 | −262.3 |
| 3 | + → + | 130.2 | −4.9 | −14.4 | −18.8 | −6.0 | −5.1 | −254.3 |
| 4 | + → + | 124.2 | −2.4 | −6.2 | −9.2 | −0.6 | −0.3 | −252.8 |
| 5 | + → + | 131.6 | −3.0 | −25.6 | −35.8 | −6.1 | −4.9 | −265.5 |
| 6 | + → + | 132.6 | −2.0 | −24.9 | −35.1 | −5.5 | −4.0 | −279.2 |
| 7 | + → + | 131.8 | 0.8 | −8.1 | −12.3 | −0.2 | 0.5 | −282.7 |
| 8 | + → + | 127.9 | −1.8 | −8.7 | −12.5 | −1.8 | −0.9 | −235.8 |
| 9 | + → + | 128.5 | −2.6 | −28.5 | −39.2 | −8.4 | −6.5 | −250.7 |
| 10 | + → + | 132.0 | −4.6 | −16.4 | −21.4 | −7.2 | −6.0 | −253.2 |
| 11 | + → + | 126.5 | 0.0 | −16.9 | −24.9 | −1.8 | −0.3 | −252.8 |
| 12 | + → + | 129.5 | −3.6 | −1.8 | −4.0 | 2.5 | 2.1 | −227.8 |
| 13 | + → + | 129.3 | −1.6 | −10.1 | −14.7 | −1.4 | −0.8 | −220.2 |
| 14 | + → + | 126.6 | −11.4 | −8.4 | −7.7 | −9.5 | −9.8 | −273.4 |
| 15 | + → + | 126.0 | 2.7 | −7.2 | −12.5 | 2.6 | 3.5 | −257.0 |
| Entry | Reactions | ΔEact a | ΔE b | ΔG298K c | ΔG450K c | ΔH298K d | ΔH450K d | If e |
|---|---|---|---|---|---|---|---|---|
| 1 | → + | 131.9 | −11.0 | −17.1 | −19.5 | −12.6 | −12.1 | −365.4 |
| 2 | → + | 141.8 | −8.0 | −8.7 | −11.1 | −3.7 | −4.2 | −455.4 |
| 3 | → + | 140.3 | −7.8 | −26.3 | −35.8 | −8.2 | −7.1 | −381.8 |
| 4 | → + | 142.3 | −4.3 | −13.5 | −18.6 | −3.8 | −3.1 | −470.3 |
| 5 | → + | 130.6 | −12.1 | −13.8 | −16.0 | −9.5 | −9.8 | −316.9 |
| 6 | → + | 140.4 | −11.8 | −10.5 | −11.1 | −9.2 | −9.8 | −412.1 |
| 7 | → + | 144.9 | −7.3 | −3.4 | −3.0 | −4.0 | −4.3 | −321.6 |
| 8 | → + | 140.9 | 1.1 | −24.3 | −36.1 | −2.0 | −0.3 | −389.9 |
| 9 | → + | 141.8 | −19.2 | −20.7 | −21.6 | −18.8 | −19.2 | −488.0 |
| 10 | → + | 144.0 | −16.5 | −0.8 | 4.4 | −10.6 | −11.7 | −298.8 |
| 11 | → + | 134.4 | −5.0 | −15.2 | −19.7 | −6.8 | −5.8 | −401.9 |
| 12 | → + | 140.4 | 0.8 | −0.9 | −5.2 | 7.5 | 7.5 | −478.8 |
| Entry | Reactions | ΔEact a | ΔE b | ΔG298K c | ΔG450K c | ΔH298K d | ΔH450K d | If e |
|---|---|---|---|---|---|---|---|---|
| 1 | + → + | 159.2 | −2.4 | −11.9 | −16.7 | −2.9 | −2.1 | −997.8 |
| 2 | + → + | 152.2 | −4.0 | −11.8 | −16.1 | −3.8 | −3.2 | −864.3 |
| 3 | + → + | 150.5 | −4.6 | −10.9 | −14.2 | −4.7 | −4.1 | −876.6 |
| 4 | + → + | 153.1 | −2.5 | 0.0 | 0.0 | 0.7 | 0.6 | −852.4 |
| 5 | + → + | 152.0 | −4.0 | −27.3 | −37.3 | −8.3 | −6.8 | −847.8 |
| 6 | + → + | 152.3 | −4.3 | −20.4 | −27.6 | −6.6 | −5.7 | −843.3 |
| 7 | + → + | 154.9 | 0.8 | −6.2 | −9.5 | −0.3 | 0.5 | −947.3 |
| 8 | + → + | 155.9 | −4.8 | −18.7 | −25.2 | −6.4 | −5.4 | −803.1 |
| 9 | + → + | 153.4 | −3.4 | −16.3 | −21.9 | −5.9 | −4.8 | −934.2 |
| 10 | + → + | 157.6 | −1.5 | −11.3 | −16.8 | −1.1 | −0.3 | −906.3 |
| 11 | + → + | 151.9 | 2.1 | −11.2 | −18.0 | 1.9 | 2.7 | −859.2 |
| 12 | + → + | 155.0 | −3.3 | −8.4 | −11.6 | −2.2 | −1.8 | −802.5 |
| 13 | + → + | 154.0 | 0.6 | −5.7 | −9.3 | 1.1 | 1.6 | −954.7 |
| 14 | + → + | 157.1 | −14.5 | −31.2 | −37.0 | −20.5 | −19.0 | −841.3 |
| 15 | + → + | 156.2 | 5.0 | −10.8 | −17.6 | 2.0 | 3.4 | −760.5 |
| Entry | Reactions | ΔEact a | ΔE b | ΔG298K c | ΔG450Kc | ΔH298K d | ΔH450K d | If e |
|---|---|---|---|---|---|---|---|---|
| 1 | → + | 136.5 | −12.5 | −14.6 | −15.8 | −12.3 | −12.2 | −793.3 |
| 2 | → + | 136.9 | −16.9 | −23.1 | −26.7 | −16.2 | −16.0 | −895.7 |
| 3 | → + | 150.9 | −4.5 | −17.0 | −24.3 | −2.0 | −2.5 | −861.9 |
| 4 | → + | 147.6 | −3.6 | −14.7 | −20.5 | −4.0 | −2.9 | −913.5 |
| 5 | → + | 133.5 | −12.8 | −9.8 | −9.9 | −9.3 | −10.11 | −928.3 |
| 6 | → + | 145.7 | −8.8 | −0.8 | 0.2 | −2.4 | −3.3 | −828.0 |
| 7 | → + | 147.2 | −7.3 | −3.4 | −3.0 | −4.0 | −4.3 | −830.2 |
| 8 | → + | 145.8 | −11.7 | −18.6 | −21.1 | −14.3 | −13.2 | −807.2 |
| 9 | → + | 147.8 | −18.8 | −13.0 | −10.6 | −17.7 | −17.8 | −884.9 |
| 10 | → + | 146.0 | −20.5 | −24.7 | −26.5 | −21.4 | −20.9 | −739.1 |
| 11 | → + | 146.7 | −19.4 | −30.8 | −34.6 | −23.8 | −22.7 | −931.1 |
| 12 | → + | 146.5 | −18.1 | −20.0 | −21.1 | −18.5 | −17.2 | −837.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Liu, C.; Meng, C. Oligomerization of Silicic Acids in Neutral Aqueous Solution: A First-Principles Investigation. Int. J. Mol. Sci. 2019, 20, 3037. https://doi.org/10.3390/ijms20123037
Liu X, Liu C, Meng C. Oligomerization of Silicic Acids in Neutral Aqueous Solution: A First-Principles Investigation. International Journal of Molecular Sciences. 2019; 20(12):3037. https://doi.org/10.3390/ijms20123037
Chicago/Turabian StyleLiu, Xin, Cai Liu, and Changgong Meng. 2019. "Oligomerization of Silicic Acids in Neutral Aqueous Solution: A First-Principles Investigation" International Journal of Molecular Sciences 20, no. 12: 3037. https://doi.org/10.3390/ijms20123037
APA StyleLiu, X., Liu, C., & Meng, C. (2019). Oligomerization of Silicic Acids in Neutral Aqueous Solution: A First-Principles Investigation. International Journal of Molecular Sciences, 20(12), 3037. https://doi.org/10.3390/ijms20123037






