Does the Epstein–Barr Virus Play a Role in the Pathogenesis of Graves’ Disease?
Abstract
:1. Introduction
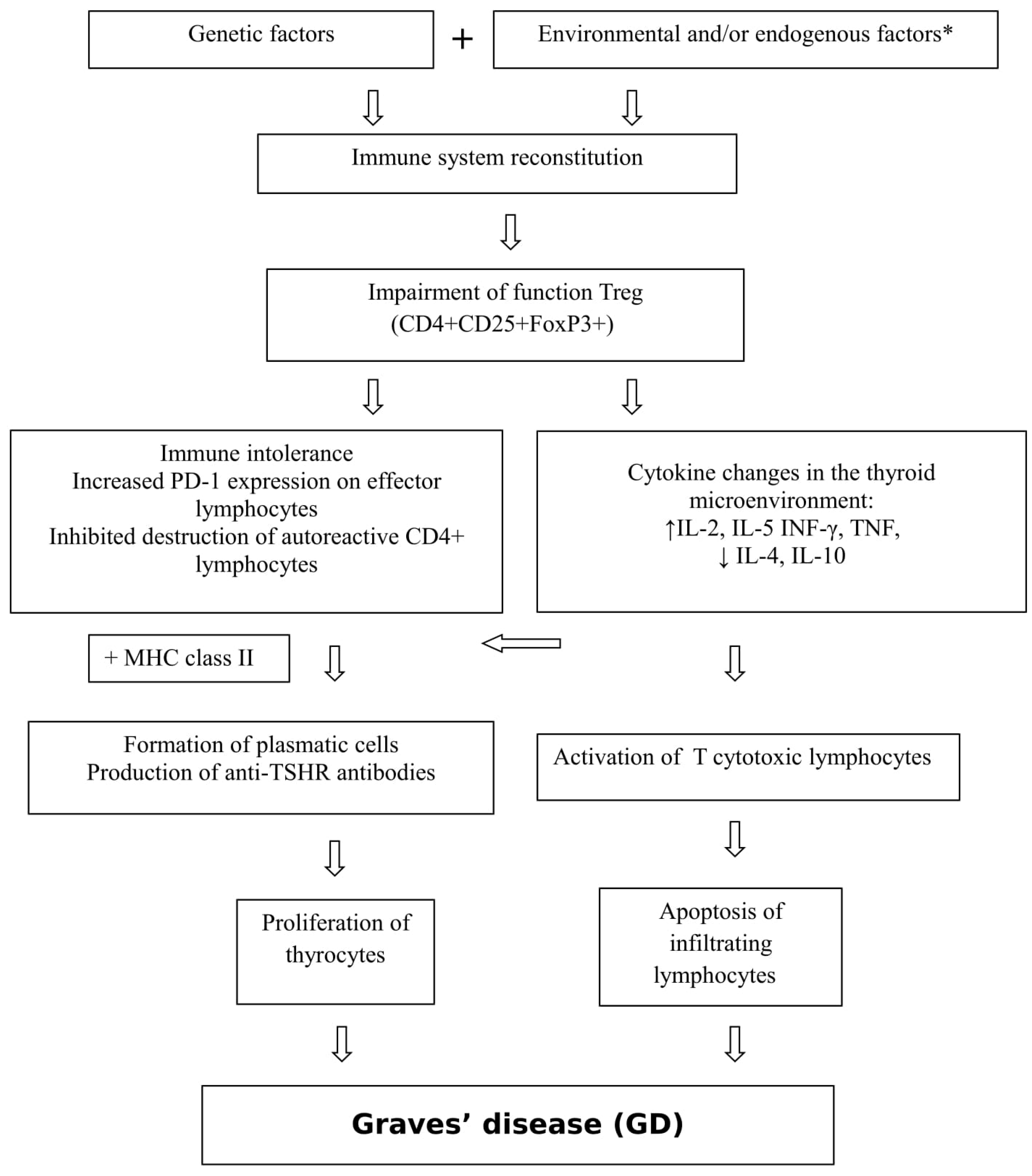
1.1. Graves’ Disease
1.2. EBV
2. Aim of the Study
3. Results
4. Discussion
5. Material and Methods
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| anti-TPO | thyroperoxidase antibodies |
| EBER | Epstein–Barr virus-encoded RNA |
| EBV | Epstein–Barr virus |
| EBNA | Epstein–Barr nuclear antigen |
| ELISA | enzyme-linked immunosorbent assay |
| FT3 | free triiodothyronine |
| FT4 | free thyroxine |
| GD | Graves’ disease |
| HLA | human leukocyte antigen |
| IL | interleukin |
| MHC | major histocompatibility complex |
| PBMCs | peripheral blood mononuclear cells |
| PCR | polymerase chain reaction |
| PD-1 | programmed cell death protein-1Tg thyroglobulin |
| TPO | thyroid peroxidase |
| Treg | regulatory T cells |
| TSH | thyroid-stimulating hormone |
| TSHR | thyroid-stimulating hormone receptor |
| TSI | thyroid-stimulating immunoglobulin |
References
- Larry, J.; Weetman, A.P. Disorders of the Thyroid gland. In Harrison’s Principles of Internal Medicine, 15th ed.; Brunwald, E., Fauci, A., Eds.; Mc Graw-Hill Publication: New York, NY, USA, 2001; Volume 2, pp. 2060–2084. [Google Scholar]
- Sowiński, J.; Gurgul, E. Nadczynność tarczycy. In Endokrynologia Kliniczna; Milewicz, A., Ed.; PTE: Wroclaw, Poland, 2012; Volume 2, pp. 252–273. [Google Scholar]
- Smith, T.J.; Hegedüs, L. Graves’ Disease. N. Engl. J. Med. 2016, 375, 1552–1565. [Google Scholar] [CrossRef] [PubMed]
- Novaes, P.; Diniz Grisolia, A.B.; Smith, T.J. Update on thyroid-associated Ophthalmopathy with a special emphasis on the ocular surface. Clin. Diabetes. Endocrinol. 2016, 2, 19. [Google Scholar] [CrossRef] [PubMed]
- Shuklaa, S.K.; Singh, G.; Ahmad, S.; Pant, P. Infections, genetic and environmental factors in pathogenesis of autoimmune thyroid diseases. Microbial. Pathogenesis 2018, 116, 279–288. [Google Scholar] [CrossRef]
- Menconi, F.; Hasham, A.; Tomer, Y. Environmental triggers of thyroiditis: Hepatitis C and interferon-α. J. Endocrinol. Investig. 2011, 34, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Rotondi, M.; Molteni, M.; Leporati, P.; Capelli, V.; Marinò, M.; Chiovato, L. Autoimmune Thyroid Diseases in Patients Treated with Alemtuzumab for Multiple Sclerosis: An Example of Selective Anti-TSH-Receptor Immune Response. Front. Endocrinol. 2017, 8, 254. [Google Scholar] [CrossRef]
- Kraszewska, A.; Abramowicz, M.; Chłopocka-Woźniak, M.; Sowiński, J.; Rybakowski, J. The effect of lithium on thyroid function in patients with bipolar disorder. Psychiatr. Pol. 2014, 48, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Kryczyk, J.; Zagrodzki, P. Selen w chorobie Gravesa-Basedowa. Post. Hig. Med. Dosw. 2013, 67, 491–498, e-ISSN 1732-2693. [Google Scholar] [CrossRef]
- Kim, D. The Role of Vitamin D in Thyroid Diseases. Int. J. Mol. Sci. 2017, 18, 1949. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, A.; Quadbeck, B.; Mueller, G.; Rettenmeier, A.W.; Hoermann, R.; Mann, K.; Steuhl, P.; Esser, J. Impact of smoking on the response to treatment of thyroid associated ophthalmopathy. Brit. J. Ophthalmol. 2004, 87, 773–776. [Google Scholar] [CrossRef] [PubMed]
- Corapcioglu, D.; Tonyukuk, V.; Kiyan, M.; Yilmaz, A.E.; Emral, R.; Kamel, N.; Erdogan, G. Relationship between thyroid autoimmunity and Yersinia enterocolitica antibodies. Thyroid 2002, 12, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Leite, J.L.; Bufalo, N.E.; Santos, R.B.; Romaldini, J.H.; Ward, L.S. Herpesvirus type 7 infection may play an important role in individuals with a genetic profile of susceptibility to Graves’ disease. Eur. J. Endocrinol. 2010, 162, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Aghili, R.; Jafarzadeh, F.; Ghorbani, R.; Khamseh, M.E.; Salami, M.A.; Malek, M. The association of Helicobacter pylori infection with Hashimoto’s thyroiditis. Acta. Med. Iran. 2013, 51, 293–296. [Google Scholar] [PubMed]
- Żuk-Wasek, A. Characterization of Epstein-Barr virus proteins – their participation in latency and relation to oncogenesis. Post. Mikrobiol. 2012, 51, 191–201. [Google Scholar]
- Chapel, H.; Haeney, M.; Misbah, S.; Snowden, N.; Senatorski, G. Immunologia Kliniczna, 1st. ed.; Czelej: Lublin, Poland, 2009; pp. 38–55. Available online: https://medbook.com.pl/ksiazka/pokaz/id/8130/tytul/immunologia-kliniczna-chapel-haeney-misbah-snowden-senatorski-czelej (accessed on 25 March 2018).
- Bocian, J.; Januszkiewicz-Lewandowska, D. Zakażenia EBV – cykl życiowy, metody diagnostyki, chorobotwórczość. Postepy Hig. Med. Dosw (online). 2011, 65, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Styczyński, J.; Gil, L.; Kyrcz-Krzemień, S.; Piątkowska-Jakubas, B.; Kałwak, K.; Wachowiak, J.; Wierzbowska, A.; Tomaszewska, A.; Drabko, K.; Czerw, T.; et al. Strategy of management in Epstein-Barr virus infections in hematology, oncology and transplantology. Guidelines of Polish Federation of Bone Marrow Transplant Centers. Acta Haematol. Pol. 2011, 43, 48–53. [Google Scholar] [CrossRef]
- Leś, B.K.; Przybylski, M.; Łazińska, B. Diagnostyka laboratoryjna mononukleozy zakaźnej u chorych leczonych ambulatoryjnie. Post. Nauk Med. 2015, 28, 42–47. [Google Scholar]
- Orgiazzi, J. Thyroid autoimmunity. Presse. Med. 2012, 41, e611–e625. [Google Scholar] [CrossRef]
- Toussirot, E.; Roudier, J. Epstein–Barr virus in autoimmune diseases. Best Pract. Res. Clin. Rheumatol. 2008, 22, 883–896. [Google Scholar] [CrossRef]
- Tarlinton, R.E.; Khaibullin, T.; Granatov, E.; Martynova, E.; Rizvanov, A.; Khaiboullin, A.S. The Interaction between Viral and Environmental Risk Factors in the Pathogenesis of Multiple Sclerosis. Int. J. Mol. Sci. 2019, 20, 303. [Google Scholar] [CrossRef]
- Thomas, D.; Karachaliou, F.; Kallergi, K.; Vlachopapadopoulou, E.; Antonaki, G.; Chatzimarkou, F.; Fotinou, A.; Kaldrymides, P.; Michalacos, S. Herpes virus antibodies seroprevalence in children with autoimmune thyroid disease. Endocrine 2008, 33, 171–175. [Google Scholar] [CrossRef]
- Volpe, R. B-lymphocytes in autoimmune thyroid disease (AITD). Rev. Endocr. Metab. Disord. 2000, 1, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Bashal, F. Hematological Disorders in Patients with Systemic Lupus Erythematosus. Open Rheumatol. J. 2013, 7, 87–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akahori, H.; Takeshita, Y.; Saito, R.; Takamura, T.; Kaneko, S. Graves’ Disease Associated with Infectious Mononucleosis due to Primary Epstein–Barr Virus Infection: Report of 3 Cases. Intern. Med. 2010, 49, 2599–2603. [Google Scholar] [CrossRef] [PubMed]
- Nagata, K.; Fukata, S.; Kanai, K.; Satoh, Y.; Segawa, T.; Kuwamoto, S.; Sugihara, H.; Kato, M.; Murakami, I.; Hayashi, K.; et al. The influence of Epstein–Barr virus reactivation in patients with Graves’ disease. Viral Immunol. 2011, 24, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Janegova, A.; Janega, P.; Rychly, B.; Kuracinova, K.; Babal, P. The role of Epstein–Barr virus infection in the development of autoimmune thyroid diseases. Endokrynol. Pol. 2015, 66, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Nagata, K.; Nakayama, Y.; Higaki, K.; Ochi, M.; Kanai, K.; Matsushita, M.; Kuwamoto, S.; Kato, M.; Murakami, I.; Iwasaki, T.; et al. Reactivation of persistent Epstein–Barr virus (EBV) causes secretion of thyrotropin receptor antibodies (TRAbs) in EBV-infected B lymphocytes with TRAbs on their surface. Autoimmunity 2015, 48, 328–335. [Google Scholar] [CrossRef]
- Moon, U.Y.; Park, S.J.; Oh, S.T.; Kim, W.-U.; Park, S.-H.; Lee, S.-H.; Cho, C.-S.; Kim, H.Y.; Lee, W.-K.; Lee, S.K. Patients with systemic lupus erythematosus have abnormally elevated Epstein–Barr virus load in blood. Arthritis Res. Ther. 2004, 6, 295–302. [Google Scholar] [CrossRef]
- Alvarez-Lafuente, R.; Fernández-Gutiérrez, B.; de Miguel, S.; Jover, J.A.; Rollin, R.; Loza, E.; Clemente, D.; Lamas, J.R. Potential relationship between herpes viruses and rheumatoid arthritis: Analysis with quantitative real time polymerase chain reaction. Ann. Rheum. Dis. 2005, 64, 1357–1359. [Google Scholar] [CrossRef]
- Morshed, S.A.; Nishioka, M.; Saito, I.; Komiyama, K.; Moro, I. Increased expression of Epstein–Barr virus in primary biliary cirrhosis patients. Gastroenterol. Jpn. 1992, 27, 751–758. [Google Scholar] [CrossRef]
- Alvarez-Lafuente, R.; García-Montojo, M.; De Las Heras, V.; Domínguez-Mozo, M.I.; Bartolome, M.; Benito-Martin, M.S.; Arroyo, R. Herpesviruses and human endogenous retroviral sequences in the cerebrospinal fluid of multiple sclerosis patients. Mult. Scler. 2008, 14, 595–601. [Google Scholar] [CrossRef]
- Pyzik, A.; Grywalska, E.; Matyjaszek-Matuszek, B.; Smoleń, A.; Pyzik, D.; Roliński, J. Frequencies of PD-1- positive T CD3+CD4+, T CD3+CD8+ and B CD19+ lymphocytes in female patients with Graves’ disease and healthy controls - preliminary study. Mol. Cell. Endocrinol. 2017, 448, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Grywalska, E.; Roliński, J.; Pasiarski, M.; Korona-Glowniak, I.; Maj, M.; Surdacka, A.; Grafka, A.; Stelmach-Gołdyś, A.; Zgurski, M.; Góźdź, S.; et al. High Viral Loads of Epstein–Barr Virus DNA in Peripheral Blood of Patients with Chronic Lymphocytic Leukemia Associated with Unfavorable Prognosis. PLoS ONE 2015, 10, e0140178. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Study Group (39) | Control Group (20) | p | χ² | |||
|---|---|---|---|---|---|---|---|
| EBV DNA Present Number (%) | Men | 2 | 12 (30.77%) | 0 (0%) | 0.01 | 5.94 | |
| women | 10 | ||||||
| Number of EBV DNA copies /mL | men | Median (min.–max.) | 4874.5 (600.8–9148.21) | ||||
| (Q1–Q3) | 2737.65–7011.36 | ||||||
| women | Median (min.–max.) | 1681 (620.44–27,339.30) | |||||
| (Q1–Q3) | 676.43–4202.35 | ||||||
| Number of EBV DNA copies (copies of EBV DNA/μgDNA) | men | Median (min.–max.) | 41.62 (22.35–60.89) | ||||
| (Q1–Q3) | 31.97–51.26 | ||||||
| women | Median (min.–max.) | 29.6 (9.27–659.1) | |||||
| (Q1–Q3) | 11.99–136.23 | ||||||
| Number of EBV DNA copies (copies of EBV DNA/100,000 cells) | men | Median (min.–max.) | 27.47 (14.75–40.19) | ||||
| (Q1–Q3) | 21.11–33.83 | ||||||
| women | Median (min.–max.) | 19.53 (6.12–435) | |||||
| (Q1–Q3) | 7.91–89.92 | ||||||
| Symptoms and Signs | EBV DNA (+) | EBV DNA (−) | p | χ² |
|---|---|---|---|---|
| Neuropsychiatric | ||||
| Irritability | 8 (20.51%) | 16 (41.03%) | 0.66 | 0.19* |
| Emotional lability | 4 (10.26%) | 12 (30.77%) | 0.77 | 0.09 ** |
| Sleep disorders | 9 (23.08%) | 17 (43.59%) | 0.46 | 0.54 * |
| Fatigue | 11 (28.21%) | 22 (56.41%) | 0.42 | 0.66 * |
| Somatic | ||||
| Weight loss | 9 (23.08%) | 20 (51.28%) | 0.95 | 0.004 * |
| Heart palpitation | 9 (23.08%) | 19 (48.72%) | 0.77 | 0.88 * |
| Heat intolerance | 7 (17.95%) | 14 (35.90%) | 0.71 | 0.14 * |
| Excessive sweating | 7 (17.95%) | 15 (38.46%) | 0.87 | 0.03 * |
| Menstrual disorder | 0 (0) | 3 (9.38%) | 0.22 | 1.50** |
| Muscle weakness | 7 (17.95%) | 13 (33.33%) | 0.56 | 0.34 * |
| Orbitopathy | 2 (5.13%) | 3 (7.69%) | 0.63 | 0.23 ** |
| Goiter | 12 (30.77%) | 27 (69.23%) | 0.77 | 1.13 * |
| Tachycardia | 6 (15.38%) | 19 (48.72%) | 0.22 | 1.50 * |
| Velvet skin | 9 (23.08%) | 23 (58.97%) | 0.44 | 0.59 * |
| Muscle trembling | 8 (20.51%) | 16 (41.03%) | 0.66 | 0.19 * |
| Superficial tendon reflexes | 1 (2.56%) | 3 (7.69%) | 0.79 | 0.07 ** |
| High amplitude of blood pressure | 2 (5.13%) | 4 (10.26%) | 0.88 | 0.02 ** |
| Pretibial myxedema | 1 (2.56%) | 0 | - | - |
| Thyroid acropachy | 1 (2.56%) | 0 | - | - |
| Parameter | Present EBV DNA | p | z | ||
|---|---|---|---|---|---|
| TSI (U/L) | value | Median (min–max) | 11.95 (2.20–38.50) | 0.68 | –0.41 |
| Q1–Q3 | 5.15–20.75 | ||||
| Anti-TPO (U/mL) | Median (min–max) | 774 (29.00–3000.00) | 0.84 | 0.20 | |
| Q1–Q3 | 120.36–2464.15 | ||||
| Anti-TG (IU/mL) | Median (min–max) | 109.42 (10.00–407.00) | 0.41 | 0.83 | |
| Q1–Q3 | 15.00–231.50 | ||||
| TSH (mIU/L) | Median (min–max) | 0.008 (0.005–0.008) | 0.82 | 0.36 | |
| Q1–Q3 | 0.008–0.008 | ||||
| FT4 (ng/dL) | Median (min–max) | 3.94 (2.23–5.08) | 0.16 | 1.39 | |
| Q1–Q3 | 3.00–4.59 | ||||
| FT3 (pg/mL) | Median (min–max) | 14.10 (5.60–20.00) | 0.28 | 1.10 | |
| Q1–Q3 | 9.30–17.60 | ||||
| Parameter | Study Group (39) | Control Group (20) | p Value | |
|---|---|---|---|---|
| Lymphocytes (1 × 109/L) | Mean ± SD | 2.01 ± 0.66 | 2.35 ± 0.59 | 0.02 |
| Median (min–max) | 1.79 (1.25–4.18) | 2.36 (1.39–3.38) | ||
| CD4+ (%) | Mean ± SD | 49.64 ± 7.5 | 44.46 ± 2.50 | <0.001 |
| Median (min–max) | 48.67 (22.85–62.63) | 44.16 (40.71–48.84) | ||
| CD4+ (103/mm3) | Mean ± SD | 0.89 ± 0.35 | 1.04 ± 0.27 | 0.04 |
| Median (min–max) | 0.87 (0.59–2.44) | 1.04 (0.62–1.54) | ||
| CD8+ (%) | Mean ± SD | 26.95 ± 4.28 | 34.36 ± 3.29 | <0.001 |
| Median (min–max) | 27.09 (20.08–38.69) | 34.7 (29.3–39.6) | ||
| CD8+ (103/mm3) | Mean ± SD | 0.56 ± 0.24 | 0.80 ± 0.20 | <0.001 |
| Median (min–max) | 0.48 (0.28–1.49) | 0.82 (0.44–1.10) | ||
| Parameter | Study Group (39) | Control Group (20) | ||
|---|---|---|---|---|
| Gender (Number and %) | Women | 32 (82.05%) | 15 (75%) | |
| Men | 7 (17.95%) | 5 (25%) | ||
| Age (years) | Mean ± SD | 41.49 ± 15.74 | 42.15 ± 10.38 | |
| Median (min.–max.) | 39 (22–95) | 40 (29–60) | ||
| Duration of hyperthyroidism symptoms (months) | Mean ± SD | 2.57 ± 1.91 | ||
| Median (min.–max.) | 2 (0–8) | |||
| TSI (U/L) | presence | present | absent | |
| value | Mean ± SD | 12.63 ± 9.41 | ||
| Median (min.–max.) | 11.2 (1.5–39.4) | |||
| Anti-TPO U/mL | Median (min.–max.) | 1009 (13.7–22810) | 11 (5–201) | |
| Q1–Q3 | 107.7–2456 | 8–17.5 | ||
| Anti-Tg (IU/mL) | Median (min.–max.) | 121.5 (10–1360) | 12 (10–364) | |
| Q1–Q3 | 15–304 | 10–45 | ||
| TSH (mIU/L) | Median (min.–max.) | 0.008 (0.005–0.03) | 1.42 (0.72–2.6) | |
| Q1–Q3 | 0.008–0.008 | 1.22–1.77 | ||
| FT4 (ng/dL) | Median (min.–max.) | 4.78 (2.14–8.02) | ||
| Q1–Q3 | 3.94–5.81 | |||
| FT3 (pg/mL) | Median (min.–max.) | 17.35 (5.6–133) | ||
| Q1–Q3 | 11.6–20 | |||
| Leukocytes (1 × 109/L) | Mean ± SD | 5.98 ± 1.72 | 6.23 ± 1.34 | |
| Median (min.–max.) | 5.66 (3.42–9.99) | 5.84 (4.12–9.68) | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pyzik, A.; Grywalska, E.; Matyjaszek-Matuszek, B.; Ludian, J.; Kiszczak-Bochyńska, E.; Smoleń, A.; Roliński, J.; Pyzik, D. Does the Epstein–Barr Virus Play a Role in the Pathogenesis of Graves’ Disease? Int. J. Mol. Sci. 2019, 20, 3145. https://doi.org/10.3390/ijms20133145
Pyzik A, Grywalska E, Matyjaszek-Matuszek B, Ludian J, Kiszczak-Bochyńska E, Smoleń A, Roliński J, Pyzik D. Does the Epstein–Barr Virus Play a Role in the Pathogenesis of Graves’ Disease? International Journal of Molecular Sciences. 2019; 20(13):3145. https://doi.org/10.3390/ijms20133145
Chicago/Turabian StylePyzik, Aleksandra, Ewelina Grywalska, Beata Matyjaszek-Matuszek, Jarosław Ludian, Ewa Kiszczak-Bochyńska, Agata Smoleń, Jacek Roliński, and Dawid Pyzik. 2019. "Does the Epstein–Barr Virus Play a Role in the Pathogenesis of Graves’ Disease?" International Journal of Molecular Sciences 20, no. 13: 3145. https://doi.org/10.3390/ijms20133145
APA StylePyzik, A., Grywalska, E., Matyjaszek-Matuszek, B., Ludian, J., Kiszczak-Bochyńska, E., Smoleń, A., Roliński, J., & Pyzik, D. (2019). Does the Epstein–Barr Virus Play a Role in the Pathogenesis of Graves’ Disease? International Journal of Molecular Sciences, 20(13), 3145. https://doi.org/10.3390/ijms20133145





