Investigations of Accessibility of T2/T3 Copper Center of Two-Domain Laccase from Streptomyces griseoflavus Ac-993
Abstract
1. Introduction
2. Results
2.1. Structural Comparison of Tunnels of Three-Domain and Two-Domain Laccases
2.2. SgfSL and Mutants: Crystal Structures and Catalytic Activity
3. Discussion
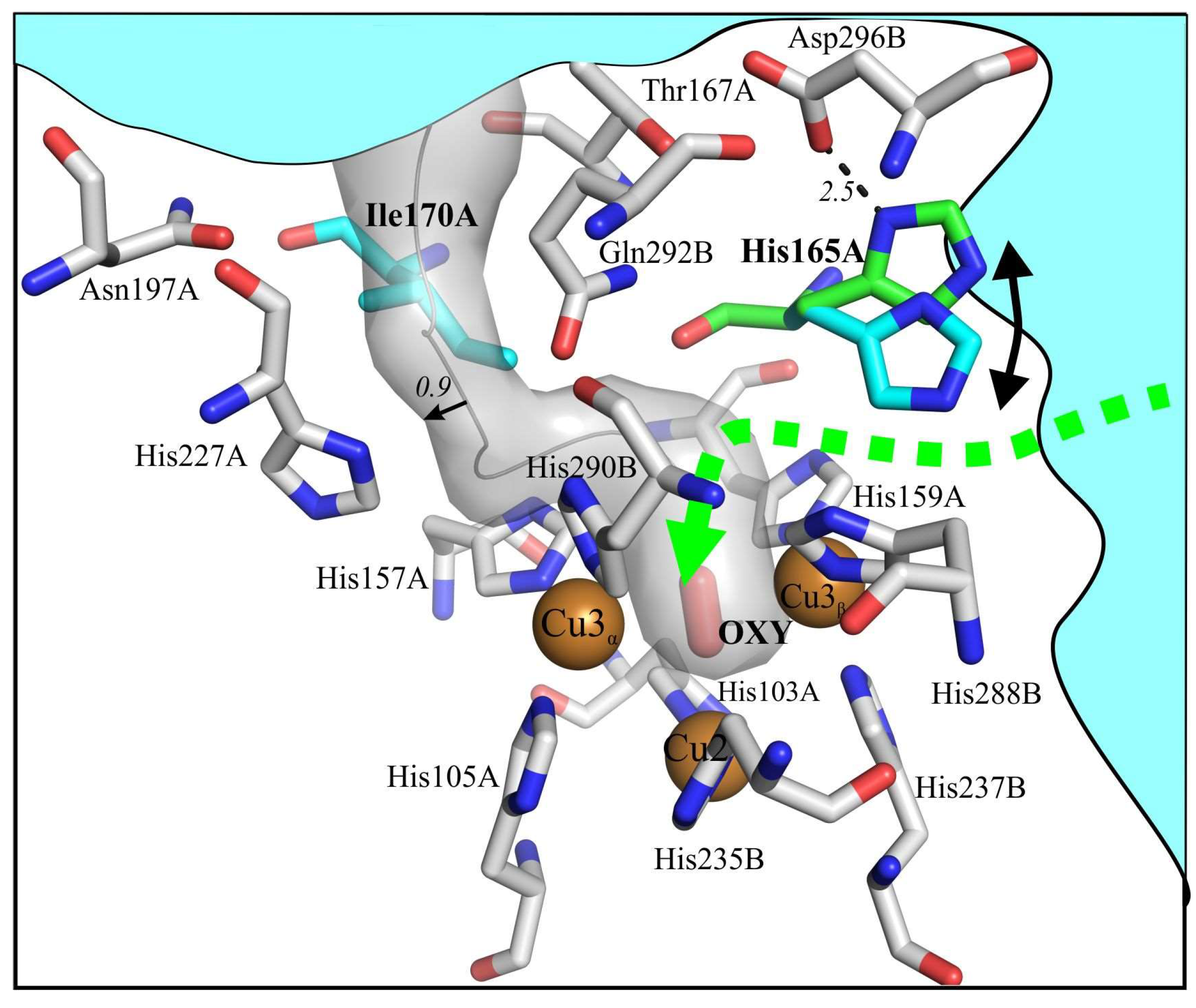
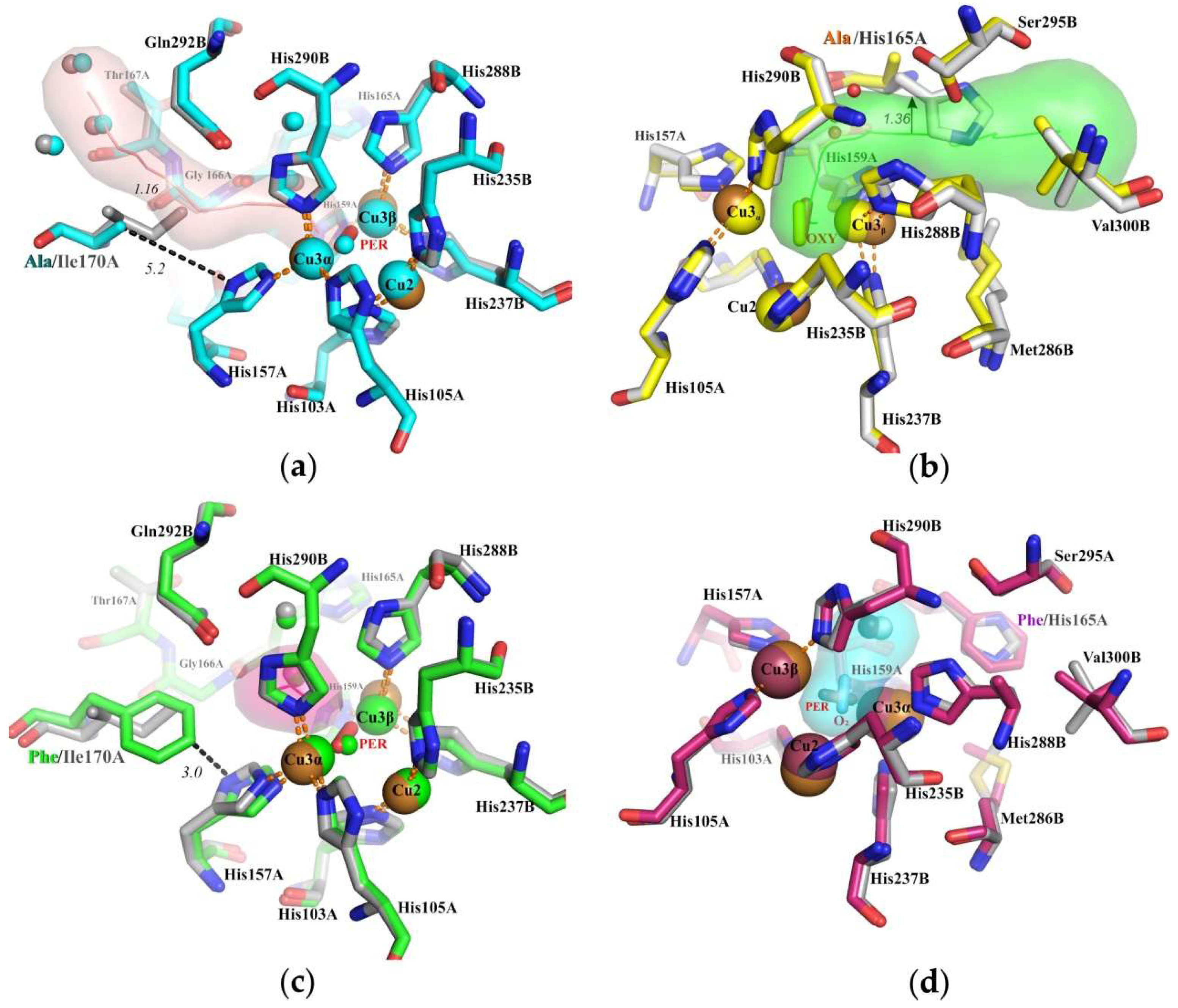
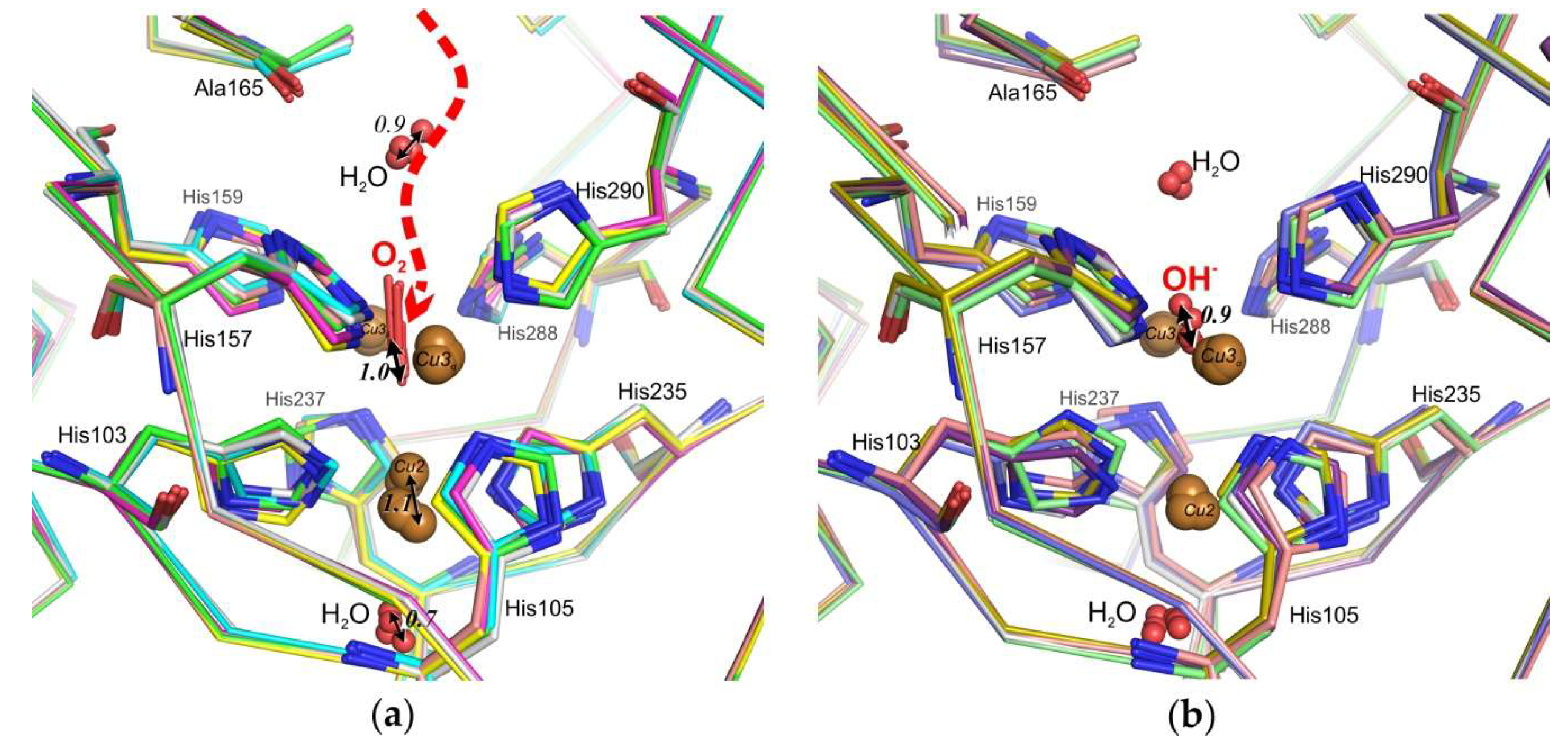
3.1. Ile170 Could Be Indirectly Involved in The Coordination of Cu3α Ion within the TNC
3.2. Histidine 165 Is A “Gateway” of T3 Tunnel Leading to Cu3β of T2/T3 Center
4. Materials and Methods
4.1. Plasmid Construction
4.2. Purification of SgfSL Mutants
4.3. Activity Assays
4.4. Crystallization and Crystallography
4.5. Noble Gas Pressurization
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| TNC | TriNuclear Center |
| 3D | Three-domain |
| 2D | Two-domain |
| SgfSL | Streptomyces griseoflavus Small Laccase |
References
- Sharma, A.; Jain, K.K.; Jain, A.; Kidwai, M.; Kuhad, R.C. Bifunctional in vivo role of laccase exploited in multiple biotechnological applications. Appl. Microbiol. Biotechnol. 2018, 102, 10327–10343. [Google Scholar] [CrossRef] [PubMed]
- Solomon, E.I.; Sundaram, U.M.; Machonkin, T.E. Multicopper Oxidases and Oxygenases. Chem. Rev. 1996, 96, 2563–2606. [Google Scholar] [CrossRef] [PubMed]
- Bento, I.; Martins, L.O.; Gato Lopes, G.; Arménia Carrondo, M.; Lindley, P.F. Dioxygen reduction by multi-copper oxidases; a structural perspective. Dalt. Trans. 2005, 4, 3507. [Google Scholar] [CrossRef] [PubMed]
- Hakulinen, N.; Kiiskinen, L.-L.; Kruus, K.; Saloheimo, M.; Paananen, A.; Koivula, A.; Rouvinen, J. Crystal structure of a laccase from Melanocarpus albomyces with an intact trinuclear copper site. Nat. Struct. Biol. 2002, 9, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Skálová, T.; Dohnálek, J.; Østergaard, L.H.; Østergaard, P.R.; Kolenko, P.; Dušková, J.; Štěpánková, A.; Hašek, J. The Structure of the Small Laccase from Streptomyces coelicolor Reveals a Link between Laccases and Nitrite Reductases. J. Mol. Biol. 2009, 385, 1165–1178. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Balda, S.; Gupta, N.; Capalash, N.; Sharma, P. Functional substitution of domain 3 (T1 copper center) of a novel laccase with Cu ions. Int. J. Biol. Macromol. 2019, 123, 1052–1061. [Google Scholar] [CrossRef]
- Solomon, E.I.; Chen, P.; Metz, M.; Lee, S.-K.; Palmer, A.E. Oxygen Binding, Activation, and Reduction to Water by Copper Proteins. Angew. Chemie Int. Ed. 2001, 40, 4570–4590. [Google Scholar] [CrossRef]
- Trubitsina, L.I.; Tishchenko, S.V.; Gabdulkhakov, A.G.; Lisov, A.V.; Zakharova, M.V.; Leontievsky, A.A. Structural and functional characterization of two-domain laccase from Streptomyces viridochromogenes. Biochimie 2015, 112, 151–159. [Google Scholar] [CrossRef]
- Tishchenko, S.; Gabdulkhakov, A.; Trubitsina, L.; Lisov, A.; Zakharova, M.; Leontievsky, A. Crystallization and X-ray diffraction studies of a two-domain laccase from Streptomyces griseoflavus. Acta Crystallogr. Sect.F Struct. Biol. Commun. 2015, 71, 1200–1204. [Google Scholar] [CrossRef]
- Gabdulkhakov, A.G.; Kostareva, O.S.; Kolyadenko, I.A.; Mikhaylina, A.O.; Trubitsina, L.I.; Tishchenko, S.V. Incorporation of Copper Ions into T2/T3 Centers of Two-Domain Laccases. Mol. Biol. 2018, 52, 23–29. [Google Scholar] [CrossRef]
- Gupta, A.; Nederlof, I.; Sottini, S.; Tepper, A.W.J.W.; Groenen, E.J.J.; Thomassen, E.A.J.; Canters, G.W. Involvement of Tyr108 in the enzyme mechanism of the small laccase from Streptomyces coelicolor. J. Am. Chem. Soc. 2012, 134, 18213–18216. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.M.; Solomon, E.I. Electron Transfer and Reaction Mechanism of Laccases. Cell Mol. Life Sci. 2015, 72, 869–883. [Google Scholar] [CrossRef] [PubMed]
- Komori, H.; Kataoka, K.; Tanaka, S.; Matsuda, N.; Higuchi, Y.; Sakurai, T. Exogenous acetate ion reaches the type II copper centre in CueO through the water-excretion channel and potentially affects the enzymatic activity. Acta Crystallogr. Sect.F Struct. Biol. Commun. 2016, 72, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Polyakov, K.M.; Gavryushov, S.; Ivanova, S.; Fedorova, T.V.; Glazunova, O.A.; Popov, A.N.; Koroleva, O.V. Structural study of the X-ray-induced enzymatic reduction of molecular oxygen to water by Steccherinum murashkinskyi laccase: Insights into the reaction mechanism. Acta Crystallogr. Sect. D Struct. Biol. 2017, 73, 388–401. [Google Scholar] [CrossRef] [PubMed]
- Brunori, M.; Cutruzzolà, F.; Savino, C.; Travaglini-Allocatelli, C.; Vallone, B.; Gibson, Q.H. Structural dynamics of ligand diffusion in the protein matrix: A study on a new myoglobin mutant Y(B10) Q(E7) R(E10). Biophys. J. 1999, 76, 1259–1269. [Google Scholar] [CrossRef]
- Luna, V.M.; Chen, Y.; Fee, J.A.; Stout, C.D. Crystallographic studies of Xe and Kr binding within the large internal cavity of cytochrome ba3 from Thermus thermophilus: Structural analysis and role of oxygen transport channels in the heme-Cu oxidases. Biochemistry 2008, 47, 4657–4665. [Google Scholar] [CrossRef] [PubMed]
- Prangé, T.; Schiltz, M.; Pernot, L.; Colloc’h, N.; Longhi, S.; Bourguet, W.; Fourme, R. Exploring hydrophobic sites in proteins with xenon or krypton. Proteins Struct. Funct. Genet. 1998, 30, 61–73. [Google Scholar] [CrossRef]
- Gabdulkhakov, A.; Guskov, A.; Broser, M.; Kern, J.; Müh, F.; Saenger, W.; Zouni, A. Probing the Accessibility of the Mn4Ca Cluster in Photosystem II: Channels Calculation, Noble Gas Derivatization, and Cocrystallization with DMSO. Structure 2009, 17, 1223–1234. [Google Scholar] [CrossRef]
- Lafumat, B.; Mueller-Dieckmann, C.; Leonard, G.; Colloc’H, N.; Prangé, T.; Giraud, T.; Dobias, F.; Royant, A.; Van Der Linden, P.; Carpentier, P. Gas-sensitive biological crystals processed in pressurized oxygen and krypton atmospheres: Deciphering gas channels in proteins using a novel “soak-and-freeze” methodology. J. Appl. Crystallogr. 2016, 49, 1478–1487. [Google Scholar] [CrossRef]
- Pavelka, A.; Sebestova, E.; Kozlikova, B.; Brezovsky, J.; Sochor, J.; Damborsky, J. CAVER: Algorithms for Analyzing Dynamics of Tunnels in Macromolecules. IEEE/ACM Trans. Comput. Biol. Bioinforma. 2016, 13, 505–517. [Google Scholar] [CrossRef]
- Vaguine, A.A.; Richelle, J.; Wodak, S.J. SFCHECK: A unified set of procedures for evaluating the quality of macromolecular structure-factor data and their agreement with the atomic model. Acta Crystallogr. Sect. D Biol. Crystallogr. 1999, 55, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Dubé, E.; Shareck, F.; Hurtubise, Y.; Daneault, C.; Beauregard, M. Homologous cloning, expression, and characterisation of a laccase from Streptomyces coelicolor and enzymatic decolourisation of an indigo dye. Appl. Microbiol. Biotechnol. 2008, 79, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Johannes, C.; Majcherczyk, A. Laccase activity tests and laccase inhibitors. J. Biotechnol. 2000, 78, 193–199. [Google Scholar] [CrossRef]
- De la Mora, E.; Lovett, J.E.; Blanford, C.F.; Garman, E.F.; Valderrama, B.; Rudino-Pinera, E. Structural changes caused by radiation-induced reduction and radiolysis: The effect of X-ray absorbed dose in a fungal multicopper oxidase. Acta Crystallogr. Sect. D Biol. Crystallogr. 2012, 68, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Rulísek, L.; Solomon, E.I.; Ryde, U. A combined quantum and molecular mechanical study of the O2 reductive cleavage in the catalytic cycle of multicopper oxidases. Inorg. Chem. 2005, 44, 5612–5628. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Posada, H.; Centeno-Leija, S.; Rojas-Trejo, S.P.; Rodríguez-Almazán, C.; Stojanoff, V.; Rudiño-Piñera, E. X-ray-induced catalytic active-site reduction of a multicopper oxidase: Structural insights into the proton-relay mechanism and O2-reduction states. Acta Crystallogr. Sect. D Biol. Crystallogr. 2015, 71, 2396–2411. [Google Scholar] [CrossRef] [PubMed]
- Augustine, A.J.; Kjaergaard, C.; Qayyum, M.; Ziegler, L.; Kosman, D.J.; Hodgson, K.O.; Hedman, B.; Solomon, E.I. Systematic perturbation of the trinuclear copper cluster in the multicopper oxidases: The role of active site asymmetry in its reduction of O2 to H2O. J. Am. Chem. Soc. 2010, 132, 6057–6067. [Google Scholar] [CrossRef] [PubMed]
- Wariishi, H.; Valli, K.; Gold, M.H. Manganese(II) oxidation by manganese peroxidase from the basidiomycete Phanerochaete chrysosporium. Kinetic mechanism and role of chelators. J. Biol. Chem. 1992, 267, 23688–23695. [Google Scholar] [PubMed]
- Heinfling, A.; Martínez, M.J.; Martínez, A.T.; Bergbauer, M.; Szewzyk, U. Purification and characterization of peroxidases from the dye-decolorizing fungus Bjerkandera adusta. FEMS Microbiol. Lett. 1998, 165, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Mueller, U.; Förster, R.; Hellmig, M.; Huschmann, F.U.; Kastner, A.; Malecki, P.; Pühringer, S.; Röwer, M.; Sparta, K.; Steffien, M.; et al. The macromolecular crystallography beamlines at BESSY II of the Helmholtz-Zentrum Berlin: Current status and perspectives. Eur. Phys. J. Plus 2015, 130, 141. [Google Scholar] [CrossRef]
- Kraft, P.; Bergamaschi, A.; Broennimann, C.; Dinapoli, R.; Eikenberry, E.F.; Henrich, B.; Johnson, I.; Mozzanica, A.; Schlepütz, C.M.; Willmott, P.R.; et al. Performance of single-photon-counting PILATUS detector modules. J. Synchrotron Radiat. 2009, 16, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Kabsch, W. XDS. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 125–132. [Google Scholar] [CrossRef] [PubMed]
- McCoy, A.J.; Grosse-Kunstleve, R.W.; Adams, P.D.; Winn, M.D.; Storoni, L.C.; Read, R.J. Phaser crystallographic software. J. Appl. Crystallogr. 2007, 40, 658–674. [Google Scholar] [CrossRef] [PubMed]
- Murshudov, G.N.; Skubák, P.; Lebedev, A.A.; Pannu, N.S.; Steiner, R.A.; Nicholls, R.A.; Winn, M.D.; Long, F.; Vagin, A.A. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr. Sect. D Biol. Crystallogr. 2011, 67, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Emsley, P.; Lohkamp, B.; Scott, W.G.; Cowtan, K. Features and development of Coot. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 486–501. [Google Scholar] [CrossRef]
- Afonine, P.V.; Grosse-Kunstleve, R.W.; Echols, N.; Headd, J.J.; Moriarty, N.W.; Mustyakimov, M.; Terwilliger, T.C.; Urzhumtsev, A.; Zwart, P.H.; Adams, P.D. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. Sect. D Biol. Crystallogr. 2012, 68, 352–367. [Google Scholar] [CrossRef]
- DeLano, W.L. The PyMOL Molecular Graphics System. Available online: http://www.pymol.org/ (accessed on 25 November 2018).
| H165Flow Cu | H165F | H165A | I170F | I170A | SgfSLwtlow Cu | |
|---|---|---|---|---|---|---|
| Data collection | ||||||
| Space group | P 21 | P1 | P1 | P1 | P 21 | P 21 |
| Cell parameters | ||||||
| a, b, c (Å) | 75.88, 95.07, 116.82 | 77.03, 94.58, 115.93 | 77.36, 95.12, 116.48 | 77.25, 94.77, 116.18 | 74.79, 94.69, 116.49 | 75.53, 94.62, 116.50 |
| α, β, γ (°) | 90.00, 90.58, 90.00 | 90.00, 90.00, 92.01 | 90.13, 90.11, 91.85 | 90.13, 89.95, 92.56 | 90.00, 90.87, 90.00 | 90.00, 90.39, 90.00 |
| Monomers in asymmetric unit | 6 | 12 | 12 | 12 | 6 | 6 |
| Resolution (Å) | 50.0–2.0 (2.12–2.0) a | 50.0–1.92 (2.05–1.92) a | 50.0–2.3 (2.45–2.30) a | 50.0–1.85 (1.90–1.85) a | 50.0–1.98 (2.03–1.98) a | 50.0–1.80 (1.84–1.80) a |
| Total No. of reflections | 206,927 (29,456) | 833,269 (147,319) | 507,815 (80,770) | 536,185 (26,532) | 757,980 (54,090) | 574,463 (45,966) |
| No. of unique reflections | 90,309 (14,288) | 235,591 (80,443) | 143,173 (22,664) | 256,815 (12,984) | 112,783 (8272) | 151,965 (11,814) |
| Rmerge (%) | 10.5 (50.8) | 9.1 (86.0) | 11.0 (83.8) | 8.2 (88.7) | 12.5 (87.5) | 6.6 (88.2) |
| I/σ (I) | 5.41 (1.49) | 7.88 (1.57) | 9.35 (1.42) | 5.89 (0.96) | 10.13 (2.30) | 9.86 (1.55) |
| Completeness (%) | 80.2 (78.9) | 94.3 (92.9) | 98.0 (95.9) | 91.4 (62.2) | 99.8 (99.9) | 99.4 (99.7) |
| CC1/2 | 0.99 (0.69) | 0.99 (0.63) | 0.99 (0.57) | 0.99 (0.35) | 0.99 (0.73) | 0.99 (0.67) |
| Redundancy | 2.29 (2.06) | 3.59 (3.55) | 3.55 (3.57) | 2.09 (2.04) | 6.72 (6.54) | 3.78 (3.89) |
| Refinement | ||||||
| Resolution (Å) | 49.77–2.00 (2.02–2.00) | 49.41–1.95 (1.98–1.95) | 49.7–2.3 (2.34–2.31) | 47.33–1.85 (1.87–1.85) | 49.60–1.98 (2.01–1.98) | 45.97–1.80 (1.84–1.80) |
| No. reflections | 90,303 (5785) | 225,948 (20,608) | 143,173 (6693) | 256,747 (5256) | 112,768 (3514) | 150,800 (10,599) |
| Rwork (%) | 19.97 (31.94) | 19.47 (30.86) | 17.09 (26.66) | 17.85 (35.78) | 17.40 (24.36) | 14.20 (27.78) |
| Rfree (%) | 23.32 (35.91) | 23.10 (32.81) | 22.21 (30.83) | 23.70 (39.29) | 21.48 (29.57) | 17.94 (32.88) |
| R.m.s. deviations | ||||||
| Bond lengths (Å) | 0.008 | 0.008 | 0.008 | 0.008 | 0.007 | 0.007 |
| Bond angles (°) | 0.928 | 0.995 | 0.961 | 0.874 | 0.854 | 0.823 |
| Ramachandran plot (%) | ||||||
| Most favored | 96.91 | 96.78 | 94.66 | 96.40 | 98.07 | 97.82 |
| Additionally allowed | 3.09 | 3.07 | 4.88 | 3.60 | 1.93 | 2.18 |
| Generously allowed | 0.00 | 0.15 | 0.46 | 0.00 | 0.00 | 0.00 |
| PDB ID | 5MKM | 6FC7 | 6FDJ | 6RH9 | 6RHQ | 6S0O |
| Protein | Cu1 | Cu3α | Cu3β | Cu2 | ||||
|---|---|---|---|---|---|---|---|---|
| Occupancy * (%) | B-factor * (Å2) | Occupancy * (%) | B-factor * (Å2) | Occupancy* (%) | B-factor * (Å2) | Occupancy * (%) | B-factor * (Å2) | |
| SgfSLwt | 0.98 | 19 | 1.00 | 21 | 1.00 | 24 | 0.48 | 56 |
| SgfSLwtlow Cu | 1.00 | 31 | 1.00 | 39 | 0.88 | 48 | 0.22 | 73 |
| H165Flow Cu | 1.00 | 22 | 0.92 | 28 | 0 | 0 | 0 | 0 |
| H165F | 0.96 | 27 | 0.96 | 34 | 0.70 | 38 | 0.27 | 40 |
| H165A | 0.98 | 28 | 0.99 | 35 | 0.95 | 40 | 0.48 | 40 |
| I170F | 1 | 31 | 1 | 35 | 0.97 | 39 | 0.53 | 100 |
| I170A | 1 | 25 | 0.97 | 31 | 0.75 | 38 | 0.19 | 50 |
| Substrate | Protein | Km (mM) | kcat (s−1) | kcat/Km (mM−1 s−1) | Activity nM O2 min−1 µg−1 |
|---|---|---|---|---|---|
| ABTS | SgfSL WT | 0.10 ± 0.008 | 6.60 ± 0.12 | 66.28 | 0.82 ± 0.01 |
| H165A | 0.12 ± 0.01 | 10.80 ± 0.21 | 90.00 | 1.45 ± 0.10 | |
| H165F | 0.45 ± 0.03 | 0.12 ± 0.009 | 0.26 | 0.13 ± 0.06 | |
| H165Flow Cu | 0.47± 0.02 | 0.11 ± 0.007 | 0.23 | 0.10 ± 0.03 | |
| I170A | 0.63 ± 0.11 | 0.70 ± 0.20 | 1.11 | 0.11 ± 0.01 | |
| I170F | 0.20 ± 0.04 | 0.08 ± 0.01 | 0.40 | 0.08 ± 0.04 | |
| 2,6-DMP | SgfSL WT | 5.20 ± 0.11 | 0.80 ± 0.09 | 0.15 | |
| H165A | 6.10 ± 0.34 | 1.10 ± 0.06 | 0.18 | ||
| H165F | - | - | - | ||
| H165Flow Cu | - | - | - | ||
| I170A | - | - | - | ||
| I170F | - | - | - | ||
| K4 [Fe(CN)6] | SgfSL WT | 2.28 ± 0.17 | |||
| H165A | 6.47 ± 0.27 | ||||
| H165F | 0.26 ± 0.12 | ||||
| I170A | 0.18 ± 0.01 | ||||
| I170F | 0.14 ± 0.04 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabdulkhakov, A.; Kolyadenko, I.; Kostareva, O.; Mikhaylina, A.; Oliveira, P.; Tamagnini, P.; Lisov, A.; Tishchenko, S. Investigations of Accessibility of T2/T3 Copper Center of Two-Domain Laccase from Streptomyces griseoflavus Ac-993. Int. J. Mol. Sci. 2019, 20, 3184. https://doi.org/10.3390/ijms20133184
Gabdulkhakov A, Kolyadenko I, Kostareva O, Mikhaylina A, Oliveira P, Tamagnini P, Lisov A, Tishchenko S. Investigations of Accessibility of T2/T3 Copper Center of Two-Domain Laccase from Streptomyces griseoflavus Ac-993. International Journal of Molecular Sciences. 2019; 20(13):3184. https://doi.org/10.3390/ijms20133184
Chicago/Turabian StyleGabdulkhakov, Azat, Ilya Kolyadenko, Olga Kostareva, Alisa Mikhaylina, Paulo Oliveira, Paula Tamagnini, Alexander Lisov, and Svetlana Tishchenko. 2019. "Investigations of Accessibility of T2/T3 Copper Center of Two-Domain Laccase from Streptomyces griseoflavus Ac-993" International Journal of Molecular Sciences 20, no. 13: 3184. https://doi.org/10.3390/ijms20133184
APA StyleGabdulkhakov, A., Kolyadenko, I., Kostareva, O., Mikhaylina, A., Oliveira, P., Tamagnini, P., Lisov, A., & Tishchenko, S. (2019). Investigations of Accessibility of T2/T3 Copper Center of Two-Domain Laccase from Streptomyces griseoflavus Ac-993. International Journal of Molecular Sciences, 20(13), 3184. https://doi.org/10.3390/ijms20133184






