Artificial Saliva: Challenges and Future Perspectives for the Treatment of Xerostomia
Abstract
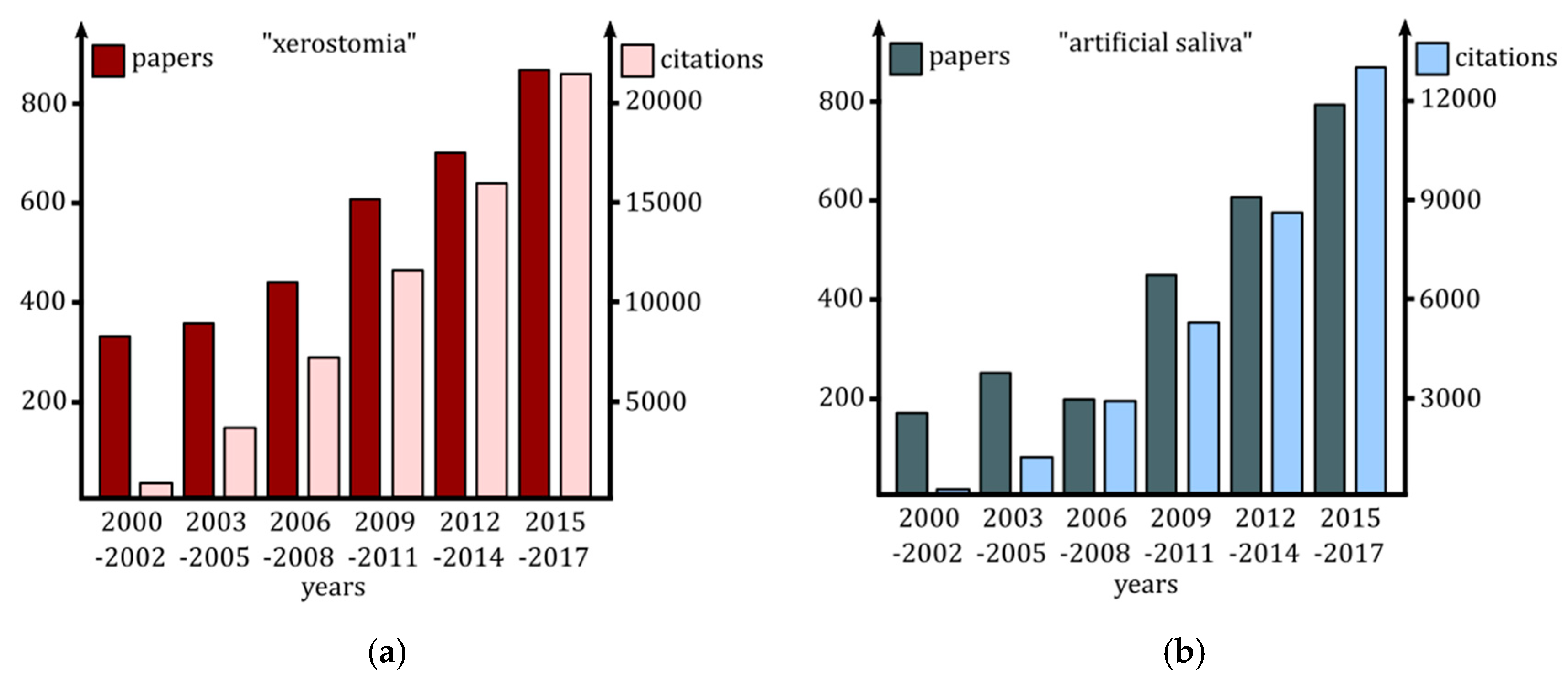
:1. Introduction
2. Xerostomia: Etiology of Salivary Glands Dysfunctions
3. Therapeutic Options
3.1. Methods for Salivary Glands Stimulation or Protection
3.2. Symptomatic Therapies: Saliva Substitutes
4. Artificial Saliva
4.1. Rheological and Lubricating Properties of Artificial Saliva
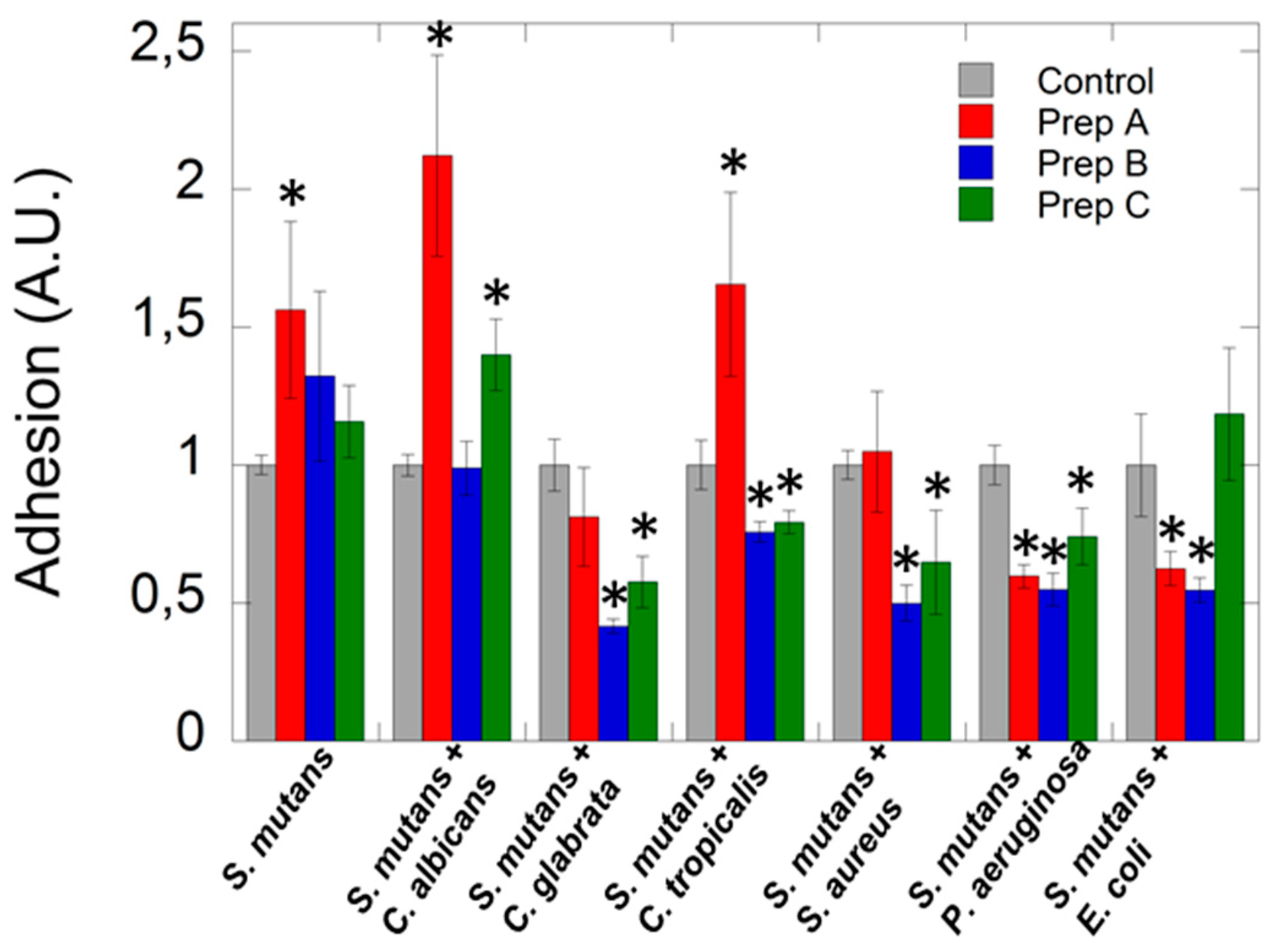
4.2. Antimicrobial Properties of Artificial Saliva
4.3. Development of Artificial Saliva
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Sreebny, L.M. Saliva in health and disease: An appraisal and update. Int. Dent. J. 2000, 50, 140–161. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, S. Salivary gland disorders: A comprehensive review. World J. Stomatol. 2015, 4, 56–71. [Google Scholar] [CrossRef]
- Aitken-Saavedra, J.; Rojas-Alcayaga, G.; Maturana-Ramírez, A.; Escobar-álvarez, A.; Cortes-Coloma, A.; Reyes-Rojas, M.; Viera-Sapiain, V.; Villablanca-Martínez, C.; Morales-Bozo, I. Salivary gland dysfunction markers in type 2 diabetes mellitus patients. J. Clin. Exp. Dent. 2015. [Google Scholar] [CrossRef] [PubMed]
- Sasportas, L.S.; Hosford, D.N.; Sodini, M.A.; Waters, D.J.; Zambricki, E.A.; Barral, J.K.; Graves, E.E.; Brinton, T.J.; Yock, P.G.; Le, Q.T.; et al. Cost-effectiveness landscape analysis of treatments addressing xerostomia in patients receiving head and neck radiation therapy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, e37–e51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva, L.; Kupek, E.; Peres, K.G. General health influences episodes of xerostomia: A prospective population-based study. community dent. Oral Epidemiol. 2017, 45, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, M.; Borgnakke, W.S.; Yoshihara, A.; Ito, K.; Ogawa, H.; Nohno, K.; Sato, M.; Minagawa, K.; Ansai, T.; Miyazaki, H. Hyposalivation and 10-year all-cause mortality in an elderly japanese population. Gerodontology 2018, 35, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, R.C.; Qazali, A.; Zaveri, J.; Chambers, M.S.; Gunn, G.B.; Fuller, C.D.; Lai, S.Y.; Mott, F.E.; Hutcheson, K.A. Self-reported oral morbidities in long-term oropharyngeal cancer survivors: A cross-sectional survey of 906 survivors. Oral Oncol. 2018, 84, 88–94. [Google Scholar] [CrossRef]
- Villa, A.; Connell, C.L.; Abati, S. Diagnosis and management of xerostomia and hyposalivation. Ther. Clin. Risk Manag. 2014, 11, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Ship, J.A.; Fox, P.C.; Baum, B.J. How much saliva is enough? “Normal” function defined. J. Am. Dent. Assoc. 1991, 122, 63–69. [Google Scholar] [CrossRef]
- Navazesh, M.; Christensen, C.; Brightman, V. Clinical criteria for the diagnosis of salivary gland hypofunction. J. Dent. Res. 1992, 71, 1363–1369. [Google Scholar] [CrossRef]
- Navazesh, M.; Kumar, S.K. Measuring salivary flow challenges and opportunities. J. Am. Dent. Assoc. 2008, 139, 35S–40S. [Google Scholar] [CrossRef] [PubMed]
- Lashley, K.S. Reflex secretion of the human parotid gland. J. Exp. Psychol. 1916, 1, 461. [Google Scholar] [CrossRef]
- Schneyer, L.H. Method for the collection of separate submaxillary and sublingual salivas in man. J. Dent. Res. 1955, 34, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Eliasson, L.; Carlén, A. An update on minor salivary gland secretions. Eur. J. Oral Sci. 2010, 118, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Saleh, J.; Figueiredo, M.A.Z.; Cherubini, K.; Salum, F.G. Salivary hypofunction: An update on aetiology, diagnosis and therapeutics. Arch. Oral Biol. 2014, 60, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.N.; Panchaksharappa, M.G.; Annigeri, R.G. Modified schirmer test-a screening tool for xerostomia among subjects on antidepressants. Arch. Oral Biol. 2014, 59, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Tardy, M.; Dold, M.; Engel, R.R.; Leucht, S. Flupenthixol versus low-potency first-generation antipsychotic drugs for schizophrenia. Cochrane Database Syst. Rev. 2014. [Google Scholar] [CrossRef] [PubMed]
- Elad, S.; Heisler, S.; Shlit, M. Saliva secretion in patients with allergic rhinitis. Int. Arch. Allergy Immunol. 2006, 141, 276–280. [Google Scholar] [CrossRef]
- Tanghe, A.; Geerts, S.; Van Dorpe, J.; Brichard, B.; Bruhwyler, J.; Géczy, J. Double-blind randomized controlled study of the efficacy and tolerability of two reversible monoamine oxidase a inhibitors, pirlindole and moclobemide, in the treatment of depression. Acta Psychiatr. Scand. 1997, 96, 134–141. [Google Scholar] [CrossRef]
- Kang, J.G.; Park, C.Y.; Kang, J.H.; Park, Y.W.; Park, S.W. Randomized controlled trial to investigate the effects of a newly developed formulation of phentermine diffuse-controlled release for obesity. Diabetes, Obes. Metab. 2010, 12, 876–882. [Google Scholar] [CrossRef]
- Oakes, T.M.; Katona, C.; Liu, P.; Robinson, M.; Raskin, J.; Greist, J.H. Safety and tolerability of duloxetine in elderly patients with major depressive disorder. Int. Clin. Psychopharmacol. 2012, 28, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nance, P.W.; Bugaresti, J.; Shellenberger, K.; Sheremata, W.; Martinez-Arizala, A. Efficacy and safety of tizanidine in the treatment of spasticity in patients with spinal cord injury. North american tizanidine study group. Neurology 1994, 44, S44–S51. [Google Scholar] [PubMed]
- Habbab, K.M.; Moles, D.R.; Porter, S.R. Potential oral manifestations of cardiovascular drugs. Oral Dis. 2010, 16, 769–773. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, P.D.V.; Grégio, A.M.T.; Brancher, J.A.; Ignácio, S.A.; Machado, M.Â.N.; de Lima, A.A.S.; Azevedo, L.R. Effects of antidepressants and benzodiazepines on stimulated salivary flow rate and biochemistry composition of the saliva. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2008, 106, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Todd, J.; Rees, E.; Gwilliam, B.; Davis, A. An assessment of the efficacy and tolerability of a “double dose” of normal-release morphine sulphate at bedtime. Palliat. Med. 2002, 16, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Looström, H.; Åkerman, S.; Ericson, D.; Tobin, G.; Götrick, B. Tramadol-induced oral dryness and pilocarpine treatment: Effects on total protein and IgA. Arch. Oral Biol. 2011, 56, 395–400. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, I.; Scully, C. Biologics in oral medicine: Sjogren syndrome. Oral Dis. 2013, 19, 121–127. [Google Scholar] [CrossRef]
- Ramos-Casals, M.; Tzioufas, A.G.; Stone, J.H.; Sisó, A.; Bosch, X. Treatment of primary sjögren syndrome: A systematic review. J. Am. Med. Assoc. 2010, 304, 452–460. [Google Scholar] [CrossRef]
- Soell, M.; Hassan, M.; Miliauskaite, A.; Haïkel, Y.; Selimovic, D. The oral cavity of elderly patients in diabetes. Diabetes Metab. 2007, 33, S10–S18. [Google Scholar] [CrossRef]
- Sreebny, L.M.; Yu, A.; Green, A.; Valdini, A. Xerostomia in diabetes mellitus. Diabetes Care 1992, 15, 900–911. [Google Scholar] [CrossRef]
- Moore, P.A.; Guggenheimer, J.; Etzel, K.R.; Weyant, R.J.; Orchard, T. Type 1 diabetes mellitus, xerostomia, and salivary flow rates. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2001, 92, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Anttila, S.S.; Knuuttila, M.L.E.; Sakki, T.K. Depressive symptoms as an underlying factor of the sensation of dry mouth. Psychosom. Med. 1998, 60, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Ohara, Y.; Hirano, H.; Yoshida, H.; Obuchi, S.; Ihara, K.; Fujiwara, Y.; Mataki, S. Prevalence and factors associated with xerostomia and hyposalivation among community-dwelling older people in Japan. Gerodontology 2016, 33, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.C.; Wang, Y.P.; Chang, J.Y.F.; Cheng, S.J.; Chen, H.M.; Sun, A. Oral manifestations and blood profile in patients with iron deficiency Anemia. J. Formos. Med. Assoc. 2014, 113, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Dynesen, A.W.; Bardow, A.; Petersson, B.; Nielsen, L.R.; Nauntofte, B. Salivary changes and dental erosion in bulimia nervosa. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2008, 106, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, W.L.; Siqueira, M.F.; Mustacchi, Z.; De Oliveira, E.; Nicolau, J. Salivary parameters in infants aged 12 to 60 months with down syndrome. Spec. Care Dent. 2007, 27, 202–205. [Google Scholar] [CrossRef]
- Saeves, R.; Nordgarden, H.; Storhaug, K.; Sandvik, L.; Espelid, I. Salivary flow rate and oral findings in prader-willi syndrome: A case-control study. Int. J. Paediatr. Dent. 2012, 22, 27–36. [Google Scholar] [CrossRef]
- Dutta, S.K.; Orestes, M.; Vengulekur, S.; Kwo, P. Ethanol and human saliva: Effect of chronic alcoholism on flow rate, composition, and epidermal growth factor. Am. J. Gastroenterol. 1992, 87, 350–354. [Google Scholar] [CrossRef]
- Dyasanoor, S.; Saddu, S.C. Association of xerostomia and assessment of salivary flow using modified schirmer test among smokers and healthy individuals: A preliminutesary study. J. Clin. Diagn. Res. 2014, 8, 211–213. [Google Scholar] [CrossRef]
- Götrick, B.; Giglio, D.; Tobin, G. Effects of amphetamine on salivary secretion. Eur. J. Oral Sci. 2009, 117, 218–223. [Google Scholar] [CrossRef]
- Saini, T.; Edwards, P.C.; Kimmes, N.S.; Carroll, L.R.; Shaner, J.W.; Dowd, F.J. Etiology of xerostomia and dental caries among methamphetamine abusers. Oral Health Prev. Dent. 2005, 3, 189–195. [Google Scholar] [PubMed]
- Dongliang, S.; Tao, Y.; Pengcgeng, R.; Shibin, Y. Prevalence and etiology of oral diseases in drug-addicted populations: A systematic review. Int. J. Clin. Exp. Med. 2018, 11, 6521–6531. [Google Scholar]
- Versteeg, P.A.; Slot, D.E.; van der Velden, U.; van der Weijden, G.A. Effect of cannabis usage on the oral environment: A review. Int. J. Dent. Hyg. 2008, 6, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Meßmer, M.B.; Thomsen, A.; Kirste, S.; Becker, G.; Momm, F. Xerostomia after radiotherapy in the head&neck area: Long-term observations. Radiother. Oncol. 2011, 98, 48–50. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.B.; Pedersen, A.M.L.; Vissink, A.; Andersen, E.; Brown, C.G.; Davies, A.N.; Dutilh, J.; Fulton, J.S.; Jankovic, L.; Lopes, N.N.F.; et al. A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: Prevalence, severity and impact on quality of life. Support. Care Cancer 2010, 18, 1039–1060. [Google Scholar] [CrossRef] [PubMed]
- Deasy, J.O.; Moiseenko, V.; Marks, L.; Chao, K.S.C.; Nam, J.; Eisbruch, A. Radiotherapy dose-volume effects on salivary gland function. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S58–S63. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Pearlstein, K.A.; Moon, D.H.; Mahbooba, Z.M.; Deal, A.M.; Wang, Y.; Sutton, S.R.; Motley, B.B.; Judy, G.D.; Holmes, J.A.; et al. Assessment of risk of xerostomia after whole-brain radiation therapy and association with parotid dose. JAMA Oncol. 2019, 5, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Åstrøm, A.N.; Lie, S.A.; Ekback, G.; Gülcan, F.; Ordell, S. Self-reported dry mouth among ageing people: A longitudinal, cross-national study. Eur. J. Oral Sci. 2019, 127, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Nederfors, T.; Isaksson, R.; Mörnstad, H.; Dahlöf, C. Prevalence of perceived symptoms of dry mouth in an adult swedish population—Relation to age, sex and pharmacotherapy. Community Dent. Oral Epidemiol. 1997, 25, 211–216. [Google Scholar] [CrossRef]
- Ghezzi, E.M.; Ship, J.A. Aging and secretory reserve capacity of major salivary glands. J. Dent. Res. 2003, 82, 844–848. [Google Scholar] [CrossRef]
- Smith, C.H.; Boland, B.; Daureeawoo, Y.; Donaldson, E.; Small, K.; Tuomainen, J. Effect of aging on stimulated salivary flow in adults. J. Am. Geriatr. Soc. 2013, 61, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Baum, B.J.; Kousvelari, E.E.; Oppenheim, F.G. Exocrine protein secretion from human parotid glands during aging: Stable release of the acidic proline-rich proteins. J. Gerontol. 1982, 37, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, M.S.; Tanner, M.; Freedman, M.L. Salivary and serum iga levels in a geriatric outpatient population. J. Clin. Immunol. 1984, 4, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Sahibzada, H.A.; Khurshid, Z.; Khan, R.S.; Naseem, M.; Siddique, K.M.; Mali, M.; Zafar, M.S. Salivary IL-8, IL-6 and TNF-α as potential diagnostic biomarkers for oral cancer. Diagnostics 2017, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Sannam Khan, R.; Khurshid, Z.; Akhbar, S.; Faraz Moin, S. Advances of salivary proteomics in oral squamous cell carcinoma (OSCC) detection: An update. Proteomes 2016, 4, 41. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Khurshid, Z.; Yahya Ibrahim Asiri, F. Advancing point-of-care (PoC) testing using human saliva as liquid biopsy. Diagnostics 2017, 7, 39. [Google Scholar] [CrossRef]
- Levine, M.J. Development of Artificial Salivas. Available online: https://journals.sagepub.com/doi/10.1177/10454411930040030401 (accessed on 1 April 1993).
- Radvansky, L.J.; Pace, M.B.; Siddiqui, A. Prevention and management of radiation-induced dermatitis, mucositis, and xerostomia. Am. J. Health-Syst. Pharm. 2013, 70, 1025–1032. [Google Scholar] [CrossRef]
- Tanigawa, T.; Yamashita, J.I.; Sato, T.; Shinohara, A.; Shibata, R.; Ueda, H.; Sasaki, H. Efficacy and safety of pilocarpine mouthwash in elderly patients with xerostomia. Spec. Care Dent. 2015, 35, 164–169. [Google Scholar] [CrossRef]
- Epstein, J.B.; Schubert, M. Synergistic effect of sialagogues in management of xerostomia after radiation therapy. Oral Surg. Oral Med. Oral Pathol. 1987, 64, 179–182. [Google Scholar] [CrossRef]
- Chambers, M.S.; Posner, M.; Jones, C.U.; Biel, M.A.; Hodge, K.M.; Vitti, R.; Armstrong, I.; Yen, C.; Weber, R.S. Cevimeline for the treatment of postirradiation xerostomia in patients with head and neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 1102–1109. [Google Scholar] [CrossRef]
- Brimhall, J.; Jhaveri, M.A.; Yepes, J.F. Efficacy of cevimeline vs. pilocarpine in the secretion of saliva: A Pilot study. Spec. Care Dent. 2013, 33, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Jham, B.C.; Teixeira, I.V.; Aboud, C.G.; Carvalho, A.L.; de Matos Coelho, M.; da Silva Freire, A.R. A randomized phase III prospective trial of bethanechol to prevent radiotherapy-induced salivary gland damage in patients with head and neck cancer. Oral Oncol. 2007, 43, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Jaguar, G.C.; Lima, E.N.P.; Kowalski, L.P.; Pellizzon, A.C.; Carvalho, A.L.; Boccaletti, K.W.; Alves, F.A. Double blind randomized prospective trial of bethanechol in the prevention of radiation-induced salivary gland dysfunction in head and neck cancer patients. Radiother. Oncol. 2015, 115, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.T.; Ferretti, G.A.; Nethery, W.J.; Valdez, I.H.; Fox, P.C.; Ng, D.; Muscoplat, C.C.; Gallagher, S.C. Oral pilocarpine for post-irradiation xerostomia in patients with head and neck cancer. N. Engl. J. Med. 2002, 329, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, L.R.; Faulds, D. Oral pilocarpine: A review of its pharmacological properties and clinical potential in xerostomia. Drugs 1995, 49, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Nanni, J.M.; Nguyen, K.H.T.; Alford, C.E.; Robinson, C.P.; Stewart, C.M.; Maeda, N.; Humphreys-Beher, M.G. Assessment of bromhexine as a treatment regimen in sjogren’s syndrome- like disease in the NOD (Non-Obese Diabetic) mouse. Clin. Exp. Rheumatol. 1997, 15, 515–521. [Google Scholar] [PubMed]
- Adachi, K.; Ono, M.; Kawamura, A.; Yuki, M.; Fujishiro, H.; Kinoshita, Y. Nizatidine and cisapride enhance salivary secretion in humans. Aliment. Pharmacol. Ther. 2002, 16, 297–301. [Google Scholar] [CrossRef]
- Lomonaco, T.; Ghimenti, S.; Biagini, D.; Bramanti, E.; Onor, M.; Bellagambi, F.G.; Fuoco, R.; Di Francesco, F. The effect of sampling procedures on the urate and lactate concentration in oral fluid. Microchem. J. 2018, 136, 255–262. [Google Scholar] [CrossRef]
- Gröschl, M.; Rauh, M. Influence of commercial collection devices for saliva on the reliability of salivary steroids analysis. Steroids 2006, 71, 1097–1100. [Google Scholar] [CrossRef]
- Strietzel, F.P.; Lafaurie, G.I.; Mendoza, G.R.B.; Alajbeg, I.; Pejda, S.; Vuletić, L.; Mantilla, R.; Falcào, D.P.; Leal, S.C.; Bezerra, A.C.B.; et al. Efficacy and safety of an intraoral electrostimulation device for xerostomia relief: A multicenter, randomized trial. Arthritis Rheum. 2011, 63, 180–190. [Google Scholar] [CrossRef]
- Johnstone, P.A.S.; Niemtzow, R.C.; Riffenburgh, R.H. Acupuncture for xerostomia: Clinical update. Cancer 2002, 94, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Fox, N.F.; Xiao, C.; Sood, A.J.; Lovelace, T.L.; Nguyen, S.A.; Sharma, A.; Day, T.A. Hyperbaric oxygen therapy for the treatment of radiation-induced xerostomia: A systematic review. Oral Surg. Oral Med., Oral Pathol. Oral Radiol. 2015, 120, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Teguh, D.N.; Levendag, P.C.; Noever, I.; Voet, P.; van der Est, H.; van Rooij, P.; Dumans, A.G.; de Boer, M.F.; van der Huls, M.P.C.; Sterk, W.; et al. Early hyperbaric oxygen therapy for reducing radiotherapy side effects: Early results of a randomized trial in oropharyngeal and nasopharyngeal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Hensley, M.L.; Hagerty, K.L.; Kewalramani, T.; Green, D.M.; Meropol, N.J.; Wasserman, T.H.; Cohen, G.I.; Emami, B.; Gradishar, W.J.; Brian Mitchell, R.; et al. American society of clinical oncology 2008 clinical practice guideline update: Use of chemotherapy and radiation therapy protectants. J. Clin. Oncol. 2009, 27, 127–145. [Google Scholar] [CrossRef] [PubMed]
- Sasse, A.D.; De Oliveira Clark, L.G.; Sasse, E.C.; Clark, O.A.C. Amifostine reduces side effects and improves complete response rate during radiotherapy: Results of a meta-analysis. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Brizel, D.M.; Wasserman, T.H.; Henke, M.; Strnad, V.; Rudat, V.; Monnier, A.; Eschwege, F.; Zhang, J.; Russell, L.; Oster, W.; et al. Phase III randomized trial of amifostine as a radioprotector in head and neck cancer. J. Clin. Oncol. 2000, 18, 3339–3345. [Google Scholar] [CrossRef]
- Shan, Z.; Li, J.; Zheng, C.; Liu, X.; Fan, Z.; Zhang, C.; Goldsmith, C.M.; Wellner, R.B.; Baum, B.J.; Wang, S. Increased fluid secretion after adenoviral-mediated transfer of the human aquaporin-1 cdna to irradiated miniature pig parotid glands. Mol. Ther. 2005, 11, 444–451. [Google Scholar] [CrossRef]
- Palaniyandi, S.; Odaka, Y.; Green, W.; Abreo, F.; Caldito, G.; Benedetti, A.D.; Sunavala-Dossabhoy, G. Adenoviral delivery of tousled kinase for the protection of salivary glands against ionizing radiation damage. Gene Ther. 2011, 18, 275–282. [Google Scholar] [CrossRef]
- Baum, B.J.; Zheng, C.; Alevizos, I.; Cotrim, A.P.; Liu, S.; McCullagh, L.; Goldsmith, C.M.; McDermott, N.; Chiorini, J.A.; Nikolov, N.P.; et al. Development of a gene transfer-based treatment for radiation-induced salivary hypofunction. Oral Oncol. 2010, 46, 4–8. [Google Scholar] [CrossRef] [Green Version]
- Lombaert, I.M.A.; Brunsting, J.F.; Wierenga, P.K.; Kampinga, H.H.; de Haan, G.; Coppes, R.P. Keratinocyte growth factor prevents radiation damage to salivary glands by expansion of the stem/progenitor pool. Stem Cells 2008, 26, 2595–2601. [Google Scholar] [CrossRef]
- Mystkowska, J.; Jałbrzykowski, M.; Dąbrowski, J.R. Tribological properties of selected self-made solutions of synthetic saliva. Solid State Phenom. 2013, 199, 567–572. [Google Scholar] [CrossRef]
- Mystkowska, J.; Karalus, W.; Sidorenko, J.; Dąbrowski, J.R.; Kalska-Szostko, B. Biotribological properties of dentures lubricated with artificial saliva. J. Frict. Wear 2016, 37, 544–551. [Google Scholar] [CrossRef]
- Hahnel, S.; Behr, M.; Handel, G.; Bürgers, R. Saliva substitutes for the treatment of radiation-induced xerostomia-a review. Support. Care Cancer. 2009, 17, 1331–1343. [Google Scholar] [CrossRef]
- Furness, S.; Worthington, H.V.; Bryan, G.; Birchenough, S.; McMillan, R. Interventions for the management of dry mouth: Topical therapies. Cochrane Database Syst. Rev. 2011. [Google Scholar] [CrossRef]
- Dost, F.; Farah, C.S. Stimulating the discussion on saliva substitutes: A clinical perspective. Aust. Dent. J. 2013, 58, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Gil-Montoya, J.A.; Silvestre, F.J.; Barrios, R.; Silvestre-Rangil, J. Treatment of xerostomia and hyposalivation in the elderly: A systematic review. Med. Oral Patol. Oral Cirugia Bucal. 2016, 21, e355. [Google Scholar] [CrossRef]
- Vissink, A.; Waterman, H.A.; ’s-Gravenmade, E.J.; Panders, A.K.; Vermey, A. Rheological properties of saliva substitutes containing mucin, carboxymethylcellulose or polyethylenoxide. J. Oral Pathol. Med. 1984, 13, 22–28. [Google Scholar] [CrossRef]
- Van der Reijden, W.A.; Veerman, E.C.I.; Nieuw Amerongen, A.V. Rheological properties of commercially available polysaccharides with potential use in saliva substitutes. Biorheology 2017, 31, 631–642. [Google Scholar] [CrossRef]
- Hatton, M.N.; Levine, M.J.; Margarone, J.E.; Aguirre, A. Lubrication and viscosity features of human saliva and commercially available saliva substitutes. J. Oral Maxillofac. Surg. 1987, 45, 496–499. [Google Scholar] [CrossRef]
- Park, M.S.; Chung, J.W.; Kim, Y.K.; Chung, S.C.; Kho, H.S. Viscosity and wettability of animal mucin solutions and human saliva. Oral Dis. 2007, 13, 181–186. [Google Scholar] [CrossRef]
- Kim, J.; Chang, J.Y.; Kim, Y.Y.; Kim, M.J.; Kho, H.S. Effects of molecular weight of hyaluronic acid on its viscosity and enzymatic activities of lysozyme and peroxidase. Arch. Oral Biol. 2018, 89, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.S. The rheological properties of saliva. Rheol. Acta 1971, 10, 28–35. [Google Scholar] [CrossRef]
- Schwarz, W.H. The rheology of saliva. J. Dent. Res. 1987, 66, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Mystkowska, J.; Łysik, D.; Klekotka, M. Effect of saliva and mucin-based saliva substitutes on fretting processes of 316 austenitic stainless steel. Metals 2019, 9, 178. [Google Scholar] [CrossRef]
- Aguirre, A.; Mendoza, B.; Reddy, M.S.; Scannapieco, F.A.; Levine, M.J.; Hatton, M.N. Lubrication of selected salivary molecules and artificial salivas. Dysphagia 1989, 4, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Reeh, E.S.; Douglas, W.H.; Levine, M.J. Lubrication of saliva substitutes at enamel-to-enamel contacts in an artificial mouth. J. Prosthet. Dent. 1996, 75, 649–656. [Google Scholar] [CrossRef]
- Briscoe, W.H. Aqueous boundary lubrication: Molecular mechanisms, design strategy, and terra incognita. Curr. Opin. Colloid Interface Sci. 2017, 27, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Briscoe, W.H.; Titmuss, S.; Tiberg, F.; Thomas, R.K.; McGillivray, D.J.; Klein, J. Boundary lubrication under water. Nature 2006, 444, 191–194. [Google Scholar] [CrossRef]
- Baker, J.L.; Bor, B.; Agnello, M.; Shi, W.; He, X. Ecology of the oral microbiome: Beyond bacteria. Trends Microbiol. 2017, 25, 362–374. [Google Scholar] [CrossRef]
- Tanasiewicz, M.; Hildebrandt, T.; Obersztyn, I. Xerostomia of various etiologies: A review of the literature. Adv. Clin. Exp. Med. 2016, 25, 199–206. [Google Scholar] [CrossRef]
- Abstracts of the 2014 International MASCC/ISOO Symposium. Available online: https://link.springer.com/article/10.1007%2Fs00520-014-2222-3 (accessed on 28 May 2014).
- Hou, J.; Zheng, H.M.; Li, P.; Liu, H.Y.; Zhou, H.W.; Yang, X.J. Distinct shifts in the oral microbiota are associated with the progression and aggravation of mucositis during radiotherapy. Radiother. Oncol. 2018, 129, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Khovidhunkit, S.O.P.; Suwantuntula, T.; Thaweboon, S.; Mitrirattanakul, S.; Chomkhakhai, U.; Khovidhunkit, W. Xerostomia, hyposalivation, and oral microbiota in type 2 diabetic patients: A preliminary study. J. Med. Assoc. Thail. 2009, 92, 1220–1228. [Google Scholar]
- Rosan, B.; Lamont, R.J. Dental plaque formation. Microbes Infect. 2000, 2, 1599–1607. [Google Scholar] [CrossRef]
- Marsh, P.D. Microbiology of dental plaque biofilms and their role in oral health and caries. Dent. Clin. N. Am. 2010, 54, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Gibbins, H.L.; Yakubov, G.E.; Proctor, G.B.; Wilson, S.; Carpenter, G.H. What interactions drive the salivary mucosal pellicle formation? Coll. Surf. B Biointerfaces 2014, 120, 184–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolenbrander, P.E.; Palmer, R.J.; Periasamy, S.; Jakubovics, N.S. Oral multispecies biofilm development and the key role of cell-cell distance. Nat. Rev. Microbiol. 2010, 8, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Glantz, P.O. Interfacial phenomena in the oral cavity. Coll. Surf. A Physicochem. Eng. Asp. 1997, 123–124, 657–670. [Google Scholar] [CrossRef]
- Tabak, L.A.; Levine, M.J.; Mandel, I.D.; Ellison, S.A. Role of salivary mucins in the protection of the oral cavity. J. Oral Pathol. Med. 1982, 11, 1–17. [Google Scholar] [CrossRef]
- Wu, A.M.; Csako, G.; Herp, A. Structure, biosynthesis, and function of salivary mucins. Mol. Cell. Biochem. 1994, 137, 39–55. [Google Scholar] [CrossRef]
- Zalewska, A.; Zwierz, K.; Zółkowski, K.; Gindzieński, A. Structure and biosynthesis of human salivary mucins. Acta Biochim. Pol. 2000, 47, 1067–1079. [Google Scholar]
- Yakubov, G.E.; Macakova, L.; Wilson, S.; Windust, J.H.C.; Stokes, J.R. Aqueous lubrication by fractionated salivary proteins: Synergistic interaction of mucin polymer brush with low molecular weight macromolecules. Tribol. Int. 2015, 89, 34–45. [Google Scholar] [CrossRef]
- Mystkowska, J.; Niemirowicz-Laskowska, K.; Łysik, D.; Tokajuk, G.; Dąbrowski, J.R.; Bucki, R. The role of oral cavity biofilm on metallic biomaterial surface destruction–corrosion and friction aspects. Int. J. Mol. Sci. 2018, 19, 743. [Google Scholar] [CrossRef]
- Slomiany, B.L.; Murty, V.L.N.; Piotrowski, J.; Slomiany, A. Salivary mucins in oral mucosal defense. Gen. Pharmacol. 1996, 27, 761–771. [Google Scholar] [CrossRef]
- Werlang, C.; Cárcarmo-Oyarce, G.; Ribbeck, K. Engineering mucus to study and influence the microbiome. Nat. Rev. Mater. 2019, 134–145. [Google Scholar] [CrossRef]
- Andrysewicz, E.; Mystkowska, J.; Dabrowski, J.R.; Olchowik, R. Influence of self-made saliva substitutes on tribological characteristics of human enamel. Acta Bioeng. Biomech. 2014, 16, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Michalak, G.; Głuszek, K.; Piktel, E.; Deptuła, P.; Puszkarz, I.; Niemirowicz, K.; Bucki, R. Polymeric Nanoparticles—A novel solution for delivery of antimicrobial agents. Med. Stud. 2016, 1, 56–62. [Google Scholar] [CrossRef]
- Niemirowicz, K.; Durnaś, B.; Tokajuk, G.; Głuszek, K.; Wilczewska, A.Z.; Misztalewska, I.; Mystkowska, J.; Michalak, G.; Sodo, A.; Wątek, M.; et al. Magnetic nanoparticles as a drug delivery system that enhance fungicidal activity of polyene antibiotics. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 2395–2404. [Google Scholar] [CrossRef]
- Xie, W.; Guo, Z.; Gao, F.; Gao, Q.; Wang, D.; Liaw, B.S.; Cai, Q.; Sun, X.; Wang, X.; Zhao, L. Shape-, size-and structure-controlled synthesis and biocompatibility of iron oxide nanoparticles for magnetic theranostics. Theranostics 2018, 8, 3284. [Google Scholar] [CrossRef]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Habib, S.S.; Memic, A. Antimicrobial activity of metal oxide nanoparticles against gram-positive and gram-negative bacteria: A comparative study. Int. J. Nanomed. 2012. [Google Scholar] [CrossRef]
- Niemirowicz, K.; Swiecicka, I.; Wilczewska, A.Z.; Misztalewska, I.; Kalska-Szostko, B.; Bienias, K.; Bucki, R.; Car, H. Gold-functionalized magnetic nanoparticles restrict growth of pseudomonas aeruginosa. Int. J. Nanomed. 2014, 7, 6003. [Google Scholar] [CrossRef]
- Taylor, E.; Webster, T.J. Reducing infections through nanotechnology and nanoparticles. Int. J. Nanomed. 2011, 6, 1463. [Google Scholar]
- Park, H.; Park, H.J.; Kim, J.A.; Lee, S.H.; Kim, J.H.; Yoon, J.; Park, T.H. Inactivation of pseudomonas aeruginosa pa01 biofilms by hyperthermia using superparamagnetic nanoparticles. J. Microbiol. Methods 2011, 84, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Tokajuk, G.; Niemirowicz, K.; Deptuła, P.; Piktel, E.; Cieśluk, M.; Wilczewska, A.Z.; Dąbrowski, J.R.; Bucki, R. Use of magnetic nanoparticles as a drug delivery system to improve chlorhexidine antimicrobial activity. Int. J. Nanomed. 2017, 12, 7833–7846. [Google Scholar] [CrossRef] [PubMed]
- Niemirowicz, K.; Durnaś, B.; Piktel, E.; Bucki, R. Development of antifungal therapies using nanomaterials. Nanomedicine 2017, 12, 1891–1905. [Google Scholar] [CrossRef] [PubMed]
- Niemirowicz, K.; Bucki, R. Enhancing the fungicidal activity of antibiotics: Are magnetic nanoparticles the key? Nanomedicine 2017, 1747–1749. [Google Scholar] [CrossRef] [PubMed]
- Niemirowicz, K.; Durnaś, B.; Tokajuk, G.; Piktel, E.; Michalak, G.; Gu, X.; Kułakowska, A.; Savage, P.B.; Bucki, R. Formulation and candidacidal activity of magnetic nanoparticles coated with cathelicidin LL-37 and ceragenin CSA-13. Sci. Rep. 2017, 7, 4610. [Google Scholar] [CrossRef] [PubMed]
- Niemirowicz, K.; Surel, U.; Wilczewska, A.Z.; Mystkowska, J.; Piktel, E.; Gu, X.; Namiot, Z.; Kulakowska, A.; Savage, P.B.; Bucki, R. Bactericidal activity and biocompatibility of ceragenin-coated magnetic nanoparticles. J. Nanobiotechnol. 2015, 13, 32. [Google Scholar] [CrossRef]
- Niemirowicz, K.; Piktel, E.; Wilczewska, A.Z.; Markiewicz, K.H.; Durnaś, B.; Wątek, M.; Puszkarz, I.; Wróblewska, M.; Niklińska, W.; Savage, P.B.; et al. Core–shell magnetic nanoparticles display synergistic antibacterial effects against pseudomonas aeruginosa and staphylococcus aureus when combined with cathelicidin LL-37 or selected ceragenins. Int. J. Nanomed. 2016, 11, 5443–5455. [Google Scholar] [CrossRef]
- Niemirowicz, K.; Car, H.; Sadowska, A.; Wątek, M.; Krȩtowski, R.; Cechowska-Pasko, M.; Wilczewska, A.Z.; Mystkowska, J.; Kasacka, I.; Torres, A.; et al. Pharmacokinetics and anticancer activity of folic acid-functionalized magnetic nanoparticles. J. Biomed. Nanotechnol. 2017, 13, 665–677. [Google Scholar] [CrossRef]
- Cole, A.J.; Yang, V.C.; David, A.E. Cancer theranostics: The rise of targeted magnetic nanoparticles. Trends Biotechnol. 2011, 29, 323–332. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łysik, D.; Niemirowicz-Laskowska, K.; Bucki, R.; Tokajuk, G.; Mystkowska, J. Artificial Saliva: Challenges and Future Perspectives for the Treatment of Xerostomia. Int. J. Mol. Sci. 2019, 20, 3199. https://doi.org/10.3390/ijms20133199
Łysik D, Niemirowicz-Laskowska K, Bucki R, Tokajuk G, Mystkowska J. Artificial Saliva: Challenges and Future Perspectives for the Treatment of Xerostomia. International Journal of Molecular Sciences. 2019; 20(13):3199. https://doi.org/10.3390/ijms20133199
Chicago/Turabian StyleŁysik, Dawid, Katarzyna Niemirowicz-Laskowska, Robert Bucki, Grażyna Tokajuk, and Joanna Mystkowska. 2019. "Artificial Saliva: Challenges and Future Perspectives for the Treatment of Xerostomia" International Journal of Molecular Sciences 20, no. 13: 3199. https://doi.org/10.3390/ijms20133199
APA StyleŁysik, D., Niemirowicz-Laskowska, K., Bucki, R., Tokajuk, G., & Mystkowska, J. (2019). Artificial Saliva: Challenges and Future Perspectives for the Treatment of Xerostomia. International Journal of Molecular Sciences, 20(13), 3199. https://doi.org/10.3390/ijms20133199





