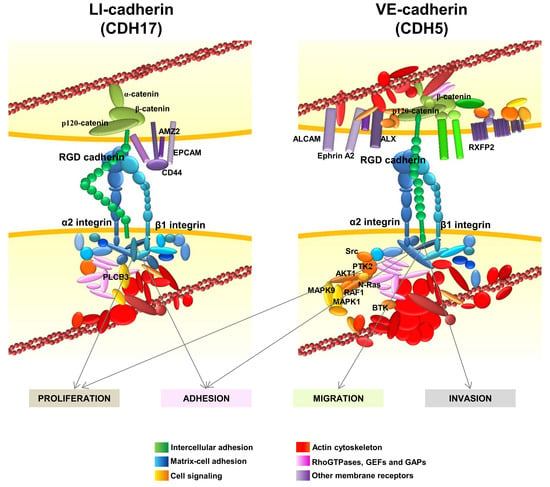
Beyond N-Cadherin, Relevance of Cadherins 5, 6 and 17 in Cancer Progression and Metastasis
Abstract
:1. Introduction
2. RGD Cadherins Structure and Function
2.1. Cadherin 17
2.2. VE-Cadherin
2.3. Cadherin 6
3. Relevance of CDH17, CDH5 and CDH6 in Cancer Progression and Metastasis
3.1. Cadherin 17 in Gastrointestinal Cancers and Other Tumors
3.2. VE-Cadherin in Breast Cancer, Melanoma and Other Tumors
3.3. Cadherin 6 Expression in Different Tumors
4. Phylogenetic Analysis of the RGD Motif in Cadherins
5. Cadherin Interaction with Integrins: Activation of the Integrin Signaling Pathway by Cadherin RGD Motifs
6. Other Interactions of RGD Cadherins Involved in Cell Signaling
7. Targeting Cadherins: Implications in Therapy
8. Final Remarks and Outlook
Acknowledgments
Conflicts of Interest
References
- Harjes, U. E-selectin fills two needs for metastasis. Nat. Rev. Cancer 2019, 19, 301. [Google Scholar] [CrossRef] [PubMed]
- Obenauf, A.C.; Massague, J. Surviving at a distance: Organ specific metastasis. Trends Cancer 2015, 1, 76–91. [Google Scholar] [CrossRef]
- Valastyan, S.; Weinberg, R.A. Tumor metastasis: Molecular insights and evolving paradigms. Cell 2011, 147, 275–292. [Google Scholar] [CrossRef] [PubMed]
- Berx, G.; van Roy, F. Involvement of members of the cadherin superfamily in cancer. Cold Spring Harb Perspect Biol. 2009, 1, a003129. [Google Scholar] [CrossRef] [PubMed]
- Nelson, W.J.; Nusse, R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science 2004, 303, 1483–1487. [Google Scholar] [CrossRef] [PubMed]
- van Roy, F.; Berx, G. The cell-cell adhesion molecule E-cadherin. Cell Mol. Life Sci. 2008, 65, 3756–3788. [Google Scholar] [CrossRef] [PubMed]
- Soncin, F.; Ward, C.M. The function of e-cadherin in stem cell pluripotency and self-renewal. Genes 2011, 2, 229–259. [Google Scholar] [CrossRef]
- Batlle, E.; Sancho, E.; Franci, C.; Dominguez, D.; Monfar, M.; Baulida, J.; Garcia De Herreros, A. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat. Cell Biol. 2000, 2, 84–89. [Google Scholar] [CrossRef]
- Wheelock, M.J.; Shintani, Y.; Maeda, M.; Fukumoto, Y.; Johnson, K.R. Cadherin switching. J. Cell Sci. 2008, 121, 727–735. [Google Scholar] [CrossRef] [Green Version]
- Hazan, R.B.; Qiao, R.; Keren, R.; Badano, I.; Suyama, K. Cadherin switch in tumor progression. Ann. N. Y. Acad. Sci. 2004, 1014, 155–163. [Google Scholar] [CrossRef]
- Shintani, Y.; Hollingsworth, M.A.; Wheelock, M.J.; Johnson, K.R. Collagen I promotes metastasis in pancreatic cancer by activating c-Jun NH(2)-terminal kinase 1 and up-regulating N-cadherin expression. Cancer Res. 2006, 66, 11745–11753. [Google Scholar] [CrossRef] [PubMed]
- Klymenko, Y.; Kim, O.; Loughran, E.; Yang, J.; Lombard, R.; Alber, M.; Stack, M.S. Cadherin composition and multicellular aggregate invasion in organotypic models of epithelial ovarian cancer intraperitoneal metastasis. Oncogene 2017, 36, 5840–5851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mrozik, K.M.; Blaschuk, O.W.; Cheong, C.M.; Zannettino, A.C.W.; Vandyke, K. N-cadherin in cancer metastasis, its emerging role in haematological malignancies and potential as a therapeutic target in cancer. BMC Cancer 2018, 18, 939. [Google Scholar] [CrossRef] [PubMed]
- Berndorff, D.; Gessner, R.; Kreft, B.; Schnoy, N.; Lajous-Petter, A.M.; Loch, N.; Reutter, W.; Hortsch, M.; Tauber, R. Liver-intestine cadherin: Molecular cloning and characterization of a novel Ca(2+)-dependent cell adhesion molecule expressed in liver and intestine. J. Cell Biol. 1994, 125, 1353–1369. [Google Scholar] [CrossRef] [PubMed]
- Jung, R.; Wendeler, M.W.; Danevad, M.; Himmelbauer, H.; Gessner, R. Phylogenetic origin of LI-cadherin revealed by protein and gene structure analysis. Cell Mol. Life Sci. 2004, 61, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Kreft, B.; Berndorff, D.; Bottinger, A.; Finnemann, S.; Wedlich, D.; Hortsch, M.; Tauber, R.; Gessner, R. LI-cadherin-mediated cell-cell adhesion does not require cytoplasmic interactions. J. Cell Biol. 1997, 136, 1109–1121. [Google Scholar] [CrossRef] [PubMed]
- Bartolome, R.A.; Barderas, R.; Torres, S.; Fernandez-Acenero, M.J.; Mendes, M.; Garcia-Foncillas, J.; Lopez-Lucendo, M.; Casal, J.I. Cadherin-17 interacts with alpha2beta1 integrin to regulate cell proliferation and adhesion in colorectal cancer cells causing liver metastasis. Oncogene 2014, 33, 1658–1669. [Google Scholar] [CrossRef]
- Cadwell, C.M.; Su, W.; Kowalczyk, A.P. Cadherin tales: Regulation of cadherin function by endocytic membrane trafficking. Traffic 2016, 17, 1262–1271. [Google Scholar] [CrossRef]
- Johnson, S.K.; Ramani, V.C.; Hennings, L.; Haun, R.S. Kallikrein 7 enhances pancreatic cancer cell invasion by shedding E-cadherin. Cancer 2007, 109, 1811–1820. [Google Scholar] [CrossRef]
- Bartolome, R.A.; Pelaez-Garcia, A.; Gomez, I.; Torres, S.; Fernandez-Acenero, M.J.; Escudero-Paniagua, B.; Imbaud, J.I.; Casal, J.I. An RGD motif present in cadherin 17 induces integrin activation and tumor growth. J. Biol. Chem. 2014, 289, 34801–34814. [Google Scholar] [CrossRef]
- Horsfield, J.; Ramachandran, A.; Reuter, K.; LaVallie, E.; Collins-Racie, L.; Crosier, K.; Crosier, P. Cadherin-17 is required to maintain pronephric duct integrity during zebrafish development. Mech. Dev. 2002, 115, 15–26. [Google Scholar] [CrossRef]
- Angres, B.; Kim, L.; Jung, R.; Gessner, R.; Tauber, R. LI-cadherin gene expression during mouse intestinal development. Dev. Dyn. 2001, 221, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, W. Possible roles of LI-Cadherin in the formation and maintenance of the intestinal epithelial barrier. Tissue Barriers 2013, 1, e23815. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, W.; Wendeler, M.W.; Weth, A.; Koob, R.; Drenckhahn, D.; Gessner, R. Heterotypic trans-interaction of LI- and E-cadherin and their localization in plasmalemmal microdomains. J. Mol. Biol. 2008, 378, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Ahl, M.; Weth, A.; Walcher, S.; Baumgartner, W. The function of 7D-cadherins: A mathematical model predicts physiological importance for water transport through simple epithelia. Biol. Med. Model. 2011, 8, 18. [Google Scholar] [CrossRef]
- Bartolmas, T.; Hirschfeld-Ihlow, C.; Jonas, S.; Schaefer, M.; Gessner, R. LI-cadherin cis-dimerizes in the plasma membrane Ca(2+) independently and forms highly dynamic trans-contacts. Cell Mol. Life Sci. 2012, 69, 3851–3862. [Google Scholar] [CrossRef] [PubMed]
- Prevarskaya, N.; Skryma, R.; Shuba, Y. Calcium in tumour metastasis: New roles for known actors. Nat. Rev. Cancer 2011, 11, 609–618. [Google Scholar] [CrossRef]
- Tanihara, H.; Kido, M.; Obata, S.; Heimark, R.L.; Davidson, M.; St John, T.; Suzuki, S. Characterization of cadherin-4 and cadherin-5 reveals new aspects of cadherins. J. Cell Sci. 1994, 107, 1697–1704. [Google Scholar]
- Hatta, K.; Okada, T.S.; Takeichi, M. A monoclonal antibody disrupting calcium-dependent cell-cell adhesion of brain tissues: Possible role of its target antigen in animal pattern formation. Proc. Natl. Acad. Sci. USA. 1985, 82, 2789–2793. [Google Scholar] [CrossRef]
- Corada, M.; Zanetta, L.; Orsenigo, F.; Breviario, F.; Lampugnani, M.G.; Bernasconi, S.; Liao, F.; Hicklin, D.J.; Bohlen, P.; Dejana, E. A monoclonal antibody to vascular endothelial-cadherin inhibits tumor angiogenesis without side effects on endothelial permeability. Blood 2002, 100, 905–911. [Google Scholar] [CrossRef]
- Bartolome, R.A.; Torres, S.; Isern de Val, S.; Escudero-Paniagua, B.; Calvino, E.; Teixido, J.; Casal, J.I. VE-cadherin RGD motifs promote metastasis and constitute a potential therapeutic target in melanoma and breast cancers. Oncotarget 2017, 8, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Giannotta, M.; Trani, M.; Dejana, E. VE-cadherin and endothelial adherens junctions: Active guardians of vascular integrity. Dev. Cell 2013, 26, 441–454. [Google Scholar] [CrossRef]
- Flemming, S.; Burkard, N.; Renschler, M.; Vielmuth, F.; Meir, M.; Schick, M.A.; Wunder, C.; Germer, C.T.; Spindler, V.; Waschke, J.; et al. Soluble VE-cadherin is involved in endothelial barrier breakdown in systemic inflammation and sepsis. Cardiovasc Res. 2015, 107, 32–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dejana, E.; Vestweber, D. The role of VE-cadherin in vascular morphogenesis and permeability control. Prog. Mol. Biol. Transl. Sci. 2013, 116, 119–144. [Google Scholar] [PubMed]
- Baumeister, U.; Funke, R.; Ebnet, K.; Vorschmitt, H.; Koch, S.; Vestweber, D. Association of Csk to VE-cadherin and inhibition of cell proliferation. Embo J. 2005, 24, 1686–1695. [Google Scholar] [CrossRef] [Green Version]
- Lagendijk, A.K.; Hogan, B.M. VE-cadherin in vascular development: A coordinator of cell signaling and tissue morphogenesis. Curr. Top. Dev. Biol. 2015, 112, 325–352. [Google Scholar] [PubMed]
- Cho, E.A.; Patterson, L.T.; Brookhiser, W.T.; Mah, S.; Kintner, C.; Dressler, G.R. Differential expression and function of cadherin-6 during renal epithelium development. Development 1998, 125, 803–812. [Google Scholar]
- Mah, S.P.; Saueressig, H.; Goulding, M.; Kintner, C.; Dressler, G.R. Kidney development in cadherin-6 mutants: Delayed mesenchyme-to-epithelial conversion and loss of nephrons. Dev. Biol. 2000, 223, 38–53. [Google Scholar] [CrossRef]
- Shimoyama, Y.; Gotoh, M.; Terasaki, T.; Kitajima, M.; Hirohashi, S. Isolation and sequence analysis of human cadherin-6 complementary DNA for the full coding sequence and its expression in human carcinoma cells. Cancer Res. 1995, 55, 2206–2211. [Google Scholar]
- Xiang, Y.Y.; Tanaka, M.; Suzuki, M.; Igarashi, H.; Kiyokawa, E.; Naito, Y.; Ohtawara, Y.; Shen, Q.; Sugimura, H.; Kino, I. Isolation of complementary DNA encoding K-cadherin, a novel rat cadherin preferentially expressed in fetal kidney and kidney carcinoma. Cancer Res. 1994, 54, 3034–3041. [Google Scholar]
- Stewart, D.B.; Barth, A.I.; Nelson, W.J. Differential regulation of endogenous cadherin expression in Madin-Darby canine kidney cells by cell-cell adhesion and activation of beta -catenin signaling. J. Biol. Chem. 2000, 275, 20707–20716. [Google Scholar] [CrossRef] [PubMed]
- Troxell, M.L.; Loftus, D.J.; Nelson, W.J.; Marrs, J.A. Mutant cadherin affects epithelial morphogenesis and invasion, but not transformation. J. Cell Sci. 2001, 114, 1237–1246. [Google Scholar] [PubMed]
- Shimazui, T.; Giroldi, L.A.; Bringuier, P.P.; Oosterwijk, E.; Schalken, J.A. Complex cadherin expression in renal cell carcinoma. Cancer Res. 1996, 56, 3234–3237. [Google Scholar] [PubMed]
- Paul, R.; Ewing, C.M.; Robinson, J.C.; Marshall, F.F.; Johnson, K.R.; Wheelock, M.J.; Isaacs, W.B. Cadherin-6, a cell adhesion molecule specifically expressed in the proximal renal tubule and renal cell carcinoma. Cancer Res. 1997, 57, 2741–2748. [Google Scholar] [CrossRef] [PubMed]
- Hinoi, T.; Lucas, P.C.; Kuick, R.; Hanash, S.; Cho, K.R.; Fearon, E.R. CDX2 regulates liver intestine-cadherin expression in normal and malignant colon epithelium and intestinal metaplasia. Gastroenterology 2002, 123, 1565–1577. [Google Scholar] [CrossRef]
- Silberg, D.G.; Swain, G.P.; Suh, E.R.; Traber, P.G. Cdx1 and cdx2 expression during intestinal development. Gastroenterology 2000, 119, 961–971. [Google Scholar] [CrossRef]
- Grotzinger, C.; Kneifel, J.; Patschan, D.; Schnoy, N.; Anagnostopoulos, I.; Faiss, S.; Tauber, R.; Wiedenmann, B.; Gessner, R. LI-cadherin: A marker of gastric metaplasia and neoplasia. Gut 2001, 49, 73–81. [Google Scholar] [CrossRef]
- Ito, R.; Oue, N.; Yoshida, K.; Kunimitsu, K.; Nakayama, H.; Nakachi, K.; Yasui, W. Clinicopathological significant and prognostic influence of cadherin-17 expression in gastric cancer. Virchows Arch. Int. J. Pathol. 2005, 447, 717–722. [Google Scholar] [CrossRef]
- Takamura, M.; Sakamoto, M.; Ino, Y.; Shimamura, T.; Ichida, T.; Asakura, H.; Hirohashi, S. Expression of liver-intestine cadherin and its possible interaction with galectin-3 in ductal adenocarcinoma of the pancreas. Cancer Sci. 2003, 94, 425–430. [Google Scholar] [CrossRef]
- Su, M.C.; Yuan, R.H.; Lin, C.Y.; Jeng, Y.M. Cadherin-17 is a useful diagnostic marker for adenocarcinomas of the digestive system. Mod. Pathol. 2008, 21, 1379–1386. [Google Scholar] [CrossRef]
- Kuhlmann, L.; Nadler, W.M.; Kerner, A.; Hanke, S.A.; Noll, E.M.; Eisen, C.; Espinet, E.; Vogel, V.; Trumpp, A.; Sprick, M.R.; et al. Identification and Validation of Novel Subtype-Specific Protein Biomarkers in Pancreatic Ductal Adenocarcinoma. Pancreas 2017, 46, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Kern, F.; Sharma, N.; McKeon, F.; Xian, W.; Yeoh, K.G.; Ho, K.Y.; Teh, M. FABP1 and Hepar expression levels in Barrett’s esophagus and associated neoplasia in an Asian population. Dig. Liver Dis. 2017, 49, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Snow, A.N.; Mangray, S.; Lu, S.; Clubwala, R.; Li, J.; Resnick, M.B.; Yakirevich, E. Expression of cadherin 17 in well-differentiated neuroendocrine tumours. Histopathology 2015, 66, 1010–1021. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.; Wright, J.P.; Zhao, Z.; Komaya, T.; Parikh, A.; Merchant, N.; Shi, C. Cadherin 17 is frequently expressed by ‘sclerosing variant’ pancreatic neuroendocrine tumour. Histopathology 2015, 66, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Seol, J.A.; Choi, H.J.; Roh, Y.H.; Choi, P.J.; Lee, K.E.; Roh, M.S. Comparison of cadherin-17 expression between primary colorectal adenocarcinomas and their corresponding metastases: The possibility of a diagnostic marker for detecting the primary site of metastatic tumour. Histopathology 2011, 58, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Ordonez, N.G. Cadherin 17 is a novel diagnostic marker for adenocarcinomas of the digestive system. Adv. Anat. Pathol. 2014, 21, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.X.; Lee, N.P.; Chan, V.W.; Xue, W.; Zender, L.; Zhang, C.; Mao, M.; Dai, H.; Wang, X.L.; Xu, M.Z.; et al. Targeting cadherin-17 inactivates Wnt signaling and inhibits tumor growth in liver carcinoma. Hepatology 2009, 50, 1453–1463. [Google Scholar] [CrossRef]
- Wong, B.W.; Luk, J.M.; Ng, I.O.; Hu, M.Y.; Liu, K.D.; Fan, S.T. Identification of liver-intestine cadherin in hepatocellular carcinoma--a potential disease marker. Biochem. Biophys. Res. Commun. 2003, 311, 618–624. [Google Scholar] [CrossRef]
- Ding, Z.B.; Shi, Y.H.; Zhou, J.; Shi, G.M.; Ke, A.W.; Qiu, S.J.; Wang, X.Y.; Dai, Z.; Xu, Y.; Fan, J. Liver-intestine cadherin predicts microvascular invasion and poor prognosis of hepatitis B virus-positive hepatocellular carcinoma. Cancer 2009, 115, 4753–4765. [Google Scholar] [CrossRef]
- Altree-Tacha, D.; Tyrrell, J.; Haas, T. CDH17 Is a More Sensitive Marker for Gastric Adenocarcinoma Than CK20 and CDX2. Arch. Pathol. Lab. Med. 2017, 141, 144–150. [Google Scholar] [CrossRef]
- Huang, L.P.; Yu, Y.H.; Sheng, C.; Wang, S.H. Up-regulation of cadherin 17 and down-regulation of homeodomain protein CDX2 correlate with tumor progression and unfavorable prognosis in epithelial ovarian cancer. Int. J. Gynecol. Cancer 2012, 22, 1170–1176. [Google Scholar] [CrossRef] [PubMed]
- Heinzelmann-Schwarz, V.A.; Gardiner-Garden, M.; Henshall, S.M.; Scurry, J.P.; Scolyer, R.A.; Smith, A.N.; Bali, A.; Vanden Bergh, P.; Baron-Hay, S.; Scott, C.; et al. A distinct molecular profile associated with mucinous epithelial ovarian cancer. Br. J. Cancer 2006, 94, 904–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uhlen, M.; Zhang, C.; Lee, S.; Sjostedt, E.; Fagerberg, L.; Bidkhori, G.; Benfeitas, R.; Arif, M.; Liu, Z.; Edfors, F.; et al. A pathology atlas of the human cancer transcriptome. Science 2017, 357. [Google Scholar] [CrossRef] [PubMed]
- Rao, Q.; Williamson, S.R.; Lopez-Beltran, A.; Montironi, R.; Huang, W.; Eble, J.N.; Grignon, D.J.; Koch, M.O.; Idrees, M.T.; Emerson, R.E.; et al. Distinguishing primary adenocarcinoma of the urinary bladder from secondary involvement by colorectal adenocarcinoma: Extended immunohistochemical profiles emphasizing novel markers. Mod. Pathol. 2013, 26, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Torres, S.; Garcia-Palmero, I.; Bartolome, R.A.; Fernandez-Acenero, M.J.; Molina, E.; Calvino, E.; Segura, M.F.; Casal, J.I. Combined miRNA profiling and proteomics demonstrates that different miRNAs target a common set of proteins to promote colorectal cancer metastasis. J. Pathol. 2017, 242, 39–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Kang, W.M.; Yu, J.C.; Liu, Y.Q.; Meng, Q.B.; Cao, Z.J. Cadherin-17 induces tumorigenesis and lymphatic metastasis in gastric cancer through activation of NFkappaB signaling pathway. Cancer Biol. 2013, 14, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.J.; Lin, J.; Cai, Q.H.; Zhao, J.F.; Zhang, H.J. CDH17 alters MMP-2 expression via canonical NF-kappaB signalling in human gastric cancer. Gene 2019, 682, 92–100. [Google Scholar] [CrossRef]
- Yoshimura, K.; Meckel, K.F.; Laird, L.S.; Chia, C.Y.; Park, J.J.; Olino, K.L.; Tsunedomi, R.; Harada, T.; Iizuka, N.; Hazama, S.; et al. Integrin alpha2 mediates selective metastasis to the liver. Cancer Res. 2009, 69, 7320–7328. [Google Scholar] [CrossRef]
- Hendrix, M.J.; Seftor, E.A.; Meltzer, P.S.; Gardner, L.M.; Hess, A.R.; Kirschmann, D.A.; Schatteman, G.C.; Seftor, R.E. Expression and functional significance of VE-cadherin in aggressive human melanoma cells: Role in vasculogenic mimicry. Proc. Natl. Acad. Sci. USA 2001, 98, 8018–8023. [Google Scholar] [CrossRef] [Green Version]
- Hendrix, M.J.; Seftor, E.A.; Hess, A.R.; Seftor, R.E. Vasculogenic mimicry and tumour-cell plasticity: Lessons from melanoma. Nat. Rev. Cancer 2003, 3, 411–421. [Google Scholar] [CrossRef]
- Wang, R.; Chadalavada, K.; Wilshire, J.; Kowalik, U.; Hovinga, K.E.; Geber, A.; Fligelman, B.; Leversha, M.; Brennan, C.; Tabar, V. Glioblastoma stem-like cells give rise to tumour endothelium. Nature 2010, 468, 829–833. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Bao, M.; Miele, L.; Sarkar, F.H.; Wang, Z.; Zhou, Q. Tumour vasculogenic mimicry is associated with poor prognosis of human cancer patients: A systemic review and meta-analysis. Eur. J. Cancer 2013, 49, 3914–3923. [Google Scholar] [CrossRef] [PubMed]
- Yue, W.Y.; Chen, Z.P. Does vasculogenic mimicry exist in astrocytoma? J. Histochem Cytochem 2005, 53, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Zhang, S.; Zhao, X.; Zhang, W.; Hao, X. Vasculogenic mimicry is associated with poor survival in patients with mesothelial sarcomas and alveolar rhabdomyosarcomas. Int. J. Oncol. 2004, 25, 1609–1614. [Google Scholar] [CrossRef] [PubMed]
- van der Schaft, D.W.; Hillen, F.; Pauwels, P.; Kirschmann, D.A.; Castermans, K.; Egbrink, M.G.; Tran, M.G.; Sciot, R.; Hauben, E.; Hogendoorn, P.C.; et al. Tumor cell plasticity in Ewing sarcoma, an alternative circulatory system stimulated by hypoxia. Cancer Res. 2005, 65, 11520–11528. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; O’Leary, H.; Fortney, J.; Gibson, L.F. Ph+/VE-cadherin+ identifies a stem cell like population of acute lymphoblastic leukemia sustained by bone marrow niche cells. Blood 2007, 110, 3334–3344. [Google Scholar] [CrossRef] [PubMed]
- Akers, S.M.; O’Leary, H.A.; Minnear, F.L.; Craig, M.D.; Vos, J.A.; Coad, J.E.; Gibson, L.F. VE-cadherin and PECAM-1 enhance ALL migration across brain microvascular endothelial cell monolayers. Exp. Hematol. 2010, 38, 733–743. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Zhang, H.X.; Wang, M.; Song, X.G.; Cao, J.; Wang, L.; Qiao, J.L.; Lu, X.Y.; Han, Z.X.; Zhu, P.; et al. Stromal cells attenuate the cytotoxicity of imatinib on Philadelphia chromosome-positive leukemia cells by up-regulating the VE-cadherin/beta-catenin signal. Leuk Res. 2014, 38, 1460–1468. [Google Scholar] [CrossRef]
- Hung, M.S.; Chen, I.C.; Lung, J.H.; Lin, P.Y.; Li, Y.C.; Tsai, Y.H. Epidermal Growth Factor Receptor Mutation Enhances Expression of Cadherin-5 in Lung Cancer Cells. PLos One 2016, 11, e0158395. [Google Scholar] [CrossRef]
- Bittner, M.; Meltzer, P.; Chen, Y.; Jiang, Y.; Seftor, E.; Hendrix, M.; Radmacher, M.; Simon, R.; Yakhini, Z.; Ben-Dor, A.; et al. Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature 2000, 406, 536–540. [Google Scholar] [CrossRef] [Green Version]
- Rochefort, P.; Chabaud, S.; Pierga, J.Y.; Tredan, O.; Brain, E.; Bidard, F.C.; Schiffler, C.; Polena, H.; Khalil-Mgharbel, A.; Vilgrain, I.; et al. Soluble VE-cadherin in metastatic breast cancer: An independent prognostic factor for both progression-free survival and overall survival. Br. J. Cancer 2017, 116, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Fry, S.A.; Robertson, C.E.; Swann, R.; Dwek, M.V. Cadherin-5: A biomarker for metastatic breast cancer with optimum efficacy in oestrogen receptor-positive breast cancers with vascular invasion. Br. J. Cancer 2016, 114, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Speisky, D.; Duces, A.; Bieche, I.; Rebours, V.; Hammel, P.; Sauvanet, A.; Richard, S.; Bedossa, P.; Vidaud, M.; Murat, A.; et al. Molecular profiling of pancreatic neuroendocrine tumors in sporadic and Von Hippel-Lindau patients. Clin. Cancer Res. 2012, 18, 2838–2849. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, K.; Inokuchi, M.; Takagi, Y.; Ishikawa, T.; Otsuki, S.; Uetake, H.; Kojima, K.; Kawano, T. Cadherin 5 expression correlates with poor survival in human gastric cancer. J. Clin. Pathol. 2017, 70, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Loges, S.; Clausen, H.; Reichelt, U.; Bubenheim, M.; Erbersdobler, A.; Schurr, P.; Yekebas, E.; Schuch, G.; Izbicki, J.; Pantel, K.; et al. Determination of microvessel density by quantitative real-time PCR in esophageal cancer: Correlation with histologic methods, angiogenic growth factor expression, and lymph node metastasis. Clin. Cancer Res. 2007, 13, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Labelle, M.; Schnittler, H.J.; Aust, D.E.; Friedrich, K.; Baretton, G.; Vestweber, D.; Breier, G. Vascular endothelial cadherin promotes breast cancer progression via transforming growth factor beta signaling. Cancer Res. 2008, 68, 1388–1397. [Google Scholar] [CrossRef] [PubMed]
- Hess, A.R.; Seftor, E.A.; Gruman, L.M.; Kinch, M.S.; Seftor, R.E.; Hendrix, M.J. VE-cadherin regulates EphA2 in aggressive melanoma cells through a novel signaling pathway: Implications for vasculogenic mimicry. Cancer Biol. 2006, 5, 228–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, X.G.; Xue, X.Y.; Wang, L.; Zhang, X.; Yan, M.; Tu, Y.Y.; Lin, W.; Jiang, X.F.; Ren, H.G.; Zhang, W.; et al. CDH5 is specifically activated in glioblastoma stemlike cells and contributes to vasculogenic mimicry induced by hypoxia. Neuro. Oncol. 2013, 15, 865–879. [Google Scholar] [CrossRef] [Green Version]
- Paul, R.; Necknig, U.; Busch, R.; Ewing, C.M.; Hartung, R.; Isaacs, W.B. Cadherin-6: A new prognostic marker for renal cell carcinoma. J. Urol. 2004, 171, 97–101. [Google Scholar] [CrossRef]
- Kobel, M.; Kalloger, S.E.; Boyd, N.; McKinney, S.; Mehl, E.; Palmer, C.; Leung, S.; Bowen, N.J.; Ionescu, D.N.; Rajput, A.; et al. Ovarian carcinoma subtypes are different diseases: Implications for biomarker studies. PLoS Med. 2008, 5, e232. [Google Scholar] [CrossRef]
- Sellar, G.C.; Li, L.; Watt, K.P.; Nelkin, B.D.; Rabiasz, G.J.; Stronach, E.A.; Miller, E.P.; Porteous, D.J.; Smyth, J.F.; Gabra, H. BARX2 induces cadherin 6 expression and is a functional suppressor of ovarian cancer progression. Cancer Res. 2001, 61, 6977–6981. [Google Scholar] [PubMed]
- Puxeddu, E.; Knauf, J.A.; Sartor, M.A.; Mitsutake, N.; Smith, E.P.; Medvedovic, M.; Tomlinson, C.R.; Moretti, S.; Fagin, J.A. RET/PTC-induced gene expression in thyroid PCCL3 cells reveals early activation of genes involved in regulation of the immune response. Endocr. Relat. Cancer 2005, 12, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Jiang, R.; Xu, M.; Zhu, P.; Mo, X.M.; Wang, N.; Chen, G.G.; Liu, Z.M. Concomitant high expression of BRAFV600E, P-cadherin and cadherin 6 is associated with High TNM stage and lymph node metastasis in conventional papillary thyroid carcinoma. Clin. Endocrinol. (Oxf) 2016, 84, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Goeppert, B.; Ernst, C.; Baer, C.; Roessler, S.; Renner, M.; Mehrabi, A.; Hafezi, M.; Pathil, A.; Warth, A.; Stenzinger, A.; et al. Cadherin-6 is a putative tumor suppressor and target of epigenetically dysregulated miR-429 in cholangiocarcinoma. Epigenetics 2016, 11, 780–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, Q.; Xu, X.; Song, Q.; Xu, Y.; Tai, Y.; Goodman, S.B.; Bi, W.; Xu, M.; Jiao, S.; Maloney, W.J.; et al. miR-223-3p Inhibits Human Osteosarcoma Metastasis and Progression by Directly Targeting CDH6. Mol. Ther. 2018, 26, 1299–1312. [Google Scholar] [CrossRef] [Green Version]
- Ciarrocchi, A.; Piana, S.; Valcavi, R.; Gardini, G.; Casali, B. Inhibitor of DNA binding-1 induces mesenchymal features and promotes invasiveness in thyroid tumour cells. Eur. J. Cancer 2011, 47, 934–945. [Google Scholar] [CrossRef]
- Zuo, L.L.; Zhang, J.; Liu, L.Z.; Zhou, Q.; Du, S.J.; Xin, S.Y.; Ning, Z.P.; Yang, J.; Yu, H.B.; Yue, W.X.; et al. Cadherin 6 is activated by Epstein-Barr virus LMP1 to mediate EMT and metastasis as an interplay node of multiple pathways in nasopharyngeal carcinoma. Oncogenesis 2017, 6, 402. [Google Scholar] [CrossRef]
- Bialucha, C.U.; Collins, S.D.; Li, X.; Saxena, P.; Zhang, X.; Durr, C.; Lafont, B.; Prieur, P.; Shim, Y.; Mosher, R.; et al. Discovery and Optimization of HKT288, a Cadherin-6-Targeting ADC for the Treatment of Ovarian and Renal Cancers. Cancer Discov. 2017, 7, 1030–1045. [Google Scholar] [CrossRef]
- de Cristofaro, T.; Di Palma, T.; Soriano, A.A.; Monticelli, A.; Affinito, O.; Cocozza, S.; Zannini, M. Candidate genes and pathways downstream of PAX8 involved in ovarian high-grade serous carcinoma. Oncotarget 2016, 7, 41929–41947. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Zhao, X.; Cui, N.; Liang, Y. Cadherins Associate with Distinct Stem Cell-Related Transcription Factors to Coordinate the Maintenance of Stemness in Triple-Negative Breast Cancer. Stem Cells Int. 2017, 2017, 5091541. [Google Scholar] [CrossRef]
- Sancisi, V.; Gandolfi, G.; Ragazzi, M.; Nicoli, D.; Tamagnini, I.; Piana, S.; Ciarrocchi, A. Cadherin 6 is a new RUNX2 target in TGF-beta signalling pathway. PLos One 2013, 8, e75489. [Google Scholar] [CrossRef] [PubMed]
- Gugnoni, M.; Sancisi, V.; Gandolfi, G.; Manzotti, G.; Ragazzi, M.; Giordano, D.; Tamagnini, I.; Tigano, M.; Frasoldati, A.; Piana, S.; et al. Cadherin-6 promotes EMT and cancer metastasis by restraining autophagy. Oncogene 2017, 36, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Dunne, E.; Spring, C.M.; Reheman, A.; Jin, W.; Berndt, M.C.; Newman, D.K.; Newman, P.J.; Ni, H.; Kenny, D. Cadherin 6 has a functional role in platelet aggregation and thrombus formation. Arter. Thromb. Vasc. Biol. 2012, 32, 1724–1731. [Google Scholar] [CrossRef] [PubMed]
- Reiss, S.; Sieber, M.; Oberle, V.; Wentzel, A.; Spangenberg, P.; Claus, R.; Kolmar, H.; Losche, W. Inhibition of platelet aggregation by grafting RGD and KGD sequences on the structural scaffold of small disulfide-rich proteins. Platelets 2006, 17, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Cortes, J.; Mrksich, M. The platelet integrin alphaIIbbeta3 binds to the RGD and AGD motifs in fibrinogen. Chem. Biol. 2009, 16, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Naci, D.; Vuori, K.; Aoudjit, F. Alpha2beta1 integrin in cancer development and chemoresistance. Semin. Cancer Biol. 2015, 35, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. Integrins: Bidirectional, allosteric signaling machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef]
- Desgrosellier, J.S.; Cheresh, D.A. Integrins in cancer: Biological implications and therapeutic opportunities. Nat. Rev. Cancer 2010, 10, 9–22. [Google Scholar] [CrossRef]
- Seguin, L.; Desgrosellier, J.S.; Weis, S.M.; Cheresh, D.A. Integrins and cancer: Regulators of cancer stemness, metastasis, and drug resistance. Trends Cell Biol. 2015, 25, 234–240. [Google Scholar] [CrossRef]
- Casal, J.I.; Bartolome, R.A. RGD cadherins and alpha2beta1 integrin in cancer metastasis: A dangerous liaison. Biochim. Biophys. Acta Rev. Cancer 2018, 1869, 321–332. [Google Scholar] [CrossRef]
- Weber, G.F.; Bjerke, M.A.; DeSimone, D.W. Integrins and cadherins join forces to form adhesive networks. J. Cell Sci. 2011, 124, 1183–1193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canonici, A.; Steelant, W.; Rigot, V.; Khomitch-Baud, A.; Boutaghou-Cherid, H.; Bruyneel, E.; Van Roy, F.; Garrouste, F.; Pommier, G.; Andre, F. Insulin-like growth factor-I receptor, E-cadherin and alpha v integrin form a dynamic complex under the control of alpha-catenin. Int. J. Cancer 2008, 122, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Hintermann, E.; Yang, N.; O’Sullivan, D.; Higgins, J.M.; Quaranta, V. Integrin alpha6beta4-erbB2 complex inhibits haptotaxis by up-regulating E-cadherin cell-cell junctions in keratinocytes. J. Biol. Chem. 2005, 280, 8004–8015. [Google Scholar] [CrossRef]
- Siret, C.; Terciolo, C.; Dobric, A.; Habib, M.C.; Germain, S.; Bonnier, R.; Lombardo, D.; Rigot, V.; Andre, F. Interplay between cadherins and alpha2beta1 integrin differentially regulates melanoma cell invasion. Br. J. Cancer 2015, 113, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Mazurek, N.; Liu, C.; Sun, Y.; Ding, Q.Q.; Liu, K.; Hung, M.C.; Bresalier, R.S. Galectin-3 mediates nuclear beta-catenin accumulation and Wnt signaling in human colon cancer cells by regulation of glycogen synthase kinase-3beta activity. Cancer Res. 2009, 69, 1343–1349. [Google Scholar] [CrossRef]
- Weichert, W.; Knosel, T.; Bellach, J.; Dietel, M.; Kristiansen, G. ALCAM/CD166 is overexpressed in colorectal carcinoma and correlates with shortened patient survival. J. Clin. Pathol. 2004, 57, 1160–1164. [Google Scholar] [CrossRef] [PubMed]
- Van Kempen, L.C.; van den Oord, J.J.; van Muijen, G.N.; Weidle, U.H.; Bloemers, H.P.; Swart, G.W. Activated leukocyte cell adhesion molecule/CD166, a marker of tumor progression in primary malignant melanoma of the skin. Am. J. Pathol. 2000, 156, 769–774. [Google Scholar] [CrossRef]
- Eiring, A.M.; Khorashad, J.S.; Anderson, D.J.; Yu, F.; Redwine, H.M.; Mason, C.C.; Reynolds, K.R.; Clair, P.M.; Gantz, K.C.; Zhang, T.Y.; et al. beta-Catenin is required for intrinsic but not extrinsic BCR-ABL1 kinase-independent resistance to tyrosine kinase inhibitors in chronic myeloid leukemia. Leukemia 2015, 29, 2328–2337. [Google Scholar] [CrossRef]
- Williams, E.; Williams, G.; Gour, B.J.; Blaschuk, O.W.; Doherty, P. A novel family of cyclic peptide antagonists suggests that N-cadherin specificity is determined by amino acids that flank the HAV motif. J. Biol. Chem. 2000, 275, 4007–4012. [Google Scholar] [CrossRef]
- Kelland, L. Drug evaluation: ADH-1, an N-cadherin antagonist targeting cancer vascularization. Curr. Opin. Mol. 2007, 9, 86–91. [Google Scholar]
- Bartolome, R.A.; Aizpurua, C.; Jaen, M.; Torres, S.; Calvino, E.; Imbaud, J.I.; Casal, J.I. Monoclonal Antibodies Directed against Cadherin RGD Exhibit Therapeutic Activity against Melanoma and Colorectal Cancer Metastasis. Clin. Cancer Res. 2018, 24, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Beasley, G.M.; Riboh, J.C.; Augustine, C.K.; Zager, J.S.; Hochwald, S.N.; Grobmyer, S.R.; Peterson, B.; Royal, R.; Ross, M.I.; Tyler, D.S. Prospective multicenter phase II trial of systemic ADH-1 in combination with melphalan via isolated limb infusion in patients with advanced extremity melanoma. J. Clin. Oncol. 2011, 29, 1210–1215. [Google Scholar] [CrossRef] [PubMed]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casal, J.I.; Bartolomé, R.A. Beyond N-Cadherin, Relevance of Cadherins 5, 6 and 17 in Cancer Progression and Metastasis. Int. J. Mol. Sci. 2019, 20, 3373. https://doi.org/10.3390/ijms20133373
Casal JI, Bartolomé RA. Beyond N-Cadherin, Relevance of Cadherins 5, 6 and 17 in Cancer Progression and Metastasis. International Journal of Molecular Sciences. 2019; 20(13):3373. https://doi.org/10.3390/ijms20133373
Chicago/Turabian StyleCasal, J. Ignacio, and Rubén A. Bartolomé. 2019. "Beyond N-Cadherin, Relevance of Cadherins 5, 6 and 17 in Cancer Progression and Metastasis" International Journal of Molecular Sciences 20, no. 13: 3373. https://doi.org/10.3390/ijms20133373
APA StyleCasal, J. I., & Bartolomé, R. A. (2019). Beyond N-Cadherin, Relevance of Cadherins 5, 6 and 17 in Cancer Progression and Metastasis. International Journal of Molecular Sciences, 20(13), 3373. https://doi.org/10.3390/ijms20133373