Magnesium Is a Key Player in Neuronal Maturation and Neuropathology
Abstract
:1. Introduction
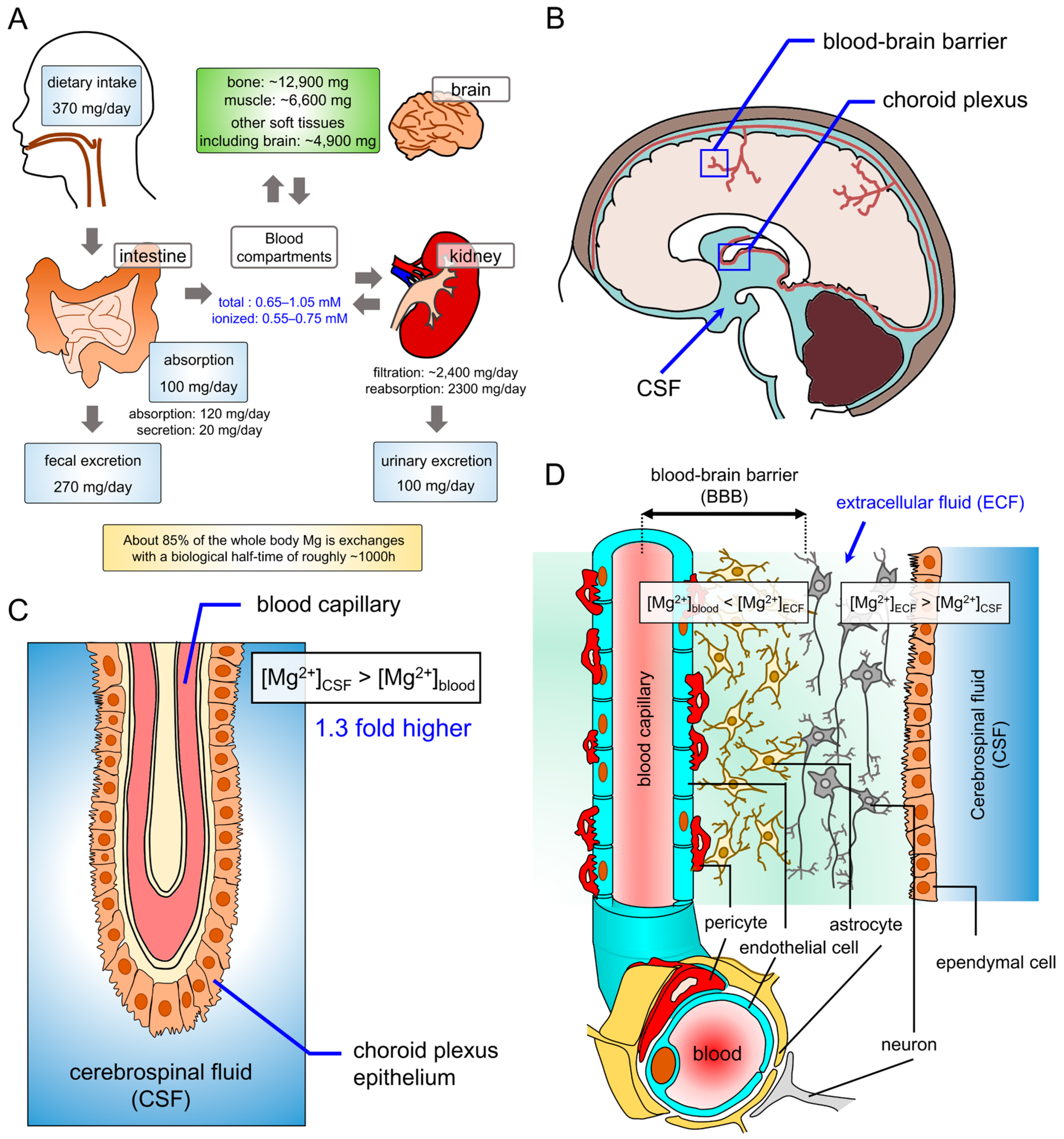
2. Magnesium Homeostasis in the Brain
2.1. Magnesium Homeostasis in the Nervous System
2.2. Mg2+ Transport in Neurons
2.3. Mg2+ Distribution of Cells
2.3.1. Cytosol
2.3.2. Nuclei
2.3.3. Mitochondria
Mitochondrial Energy Metabolism
Apoptotic Process
Mitochondrial Ca2+ Homeostasis
Mitochondrial DNA Functions
2.3.4. Endo(sarco)plasmic Reticulum
2.3.5. Ribosome
3. Physiological Roles of Cellular Mg2+
3.1. Biochemical Reactions in Cells
3.2. Intracellular Signaling
3.3. ROS Toxicity
3.4. Channel Regulation
3.5. DNA Protection and Genome Stability
4. Effects of Mg2+ on the Cellular Fate and Phenotype
4.1. Formation of Neural Networks and Synaptic Activities
4.2. Neural Cell Fate Determination
5. Neuropathology of Mg2+ Homeostasis
5.1. Parkinson’s Disease
5.2. Alzheimer’s Disease and Cognitive Functions
5.3. Demyelination
6. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| [Ca2+] | Ca2+ concentration |
| [H+] | H+ concentration |
| [Mg2+] | Mg2+ concentration |
| [Mg2+]cyto | cytosolic free Mg2+ concentration |
| [Mg2+]ex | extracellular free Mg2+ concentration |
| [Mg2+]mito | free [Mg2+] in the mitochondrial matrix |
| [Mg2+]nuc | nuclear free [Mg2+] |
| ACDP2 | cyclin M2 |
| AD | Alzheimer’s disease |
| APP | Aβ precursor protein |
| APPβ | β-amyloid precursor protein β |
| ATP | adenosine 5’-triphosphate |
| Aβ | amyloid β |
| BBB | blood–brain barrier |
| CNNM2 | cyclin M2 |
| CNS | central nervous system |
| CREB | cAMP response element binding |
| CSF | cerebrospinal fluid |
| CTFα | C terminal fragment α |
| CTFβ | C terminal fragment β |
| ECF | extracellular cellular fluid |
| ERK | extracellular signal-regulated kinase |
| GABA | gamma-aminobutyric acid |
| GSK-3β | glycogen synthase kinase-3β |
| IP3R | inositol-1,4,5-trisphosphate receptor |
| JNK | c-Jun N-terminal kinase |
| LTP | long-term potentiation |
| Mg | magnesium |
| Mg2+ | magnesium ion |
| MgT | magnesium-L-threonate |
| mitoKATP | mitochondrial ATP-sensitive potassium channel |
| MPP+ | N-methyl-4-phenylpyridinium iodide |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| Mrs2 | mitochondrial RNA splicing 2 |
| mtDNA | mitochondrial DNA |
| mTOR | mammalian target of rapamycin |
| NF-κB | nuclear factor-kappa B |
| NMDA | N-methyl-D-aspartate |
| NSCs | neural stem cells |
| OGDH | 2-oxoglutarate dehydrogenase |
| OXPHOS | oxidative phosphorylation |
| PC12 cell | pheochromocytoma cell |
| PD | Parkinson’s disease |
| PI3K | phosphatidylinositol-3 kinase |
| PKC | protein kinase C |
| PTP | permeability transition pore |
| ROS | reactive oxygen species |
| RyR | ryanodine receptor |
| sAPPα | soluble APPα |
| TCA | tricarboxylic acid |
| TRPM6 | transient receptor potential melastatin 6 |
| TRPM7 | transient receptor potential melastatin 7 |
| ΔG | Gibbs free energy change |
| ΔΨm | mitochondrial membrane potential |
References
- Romani, A.M.P. Cellular magnesium homeostasis. Arch. Biochem. Biophys. 2011, 512, 1–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolf, F.I.; Trapani, V. Cell (patho)physiology of magnesium. Clin. Sci. (Lond.) 2008, 114, 27–35. [Google Scholar] [CrossRef] [PubMed]
- De Baaij, J.H.F.; Hoenderop, J.G.J.; Bindels, R.J.M. Magnesium in man: implications for health and disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Anastassopoulou, J.; Theophanides, T. Magnesium-DNA interactions and the possible relation of magnesium to carcinogenesis. Irradiation and free radicals. Crit. Rev. Oncol. Hematol. 2002, 42, 79–91. [Google Scholar] [CrossRef]
- Holm, N.G. The significance of Mg in prebiotic geochemistry. Geobiology 2012, 10, 269–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbert, W. Origin of life: The RNA world. Nature 1986, 319, 618. [Google Scholar] [CrossRef]
- Doudna, J.A.; Cech, T.R. The chemical repertoire of natural ribozymes. Nature 2002, 418, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Oyanagi, K.; Kawakami, E.; Kikuchi-Horie, K.; Ohara, K.; Ogata, K.; Takahama, S.; Wada, M.; Kihira, T.; Yasui, M. Magnesium deficiency over generations in rats with special references to the pathogenesis of the parkinsonism-dementia complex and amyotrophic lateral sclerosis of Guam. Neuropathology 2006, 26, 115–128. [Google Scholar] [CrossRef]
- Barbagallo, M.; Dominguez, L.J. Magnesium Role in Health and Longevity. In Trace Elements and Minerals in Health and Longevity; Springer: Cham, Switzerland, 2018; pp. 235–264. [Google Scholar]
- Komiya, Y.; Su, L.-T.; Chen, H.-C.; Habas, R.; Runnels, L.W. Magnesium and embryonic development. Magnes. Res. 2014, 27, 1–8. [Google Scholar]
- Lowenstein, F.W.; Stanton, M.F. Serum magnesium levels in the United States, 1971-1974. J. Am. Coll. Nutr. 1986, 5, 399–414. [Google Scholar] [CrossRef]
- Jahnen-Dechent, W.; Ketteler, M. Magnesium basics. Clin. Kidney J. 2012, 5, i3–i14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, L.A.; Caesar, J.J.; Burgen, A.S. Gastrointestinal absorption and excretion of Mg 28 in man. Metabolism. 1960, 9, 646–659. [Google Scholar] [PubMed]
- Avioli, L.V.; Berman, M. Mg28 kinetics in man. J. Appl. Physiol. 1966, 21, 1688–1694. [Google Scholar] [CrossRef] [PubMed]
- Rusakov, D.A.; Kullmann, D.M. Extrasynaptic glutamate diffusion in the hippocampus: ultrastructural constraints, uptake, and receptor activation. J. Neurosci. 1998, 18, 3158–3170. [Google Scholar] [CrossRef] [PubMed]
- Van Harreveld, A.; Malhotra, S.K. Extracellular space in the cerebral cortex of the mouse. J. Anat. 1967, 101, 197–207. [Google Scholar] [PubMed]
- Ghabriel, M.N.; Vink, R. Magnesium Transport across the Blood-brain Barriers; Vink, R., Nechifor, M., Eds.; University of Adelaide Press: Adelaide, Australia, 2011; ISBN 9780987073051. [Google Scholar]
- Bito, L.Z. Blood-Brain Barrier: Evidence for Active Cation Transport between Blood and the Extraceliular Fluid of Brain. Science 1969, 165, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Su, Y.; Fu, B.; Xu, H. Magnesium Reduces Blood-Brain Barrier Permeability and Regulates Amyloid-beta Transcytosis. Mol. Neurobiol. 2018, 55, 7118–7131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.L.; Yue, Z.; Arnold, D.M.; Artiushin, G.; Sehgal, A. A Circadian Clock in the Blood-Brain Barrier Regulates Xenobiotic Efflux. Cell 2018, 173, 130–139.e10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hladky, S.B.; Barrand, M.A. Mechanisms of fluid movement into, through and out of the brain: evaluation of the evidence. Fluids Barriers CNS 2014, 11, 26. [Google Scholar] [CrossRef]
- Praetorius, J.; Damkier, H.H. Transport across the choroid plexus epithelium. Am. J. Physiol. Cell Physiol. 2017, 312, C673–C686. [Google Scholar] [CrossRef]
- Bradbury, M.W.; Sarna, G.S. Homeostasis of the ionic composition of the cerebrospinal fluid. Exp. Eye Res. 1977, 25 (Suppl. 1), 249–257. [Google Scholar] [CrossRef]
- Morris, M.E. Brain and CSF magnesium concentrations during magnesium deficit in animals and humans: neurological symptoms. Magnes. Res. 1992, 5, 303–313. [Google Scholar] [PubMed]
- Allsop, T.F.; Pauli, J.V. Magnesium concentrations in the ventricular and lumbar cerebrospinal fluid of hypomagnesaemic cows. Res. Vet. Sci. 1985, 38, 61–64. [Google Scholar] [CrossRef]
- Mori, K.; Yamamoto, T.; Miyazaki, M.; Hara, Y.; Koike, N.; Nakao, Y. Potential risk of artificial cerebrospinal fluid solution without magnesium ion for cerebral irrigation and perfusion in neurosurgical practice. Neurol. Med. Chir. (Tokyo) 2013, 53, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.K.C.; Lam, C.W.K.; Chan, M.T.V.; Gin, T.; Poon, W.S. The effect of hypermagnesemic treatment on cerebrospinal fluid magnesium level in patients with aneurysmal subarachnoid hemorrhage. Magnes. Res. 2009, 22, 60–65. [Google Scholar] [PubMed]
- Sun, Q.; Weinger, J.G.; Mao, F.; Liu, G. Regulation of structural and functional synapse density by L-threonate through modulation of intraneuronal magnesium concentration. Neuropharmacology 2016, 108, 426–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Yu, J.; Liu, Y.; Huang, X.; Abumaria, N.; Zhu, Y.; Huang, X.; Xiong, W.; Ren, C.; Liu, X.-G.; et al. Elevation of brain magnesium prevents synaptic loss and reverses cognitive deficits in Alzheimer’s disease mouse model. Mol. Brain 2014, 7, 65. [Google Scholar] [CrossRef] [PubMed]
- Slutsky, I.; Abumaria, N.; Wu, L.-J.; Huang, C.; Zhang, L.; Li, B.; Zhao, X.; Govindarajan, A.; Zhao, M.-G.; Zhuo, M.; et al. Enhancement of learning and memory by elevating brain magnesium. Neuron 2010, 65, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Bush, A.I. Kalzium ist nicht alles. Neuron 2010, 65, 143–144. [Google Scholar] [CrossRef]
- Miyashita, T.; Oda, Y.; Horiuchi, J.; Yin, J.C.P.; Morimoto, T.; Saitoe, M. Mg2+ block of Drosophila NMDA receptors is required for long-term memory formation and CREB-dependent gene expression. Neuron 2012, 74, 887–898. [Google Scholar] [CrossRef]
- Xiong, W.; Liang, Y.; Li, X.; Liu, G.; Wang, Z. Erythrocyte intracellular Mg2+ concentration as an index of recognition and memory. Sci. Rep. 2016, 6, 26975. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Liang, Y.; Li, X.; Liu, G.; Wang, Z. A Direct Quantitative Analysis of Erythrocyte Intracellular Ionized Magnesium in Physiological and Pathological Conditions. Biol. Pharm. Bull. 2019, 42, 357–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaigne-Delalande, B.; Lenardo, M.J. Divalent cation signaling in immune cells. Trends Immunol. 2014, 35, 332–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trapani, V.; Farruggia, G.; Marraccini, C.; Iotti, S.; Cittadini, A.; Wolf, F.I. Intracellular magnesium detection: imaging a brighter future. Analyst 2010, 135, 1855–1866. [Google Scholar] [CrossRef]
- Romani, A. Regulation of magnesium homeostasis and transport in mammalian cells. Arch. Biochem. Biophys. 2007, 458, 90–102. [Google Scholar] [CrossRef]
- Yamanaka, R.; Shindo, Y.; Hotta, K.; Suzuki, K.; Oka, K. GABA-Induced Intracellular Mg2+ Mobilization Integrates and Coordinates Cellular Information Processing for the Maturation of Neural Networks. Curr. Biol. 2018, 28, 3984–3991.e5. [Google Scholar] [CrossRef]
- Shindo, Y.; Yamanaka, R.; Suzuki, K.; Hotta, K.; Oka, K. Intracellular magnesium level determines cell viability in the MPP+ model of Parkinson’s disease. Biochim. Biophys. Acta 2015, 1853, 3182–3191. [Google Scholar] [CrossRef]
- Maeshima, K.; Matsuda, T.; Shindo, Y.; Imamura, H.; Tamura, S.; Imai, R.; Kawakami, S.; Nagashima, R.; Soga, T.; Noji, H.; et al. A Transient Rise in Free Mg2+ Ions Released from ATP-Mg Hydrolysis Contributes to Mitotic Chromosome Condensation. Curr. Biol. 2018, 28, 444–451.e6. [Google Scholar] [CrossRef]
- Yatsimirskii, K.B. Electronic structure, energy of hydration, and stability of metal aquo ions. Theor. Exp. Chem. 1994, 30, 1–9. [Google Scholar] [CrossRef]
- Winkelmann, G. Microbial Transport Systems; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 3527612726. [Google Scholar]
- Maret, W.; Wedd, A. Binding, Transport and Storage of Metal Ions in Biological Cells; Royal Society of Chemistry: Cambridge, UK, 2014; Volume 2, ISBN 1849735999. [Google Scholar]
- Politi, H.C.; Preston, R.R. Is it time to rethink the role of Mg2+ in membrane excitability? Neuroreport 2003, 14, 659–668. [Google Scholar] [CrossRef]
- Quamme, G.A. Molecular identification of ancient and modern mammalian magnesium transporters. Am. J. Physiol. Cell Physiol. 2010, 298, C407–C429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gout, E.; Rébeillé, F.; Douce, R.; Bligny, R. Interplay of Mg2+, ADP, and ATP in the cytosol and mitochondria: unravelling the role of Mg2+ in cell respiration. Proc. Natl. Acad. Sci. USA 2014, 111, E4560–E4567. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, R.; Shindo, Y.; Hotta, K.; Suzuki, K.; Oka, K. NO/cGMP/PKG signaling pathway induces magnesium release mediated by mitoKATP channel opening in rat hippocampal neurons. FEBS Lett. 2013, 587, 2643–2648. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, R.; Tabata, S.; Shindo, Y.; Hotta, K.; Suzuki, K.; Soga, T.; Oka, K. Mitochondrial Mg2+ homeostasis decides cellular energy metabolism and vulnerability to stress. Sci. Rep. 2016, 6, 30027. [Google Scholar] [CrossRef] [PubMed]
- Shindo, Y.; Fujimoto, A.; Hotta, K.; Suzuki, K.; Oka, K. Glutamate-induced calcium increase mediates magnesium release from mitochondria in rat hippocampal neurons. J. Neurosci. Res. 2010, 88, 3125–3132. [Google Scholar] [CrossRef] [PubMed]
- Kroeger, H.; Trösch, W. Influence of the explantation milieu on intranuclear [Na], [K] and [Mg] of Chironomus thummi salivary gland cells. J. Cell. Physiol. 1974, 83, 19–25. [Google Scholar] [CrossRef]
- Pasternak, K.; Kocot, J.; Horecka, A. Biochemistry of magnesium. J. Elem. 2010, 15, 601–616. [Google Scholar] [CrossRef]
- Korolev, N.; Lyubartsev, A.P.; Rupprecht, A.; Nordenskiold, L. Competitive binding of Mg2+, Ca2+, Na+, and K+ ions to DNA in oriented DNA fibers: experimental and Monte Carlo simulation results. Biophys. J. 1999, 77, 2736–2749. [Google Scholar] [CrossRef]
- Bloom, K. Cell Division: Single-Cell Physiology Reveals Secrets of Chromosome Condensation. Curr. Biol. 2018, 28, R117–R119. [Google Scholar] [CrossRef]
- Uz, G.; Sarikaya, A.T. The effect of magnesium on mitotic spindle formation in Schizosaccharomyces pombe. Genet. Mol. Biol. 2016, 39, 459–464. [Google Scholar] [CrossRef]
- Chandra, S.; Parker, D.J.; Barth, R.F.; Pannullo, S.C. Quantitative imaging of magnesium distribution at single-cell resolution in brain tumors and infiltrating tumor cells with secondary ion mass spectrometry (SIMS). J. Neurooncol. 2016, 127, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Lukacs, G.L.; Zs-Nagy, I.; Steiber, J.; Gyori, F.; Balazs, G. Relative intranuclear magnesium and phosphorus contents in normal and tumor cells of the human thyroid gland as revealed by energy-dispersive X-ray microanalysis. Scanning Microsc. 1996, 10, 1191–1200. [Google Scholar] [PubMed]
- Rubin, H. Central roles of Mg2+ and MgATP2- in the regulation of protein synthesis and cell proliferation: significance for neoplastic transformation. Adv. Cancer Res. 2005, 93, 1–58. [Google Scholar] [PubMed]
- Wolf, F.I.; Trapani, V.; Cittadini, A. Magnesium and the control of cell proliferation: Looking for a needle in a haystack. Magnes. Res. 2008, 21, 83–91. [Google Scholar] [PubMed]
- Hartwig, A. Role of magnesium in genomic stability. Mutat. Res. 2001, 475, 113–121. [Google Scholar] [CrossRef]
- Wright, R.H.G.; Le Dily, F.; Beato, M. ATP, Mg2+, Nuclear Phase Separation, and Genome Accessibility. Trends Biochem. Sci. 2019, 44, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Kubota, T.; Shindo, Y.; Tokuno, K.; Komatsu, H.; Ogawa, H.; Kudo, S.; Kitamura, Y.; Suzuki, K.; Oka, K. Mitochondria are intracellular magnesium stores: investigation by simultaneous fluorescent imagings in PC12 cells. Biochim. Biophys. Acta 2005, 1744, 19–28. [Google Scholar] [CrossRef] [Green Version]
- Shindo, Y.; Fujii, T.; Komatsu, H.; Citterio, D.; Hotta, K.; Suzuki, K.; Oka, K. Newly developed Mg2+-selective fluorescent probe enables visualization of Mg2+ dynamics in mitochondria. PLoS ONE 2011, 6, e23684. [Google Scholar] [CrossRef]
- Pilchova, I.; Klacanova, K.; Tatarkova, Z.; Kaplan, P.; Racay, P. The Involvement of Mg2+ in Regulation of Cellular and Mitochondrial Functions. Oxid. Med. Cell. Longev. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Jung, D.W.; Brierley, G.P. Magnesium transport by mitochondria. J. Bioenerg. Biomembr. 1994, 26, 527–535. [Google Scholar] [CrossRef]
- Rutter, G.A.; Osbaldeston, N.J.; Mccormackt, J.G.; Denton, R.M. Measurement of matrix free Mg2+ concentration in rat heart mitochondria by using entrapped fluorescent probes. Biochem. J. 1990, 271, 627–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolisek, M.; Zsurka, G.; Samaj, J.; Weghuber, J.; Schweyen, R.J.; Schweigel, M. Mrs2p is an essential component of the major electrophoretic Mg2+ influx system in mitochondria. EMBO J. 2003, 22, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Mastrototaro, L.; Smorodchenko, A.; Aschenbach, J.R.; Kolisek, M.; Sponder, G. Solute carrier 41A3 encodes for a mitochondrial Mg2+ efflux system. Sci. Rep. 2016, 6, 27999. [Google Scholar] [CrossRef] [PubMed]
- Panov, A.; Scarpa, A. Independent modulation of the activity of alpha-ketoglutarate dehydrogenase complex by Ca2+ and Mg2+. Biochemistry 1996, 35, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Aprille, J.R. Mechanism and regulation of the mitochondrial ATP-Mg/Pi carrier. J. Bioenerg. Biomembr. 1993, 25, 473–481. [Google Scholar] [CrossRef] [PubMed]
- LaNoue, K.F.; Bryla, J.; Williamson, J.R. Feedback interactions in the control of citric acid cycle activity in rat heart mitochondria. J. Biol. Chem. 1972, 247, 667–679. [Google Scholar] [PubMed]
- Piskacek, M.; Zotova, L.; Zsurka, G.; Schweyen, R.J. Conditional knockdown of hMRS2 results in loss of mitochondrial Mg2+ uptake and cell death. J. Cell. Mol. Med. 2009, 13, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Matsui, Y.; Funato, Y.; Imamura, H.; Miki, H.; Mizukami, S.; Kikuchi, K. Visualization of long-term Mg2+ dynamics in apoptotic cells using a novel targetable fluorescent probe. Chem. Sci. 2017, 8, 8255–8264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, F.; Zhao, Y.; Wang, J.; Pei, L.; Sun, N.; Shi, J. Hypoxia induces an increase in intracellular magnesium via transient receptor potential melastatin 7 (TRPM7) channels in rat hippocampal neurons in vitro. J. Biol. Chem. 2011, 286, 20194–20207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dong, Y.; Xu, Z.; Xie, Z. Propofol and magnesium attenuate isoflurane-induced caspase-3 activation via inhibiting mitochondrial permeability transition pore. Med. Gas Res. 2012, 2, 20. [Google Scholar] [CrossRef]
- Chen, Y.; Wei, X.; Yan, P.; Han, Y.; Sun, S.; Wu, K.; Fan, D. Human mitochondrial Mrs2 protein promotes multidrug resistance in gastric cancer cells by regulating p27, cyclin D1 expression and cytochrome C release. Cancer Biol. Ther. 2009, 8, 607–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Testai, L.; Rapposelli, S.; Martelli, A.; Breschi, M.C.; Calderone, V. Mitochondrial potassium channels as pharmacological target for cardioprotective drugs. Med. Res. Rev. 2015, 35, 520–553. [Google Scholar] [CrossRef]
- Kalogeris, T.; Bao, Y.; Korthuis, R.J. Mitochondrial reactive oxygen species: a double edged sword in ischemia/reperfusion vs preconditioning. Redox Biol. 2014, 2, 702–714. [Google Scholar] [CrossRef] [PubMed]
- Langston, J.W.; Irwin, I.; Langston, E.B.; Forno, L.S. 1-Methyl-4-phenylpyridinium ion (MPP+): identification of a metabolite of MPTP, a toxin selective to the substantia nigra. Neurosci. Lett. 1984, 48, 87–92. [Google Scholar] [CrossRef]
- Zoratti, M.; Szabo, I. The mitochondrial permeability transition. Biochim. Biophys. Acta 1995, 1241, 139–176. [Google Scholar] [CrossRef]
- Seo, Y.-W.; Shin, J.N.; Ko, K.H.; Cha, J.H.; Park, J.Y.; Lee, B.R.; Yun, C.-W.; Kim, Y.M.; Seol, D.; Kim, D.; et al. The molecular mechanism of Noxa-induced mitochondrial dysfunction in p53-mediated cell death. J. Biol. Chem. 2003, 278, 48292–48299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Gruskos, J.J.; Afzal, M.S.; Buccella, D. Visualizing changes in mitochondrial Mg2+ during apoptosis with organelle-targeted triazole-based ratiometric fluorescent sensors. Chem. Sci. 2015, 6, 6841–6846. [Google Scholar] [CrossRef]
- Cappadone, C.; Merolle, L.; Marraccini, C.; Farruggia, G.; Sargenti, A.; Locatelli, A.; Morigi, R.; Iotti, S. Intracellular magnesium content decreases during mitochondria-mediated apoptosis induced by a new indole-derivative in human colon cancer cells. Magnes. Res. 2012, 25, 104–111. [Google Scholar] [Green Version]
- Eskes, R.; Antonsson, B.; Osen-Sand, A.; Montessuit, S.; Richter, C.; Sadoul, R.; Mazzei, G.; Nichols, A.; Martinou, J.C. Bax-induced cytochrome C release from mitochondria is independent of the permeability transition pore but highly dependent on Mg2+ ions. J. Cell Biol. 1998, 143, 217–224. [Google Scholar] [CrossRef]
- Kim, T.H.; Zhao, Y.; Barber, M.J.; Kuharsky, D.K.; Yin, X.M. Bid-induced cytochrome c release is mediated by a pathway independent of mitochondrial permeability transition pore and Bax. J. Biol. Chem. 2000, 275, 39474–39481. [Google Scholar] [CrossRef]
- Lee, S.K.; Shanmughapriya, S.; Mok, M.C.Y.; Dong, Z.; Tomar, D.; Carvalho, E.; Rajan, S.; Junop, M.S.; Madesh, M.; Stathopulos, P.B. Structural Insights into Mitochondrial Calcium Uniporter Regulation by Divalent Cations. Cell Chem. Biol. 2016, 23, 1157–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szanda, G.; Rajki, A.; Gallego-Sandín, S.; Garcia-Sancho, J.; Spät, A. Effect of cytosolic Mg2+ on mitochondrial Ca2+ signaling. Pflugers Arch. 2009, 457, 941–954. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, R.K.; Qi, F.; Beard, D.A.; Dash, R.K. Characterization of Mg2+ inhibition of mitochondrial Ca2+ uptake by a mechanistic model of mitochondrial Ca2+ uniporter. Biophys. J. 2011, 101, 2071–2081. [Google Scholar] [CrossRef] [PubMed]
- Boelens, A.D.; Pradhan, R.K.; Blomeyer, C.A.; Camara, A.K.S.; Dash, R.K.; Stowe, D.F. Extra-matrix Mg2+ limits Ca2+ uptake and modulates Ca2+ uptake-independent respiration and redox state in cardiac isolated mitochondria. J. Bioenerg. Biomembr. 2013, 45, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Carreras-Sureda, A.; Pihan, P.; Hetz, C. Calcium signaling at the endoplasmic reticulum: fine-tuning stress responses. Cell Calcium 2018, 70, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Luarte, A.; Cornejo, V.H.; Bertin, F.; Gallardo, J.; Couve, A. The axonal endoplasmic reticulum: One organelle-many functions in development, maintenance, and plasticity. Dev. Neurobiol. 2018, 78, 181–208. [Google Scholar] [CrossRef]
- Kwon, S.-K.; Hirabayashi, Y.; Polleux, F. Organelle-Specific Sensors for Monitoring Ca2+ Dynamics in Neurons. Front. Synaptic Neurosci. 2016, 8, 29. [Google Scholar] [CrossRef]
- Volpe, P.; Alderson-Lang, B.H.; Nickols, G.A. Regulation of inositol 1,4,5-trisphosphate-induced Ca2+ release. I. Effect of Mg2+. Am. J. Physiol. 1990, 258, C1077–C1085. [Google Scholar] [CrossRef]
- Gusev, K.; Niggli, E. Modulation of the local SR Ca2+ release by intracellular Mg2+ in cardiac myocytes. J. Gen. Physiol. 2008, 132, 721–730. [Google Scholar] [CrossRef]
- Bull, R.; Finkelstein, J.P.; Humeres, A.; Behrens, M.I.; Hidalgo, C. Effects of ATP, Mg2+, and redox agents on the Ca2+ dependence of RyR channels from rat brain cortex. Am. J. Physiol. Cell Physiol. 2007, 293, C162–C171. [Google Scholar] [CrossRef]
- Laver, D.R. Regulation of the RyR channel gating by Ca2+ and Mg2+. Biophys. Rev. 2018, 10, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- De Juan-Sanz, J.; Holt, G.T.; Schreiter, E.R.; de Juan, F.; Kim, D.S.; Ryan, T.A. Axonal Endoplasmic Reticulum Ca2+ Content Controls Release Probability in CNS Nerve Terminals. Neuron 2017, 93, 867–881.e6. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Quamme, G.A. Caffeine decreases intracellular free Mg2+ in isolated adult rat ventricular myocytes. Biochim. Biophys. Acta 1997, 1355, 61–68. [Google Scholar] [CrossRef]
- Schuwirth, B.S.; Borovinskaya, M.A.; Hau, C.W.; Zhang, W.; Vila-Sanjurjo, A.; Holton, J.M.; Cate, J.H.D. Structures of the bacterial ribosome at 3.5 A resolution. Science 2005, 310, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Pontes, M.H.; Sevostyanova, A.; Groisman, E.A. When Too Much ATP Is Bad for Protein Synthesis. J. Mol. Biol. 2015, 427, 2586–2594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nierhaus, K.H. Mg2+, K+, and the ribosome. J. Bacteriol. 2014, 196, 3817–3819. [Google Scholar] [CrossRef]
- Weiss, R.L.; Morris, D.R. Cations and ribosome structure. I. Effects on the 30S subunit of substituting polyamines for magnesium ion. Biochemistry 1973, 12, 435–441. [Google Scholar] [CrossRef]
- Weiss, R.L.; Kimes, B.W.; Morris, D.R. Cations and ribosome structure. 3. Effects on the 30S and 50S subunits of replacing bound Mg2+ by inorganic cations. Biochemistry 1973, 12, 450–456. [Google Scholar] [CrossRef]
- Fagerbakke, K.M.; Norland, S.; Heldal, M. The inorganic ion content of native aquatic bacteria. Can. J. Microbiol. 1999, 45, 304–311. [Google Scholar] [CrossRef]
- Lee, D.-Y.D.; Galera-Laporta, L.; Bialecka-Fornal, M.; Moon, E.C.; Shen, Z.; Briggs, S.P.; Garcia-Ojalvo, J.; Suel, G.M. Magnesium Flux Modulates Ribosomes to Increase Bacterial Survival. Cell 2019, 177, 352–360.e13. [Google Scholar] [CrossRef] [Green Version]
- Akanuma, G.; Yamazaki, K.; Yagishi, Y.; Iizuka, Y.; Ishizuka, M.; Kawamura, F.; Kato-Yamada, Y. Magnesium Suppresses Defects in the Formation of 70S Ribosomes as Well as in Sporulation Caused by Lack of Several Individual Ribosomal Proteins. J. Bacteriol. 2018, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akanuma, G.; Kobayashi, A.; Suzuki, S.; Kawamura, F.; Shiwa, Y.; Watanabe, S.; Yoshikawa, H.; Hanai, R.; Ishizuka, M. Defect in the formation of 70S ribosomes caused by lack of ribosomal protein L34 can be suppressed by magnesium. J. Bacteriol. 2014, 196, 3820–3830. [Google Scholar] [CrossRef]
- Pontes, M.H.; Groisman, E.A. Protein synthesis controls phosphate homeostasis. Genes Dev. 2018, 32, 79–92. [Google Scholar] [CrossRef] [Green Version]
- Gesteland, R.F. Unfolding of Escherichia coli ribosomes by removal of magnesium. J. Mol. Biol. 1966, 18, 356–371. [Google Scholar] [CrossRef]
- Terasaki, M.; Rubin, H. Evidence that intracellular magnesium is present in cells at a regulatory concentration for protein synthesis. Proc. Natl. Acad. Sci. USA 1985, 82, 7324–7326. [Google Scholar] [CrossRef]
- Pontes, M.H.; Yeom, J.; Groisman, E.A. Reducing Ribosome Biosynthesis Promotes Translation during Low Mg2+ Stress. Mol. Cell 2016, 64, 480–492. [Google Scholar] [CrossRef] [PubMed]
- Gall, A.R.; Datsenko, K.A.; Figueroa-Bossi, N.; Bossi, L.; Masuda, I.; Hou, Y.-M.; Csonka, L.N. Mg2+ regulates transcription of mgtA in Salmonella Typhimurium via translation of proline codons during synthesis of the MgtL peptide. Proc. Natl. Acad. Sci. USA 2016, 113, 15096–15101. [Google Scholar] [CrossRef] [PubMed]
- Hughes, K.T. Mg2+-dependent translational speed bump acts to regulate gene transcription. Proc. Natl. Acad. Sci. USA 2016, 113, 14881–14883. [Google Scholar] [CrossRef]
- Park, M.; Nam, D.; Kweon, D.-H.; Shin, D. ATP reduction by MgtC and Mg2+ homeostasis by MgtA and MgtB enables Salmonella to accumulate RpoS upon low cytoplasmic Mg2+ stress. Mol. Microbiol. 2018, 110, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Mayer, C.; Grummt, I. Ribosome biogenesis and cell growth: mTOR coordinates transcription by all three classes of nuclear RNA polymerases. Oncogene 2006, 25, 6384–6391. [Google Scholar] [CrossRef] [Green Version]
- Vidair, C.; Rubin, H. Mg2+ as activator of uridine phosphorylation in coordination with other cellular responses to growth factors. Proc. Natl. Acad. Sci. USA 2005, 102, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Rubin, H. The membrane, magnesium, mitosis (MMM) model of cell proliferation control. Magnes. Res. 2005, 18, 268–274. [Google Scholar] [PubMed]
- Slomnicki, L.P.; Pietrzak, M.; Vashishta, A.; Jones, J.; Lynch, N.; Elliot, S.; Poulos, E.; Malicote, D.; Morris, B.E.; Hallgren, J.; et al. Requirement of Neuronal Ribosome Synthesis for Growth and Maintenance of the Dendritic Tree. J. Biol. Chem. 2016, 291, 5721–5739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koley, S.; Rozenbaum, M.; Fainzilber, M.; Terenzio, M. Translating regeneration: Local protein synthesis in the neuronal injury response. Neurosci. Res. 2019, 139, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Honda, K.; Smith, M.A.; Zhu, X.; Baus, D.; Merrick, W.C.; Tartakoff, A.M.; Hattier, T.; Harris, P.L.; Siedlak, S.L.; Fujioka, H.; et al. Ribosomal RNA in Alzheimer disease is oxidized by bound redox-active iron. J. Biol. Chem. 2005, 280, 20978–20986. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Markesbery, W.R.; Chen, Q.; Li, F.; Keller, J.N. Ribosome dysfunction is an early event in Alzheimer’s disease. J. Neurosci. 2005, 25, 9171–9175. [Google Scholar] [CrossRef] [PubMed]
- Rieker, C.; Engblom, D.; Kreiner, G.; Domanskyi, A.; Schober, A.; Stotz, S.; Neumann, M.; Yuan, X.; Grummt, I.; Schutz, G.; et al. Nucleolar disruption in dopaminergic neurons leads to oxidative damage and parkinsonism through repression of mammalian target of rapamycin signaling. J. Neurosci. 2011, 31, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Vilotti, S.; Codrich, M.; Dal Ferro, M.; Pinto, M.; Ferrer, I.; Collavin, L.; Gustincich, S.; Zucchelli, S. Parkinson’s disease DJ-1 L166P alters rRNA biogenesis by exclusion of TTRAP from the nucleolus and sequestration into cytoplasmic aggregates via TRAF6. PLoS One 2012, 7, e35051. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Shin, J.-H. Repression of rRNA transcription by PARIS contributes to Parkinson’s disease. Neurobiol. Dis. 2015, 73, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Rubin, H. The logic of the Membrane, Magnesium, Mitosis (MMM) model for the regulation of animal cell proliferation. Arch. Biochem. Biophys. 2007, 458, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Bhagavan, N.V. Medical Biochemistry; Academic Press: Cambridge, MA, USA, 2002; ISBN 0120954400. [Google Scholar]
- Milo, R.; Phillips, R. Cell Biology by the Numbers; Garland Science: Boca Raton, FL, USA, 2015; ISBN 1317230698. [Google Scholar]
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. Biochemistry, 5th ed.; W. H. Freeman: New York, NY, USA, 2002; ISBN 0-7167-3051-0. [Google Scholar]
- Feeney, K.A.; Hansen, L.L.; Putker, M.; Olivares-Yanez, C.; Day, J.; Eades, L.J.; Larrondo, L.F.; Hoyle, N.P.; O’Neill, J.S.; van Ooijen, G. Daily magnesium fluxes regulate cellular timekeeping and energy balance. Nature 2016, 532, 375–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Ooijen, G.; O’Neill, J.S. Intracellular magnesium and the rhythms of life. Cell Cycle 2016, 15, 2997–2998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, L.; Xu, L.; Xu, M.; Wan, B.; Yu, L.; Huang, Q. Role of Mg2+ ions in protein kinase phosphorylation: insights from molecular dynamics simulations of ATP-kinase complexes. Mol. Simul. 2011, 37, 1143–1150. [Google Scholar] [CrossRef]
- Takaya, J.; Higashino, H.; Kobayashi, Y. Can magnesium act as a second messenger? Current data on translocation induced by various biologically active substances. Magnes. Res. 2000, 13, 139–146. [Google Scholar] [PubMed]
- Grubbs, R.D.; Maguire, M.E. Magnesium as a regulatory cation: criteria and evaluation. Magnesium 1987, 6, 113–127. [Google Scholar] [PubMed]
- Li, F.-Y.; Chaigne-Delalande, B.; Kanellopoulou, C.; Davis, J.C.; Matthews, H.F.; Douek, D.C.; Cohen, J.I.; Uzel, G.; Su, H.C.; Lenardo, M.J. Second messenger role for Mg2+ revealed by human T-cell immunodeficiency. Nature 2011, 475, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.M.; Huganir, R.L. MAPK cascade signalling and synaptic plasticity. Nat. Rev. Neurosci. 2004, 5, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Zhai, S.; Ark, E.D.; Parra-Bueno, P.; Yasuda, R. Long-distance integration of nuclear ERK signaling triggered by activation of a few dendritic spines. Science 2013, 342, 1107–1111. [Google Scholar] [CrossRef] [PubMed]
- Stangherlin, A.; O’Neill, J.S. Signal Transduction: Magnesium Manifests as a Second Messenger. Curr. Biol. 2018, 28, R1403–R1405. [Google Scholar] [CrossRef] [Green Version]
- Tarafdar, A.; Pula, G. The Role of NADPH Oxidases and Oxidative Stress in Neurodegenerative Disorders. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef]
- Cid-Castro, C.; Hernandez-Espinosa, D.R.; Moran, J. ROS as Regulators of Mitochondrial Dynamics in Neurons. Cell. Mol. Neurobiol. 2018, 38, 995–1007. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Davies, L.R.; Martin, S.M.; Bawaney, I.M.; Buettner, G.R.; Kerber, R.E. Magnesium reduces free radical concentration and preserves left ventricular function after direct current shocks. Resuscitation 2003, 56, 199–206. [Google Scholar] [CrossRef]
- Zheltova, A.A.; Kharitonova, M.V.; Iezhitsa, I.N.; Spasov, A.A. Magnesium deficiency and oxidative stress: an update. BioMedicine (Taipei) 2016, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, M.; Belvedere, M.; Dominguez, L.J. Magnesium homeostasis and aging. Magnes. Res. 2009, 22, 235–246. [Google Scholar] [PubMed] [Green Version]
- Nielsen, F.H. Magnesium deficiency and increased inflammation: current perspectives. J. Inflamm. Res. 2018, 11, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Guiet-Bara, A.; Durlach, J.; Bara, M. Magnesium ions and ionic channels: activation, inhibition or block - a hypothesis. Magnes. Res. 2007, 20, 100–106. [Google Scholar]
- Kumar, A. NMDA Receptor Function During Senescence: Implication on Cognitive Performance. Front. Neurosci. 2015, 9, 473. [Google Scholar] [CrossRef]
- Iacobucci, G.J.; Popescu, G.K. NMDA receptors: linking physiological output to biophysical operation. Nat. Rev. Neurosci. 2017, 18, 236–249. [Google Scholar] [CrossRef]
- Cull-Candy, S.; Brickley, S.; Farrant, M. NMDA receptor subunits: diversity, development and disease. Curr. Opin. Neurobiol. 2001, 11, 327–335. [Google Scholar] [CrossRef]
- Nechifor, M. Magnesium in addiction - a general view. Magnes. Res. 2018, 31, 90–98. [Google Scholar]
- Song, Q.; Feng, G.; Zhang, J.; Xia, X.; Ji, M.; Lv, L.; Ping, Y. NMDA Receptor-mediated Ca2+ Influx in the Absence of Mg2+ Block Disrupts Rest: Activity Rhythms in Drosophila. Sleep 2017, 40. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, H.; Sawaya, M.R.; Kumar, A.; Wilson, S.H.; Kraut, J. Structures of ternary complexes of rat DNA polymerase beta, a DNA template-primer, and ddCTP. Science 1994, 264, 1891–1903. [Google Scholar] [CrossRef] [PubMed]
- Blaszczyk, U.; Duda-Chodak, A. Magnesium: its role in nutrition and carcinogenesis. Rocz. Panstw. Zakl. Hig. 2013, 64, 165–171. [Google Scholar] [PubMed]
- Gao, Y.; Yang, W. Capture of a third Mg2+ is essential for catalyzing DNA synthesis. Science 2016, 352, 1334–1337. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Davies, K.J.A. Oxidative DNA damage & repair: an introduction. Free Radic. Biol. Med. 2017, 107, 2–12. [Google Scholar] [PubMed]
- LiCausi, F.; Hartman, N. Role of mTOR Complexes in Neurogenesis. Int. J. Mol. Sci. 2018, 19, 1544. [Google Scholar] [CrossRef] [PubMed]
- Schlingmann, K.P.; Waldegger, S.; Konrad, M.; Chubanov, V.; Gudermann, T. TRPM6 and TRPM7-Gatekeepers of human magnesium metabolism. Biochim. Biophys. Acta 2007, 1772, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Paravicini, T.M.; Chubanov, V.; Gudermann, T. TRPM7: a unique channel involved in magnesium homeostasis. Int. J. Biochem. Cell Biol. 2012, 44, 1381–1384. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, C.; Deason, F.; Perraud, A. Molecular components of vertebrate Mg2+ homeostasis regulation. Magnes. Res. 2007, 20, 6–18. [Google Scholar]
- Schmitz, C.; Perraud, A.; Johnson, C.O.; Inabe, K.; Smith, M.K.; Penner, R.; Kurosaki, T.; Fleig, A.; Scharenberg, A.M. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell 2003, 114, 191–200. [Google Scholar] [CrossRef]
- Clark, K.; Langeslag, M.; van Leeuwen, B.; Ran, L.; Ryazanov, A.G.; Figdor, C.G.; Moolenaar, W.H.; Jalink, K.; van Leeuwen, F.N. TRPM7, a novel regulator of actomyosin contractility and cell adhesion. EMBO J. 2006, 25, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Ryazanova, L.V.; Rondon, L.J.; Zierler, S.; Hu, Z.; Galli, J.; Yamaguchi, T.P.; Mazur, A.; Fleig, A.; Ryazanov, A.G. TRPM7 is essential for Mg2+ homeostasis in mammals. Nat. Commun. 2010, 1, 109. [Google Scholar] [CrossRef] [PubMed]
- Runnels, L.W.; Yue, L.; Clapham, D.E. The TRPM7 channel is inactivated by PIP(2) hydrolysis. Nat. Cell Biol. 2002, 4, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Demeuse, P.; Penner, R.; Fleig, A. TRPM7 channel is regulated by magnesium nucleotides via its kinase domain. J. Gen. Physiol. 2006, 127, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Takezawa, R.; Schmitz, C.; Demeuse, P.; Scharenberg, A.M.; Penner, R.; Fleig, A. Receptor-mediated regulation of the TRPM7 channel through its endogenous protein kinase domain. Proc. Natl. Acad. Sci. USA 2004, 101, 6009–6014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, J.; Wu, L.-J.; Jun, J.; Cheng, X.; Xu, H.; Andrews, N.C.; Clapham, D.E. The channel kinase, TRPM7, is required for early embryonic development. Proc. Natl. Acad. Sci. USA 2012, 109, E225–E233. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Desai, B.N.; Navarro, B.; Donovan, A.; Andrews, N.C.; Clapham, D.E. Deletion of Trpm7 disrupts embryonic development and thymopoiesis without altering Mg2+ homeostasis. Science 2008, 322, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Mittermeier, L.; Demirkhanyan, L.; Stadlbauer, B.; Breit, A.; Recordati, C.; Hilgendorff, A.; Matsushita, M.; Braun, A.; Simmons, D.G.; Zakharian, E.; et al. TRPM7 is the central gatekeeper of intestinal mineral absorption essential for postnatal survival. Proc. Natl. Acad. Sci. USA 2019, 116. [Google Scholar] [CrossRef]
- Ogata, K.; Tsumuraya, T.; Oka, K.; Shin, M.; Okamoto, F.; Kajiya, H.; Katagiri, C.; Ozaki, M.; Matsushita, M.; Okabe, K. The crucial role of the TRPM7 kinase domain in the early stage of amelogenesis. Sci. Rep. 2017, 7, 18099. [Google Scholar] [CrossRef]
- Dribben, W.H.; Eisenman, L.N.; Mennerick, S. Magnesium induces neuronal apoptosis by suppressing excitability. Cell Death Dis. 2010, 1, e63. [Google Scholar] [CrossRef]
- Shindo, Y.; Yamanaka, R.; Suzuki, K.; Hotta, K.; Oka, K. Altered expression of Mg2+ transport proteins during Parkinson’s disease-like dopaminergic cell degeneration in PC12 cells. Biochim. Biophys. Acta 2016, 1863, 1979–1984. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Furuya, H.; Faouzi, M.; Zhang, Z.; Monteilh-Zoller, M.; Kawabata, K.G.; Horgen, F.D.; Kawamori, T.; Penner, R.; Fleig, A. Inhibition of TRPM7 suppresses cell proliferation of colon adenocarcinoma in vitro and induces hypomagnesemia in vivo without affecting azoxymethane-induced early colon cancer in mice. Cell Commun. Signal. 2017, 15, 30. [Google Scholar] [CrossRef] [PubMed]
- Komiya, Y.; Runnels, L.W. TRPM channels and magnesium in early embryonic development. Int. J. Dev. Biol. 2015, 59, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Turlova, E.; Bae, C.Y.J.; Deurloo, M.; Chen, W.; Barszczyk, A.; Horgen, F.D.; Fleig, A.; Feng, Z.-P.; Sun, H.-S. TRPM7 Regulates Axonal Outgrowth and Maturation of Primary Hippocampal Neurons. Mol. Neurobiol. 2016, 53, 595–610. [Google Scholar] [CrossRef] [PubMed]
- Su, L.-T.; Liu, W.; Chen, H.-C.; Gonzalez-Pagan, O.; Habas, R.; Runnels, L.W. TRPM7 regulates polarized cell movements. Biochem. J. 2011, 434, 513–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahni, J.; Tamura, R.; Sweet, I.R.; Scharenberg, A.M. TRPM7 regulates quiescent/proliferative metabolic transitions in lymphocytes. Cell Cycle 2010, 9, 3565–3574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahni, J.; Scharenberg, A.M. TRPM7 ion channels are required for sustained phosphoinositide 3-kinase signaling in lymphocytes. Cell Metab. 2008, 8, 84–93. [Google Scholar] [CrossRef]
- Wilson, C.; González-Billault, C. Regulation of cytoskeletal dynamics by redox signaling and oxidative stress: implications for neuronal development and trafficking. Front. Cell. Neurosci. 2015, 9, 381. [Google Scholar] [CrossRef] [Green Version]
- Oswald, M.C.W.; Garnham, N.; Sweeney, S.T.; Landgraf, M. Regulation of neuronal development and function by ROS. FEBS Lett. 2018, 592, 679–691. [Google Scholar] [CrossRef]
- Oancea, E.; Wolfe, J.T.; Clapham, D.E. Functional TRPM7 channels accumulate at the plasma membrane in response to fluid flow. Circ. Res. 2006, 98, 245–253. [Google Scholar] [CrossRef]
- Visser, D.; Middelbeek, J.; van Leeuwen, F.N.; Jalink, K. Function and regulation of the channel-kinase TRPM7 in health and disease. Eur. J. Cell Biol. 2014, 93, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Prado, N.; Chapuis, S.; Panjkovich, A.; Fregeac, J.; Nagy, J.I.; Bukauskas, F.F. Molecular determinants of magnesium-dependent synaptic plasticity at electrical synapses formed by connexin36. Nat. Commun. 2014, 5, 4667. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Prado, N.; Hoge, G.; Marandykina, A.; Rimkute, L.; Chapuis, S.; Paulauskas, N.; Skeberdis, V.A.; O’Brien, J.; Pereda, A.E.; Bennett, M.V.L.; et al. Intracellular magnesium-dependent modulation of gap junction channels formed by neuronal connexin36. J. Neurosci. 2013, 33, 4741–4753. [Google Scholar] [CrossRef] [PubMed]
- Snipas, M.; Rimkute, L.; Kraujalis, T.; Maciunas, K.; Bukauskas, F.F. Functional asymmetry and plasticity of electrical synapses interconnecting neurons through a 36-state model of gap junction channel gating. PLoS Comput. Biol. 2017, 13, e1005464. [Google Scholar] [CrossRef]
- Yamanaka, R.; Shindo, Y.; Karube, T.; Hotta, K.; Suzuki, K.; Oka, K. Neural depolarization triggers Mg2+ influx in rat hippocampal neurons. Neuroscience 2015, 310, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Liu, Y.; Shi, Y.; Ma, Y.; Hu, Y.; Wang, M.; Li, X. Elevation of Brain Magnesium Potentiates Neural Stem Cell Proliferation in the Hippocampus of Young and Aged Mice. J. Cell. Physiol. 2016, 231, 1903–1912. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Xue, L.-D.; Su, L.-W.; Xie, J.-L.; Jiang, H.; Yu, X.-J.; Liu, H.-M. Magnesium promotes the viability and induces differentiation of neural stem cells both in vitro and in vivo. Neurol. Res. 2019, 41, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Jiang, M.; Li, M.; Jin, C.; Xiao, S.; Fan, S.; Fang, W.; Zheng, Y.; Liu, J. Magnesium Elevation Promotes Neuronal Differentiation While Suppressing Glial Differentiation of Primary Cultured Adult Mouse Neural Progenitor Cells through ERK/CREB Activation. Front. Neurosci. 2017, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.W.; Minski, M.J.; Lim, L.; Lai, J.C. Changes in brain regional manganese and magnesium levels during postnatal development: modulations by chronic manganese administration. Metab. Brain Dis. 1992, 7, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Miller, F.D.; Gauthier, A.S. Timing is everything: making neurons versus glia in the developing cortex. Neuron 2007, 54, 357–369. [Google Scholar] [CrossRef]
- Cavaliere, F.; Benito-Muñoz, M.; Panicker, M.; Matute, C. NMDA modulates oligodendrocyte differentiation of subventricular zone cells through PKC activation. Front. Cell. Neurosci. 2013, 7, 261. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Leng, T.; Feng, X.; Sun, H.; Inoue, K.; Zhu, L.; Xiong, Z.-G. Silencing TRPM7 in mouse cortical astrocytes impairs cell proliferation and migration via ERK and JNK signaling pathways. PLoS ONE 2015, 10, e0119912. [Google Scholar] [CrossRef] [PubMed]
- Verstraeten, A.; Theuns, J.; Van Broeckhoven, C. Progress in unraveling the genetic etiology of Parkinson disease in a genomic era. Trends Genet. 2015, 31, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.; Wong, Y.C.; Ysselstein, D.; Severino, A.; Krainc, D. Synaptic, Mitochondrial, and Lysosomal Dysfunction in Parkinson’s Disease. Trends Neurosci. 2019, 42, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.-S.; Geng, W.-S.; Jia, J.-J.; Chen, L.; Zhang, P.-P. Cellular and Molecular Basis of Neurodegeneration in Parkinson Disease. Front. Aging Neurosci. 2018, 10, 109. [Google Scholar] [CrossRef] [Green Version]
- Yasui, M.; Kihira, T.; Ota, K. Calcium, magnesium and aluminum concentrations in Parkinson’s disease. Neurotoxicology 1992, 13, 593–600. [Google Scholar] [PubMed]
- Oyanagi, K. The nature of the parkinsonism-dementia complex and amyotrophic lateral sclerosis of Guam and magnesium deficiency. Parkinsonism Relat. Disord. 2005, 11 (Suppl. 1), S17–S23. [Google Scholar] [CrossRef]
- Aden, E.; Carlsson, M.; Poortvliet, E.; Stenlund, H.; Linder, J.; Edstrom, M.; Forsgren, L.; Haglin, L. Dietary intake and olfactory function in patients with newly diagnosed Parkinson’s disease: a case-control study. Nutr. Neurosci. 2011, 14, 25–31. [Google Scholar] [CrossRef]
- Miyake, Y.; Tanaka, K.; Fukushima, W.; Sasaki, S.; Kiyohara, C.; Tsuboi, Y.; Yamada, T.; Oeda, T.; Miki, T.; Kawamura, N.; et al. Dietary intake of metals and risk of Parkinson’s disease: a case-control study in Japan. J. Neurol. Sci. 2011, 306, 98–102. [Google Scholar] [CrossRef]
- Hermosura, M.C.; Garruto, R.M. TRPM7 and TRPM2 - Candidate susceptibility genes for Western Pacific ALS and PD? Biochim. Biophys. Acta 2007, 1772, 822–835. [Google Scholar] [CrossRef]
- Hermosura, M.C.; Nayakanti, H.; Dorovkov, M.V.; Calderon, F.R.; Ryazanov, A.G.; Haymer, D.S.; Garruto, R.M. A TRPM7 variant shows altered sensitivity to magnesium that may contribute to the pathogenesis of two Guamanian neurodegenerative disorders. Proc. Natl. Acad. Sci. USA 2005, 102, 11510–11515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolisek, M.; Sponder, G.; Mastrototaro, L.; Smorodchenko, A.; Launay, P.; Vormann, J.; Schweigel-Röntgen, M. Substitution p.A350V in Na+/Mg2+ exchanger SLC41A1, potentially associated with Parkinson’s disease, is a gain-of-function mutation. PLoS One 2013, 8, e71096. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Wu, Y.-R.; Chen, W.-L.; Wang, H.-C.; Lee, C.-M.; Lee-Chen, G.-J.; Chen, C.-M. Variant R244H in Na+/Mg2+ exchanger SLC41A1 in Taiwanese Parkinson’s disease is associated with loss of Mg2+ efflux function. Parkinsonism Relat. Disord. 2014, 20, 600–603. [Google Scholar] [CrossRef] [PubMed]
- Decker, A.R.; McNeill, M.S.; Lambert, A.M.; Overton, J.D.; Chen, Y.-C.; Lorca, R.A.; Johnson, N.A.; Brockerhoff, S.E.; Mohapatra, D.P.; MacArthur, H.; et al. Abnormal differentiation of dopaminergic neurons in zebrafish trpm7 mutant larvae impairs development of the motor pattern. Dev. Biol. 2014, 386, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Muroyama, A.; Inaka, M.; Matsushima, H.; Sugino, H.; Marunaka, Y.; Mitsumoto, Y. Enhanced susceptibility to MPTP neurotoxicity in magnesium-deficient C57BL/6N mice. Neurosci. Res. 2009, 63, 72–75. [Google Scholar] [CrossRef]
- Hashimoto, T.; Nishi, K.; Nagasao, J.; Tsuji, S.; Oyanagi, K. Magnesium exerts both preventive and ameliorating effects in an in vitro rat Parkinson disease model involving 1-methyl-4-phenylpyridinium (MPP+) toxicity in dopaminergic neurons. Brain Res. 2008, 1197, 143–151. [Google Scholar] [CrossRef]
- Lin, L.; Yan, M.; Wu, B.; Lin, R.; Zheng, Z. Expression of magnesium transporter SLC41A1 in the striatum of 6-hydroxydopamine-induced parkinsonian rats. Brain Res. Bull. 2018, 142, 338–343. [Google Scholar] [CrossRef]
- Xu, R.; Zhou, Y.; Fang, X.; Lu, Y.; Li, J.; Zhang, J.; Deng, X.; Li, S. The possible mechanism of Parkinson’s disease progressive damage and the preventive effect of GM1 in the rat model induced by 6-hydroxydopamine. Brain Res. 2014, 1592, 73–81. [Google Scholar] [CrossRef]
- Faustini, G.; Bono, F.; Valerio, A.; Pizzi, M.; Spano, P.; Bellucci, A. Mitochondria and α-Synuclein: Friends or Foes in the Pathogenesis of Parkinson’s Disease? Genes (Basel) 2017, 8, 377. [Google Scholar] [CrossRef]
- Golts, N.; Snyder, H.; Frasier, M.; Theisler, C.; Choi, P.; Wolozin, B. Magnesium inhibits spontaneous and iron-induced aggregation of alpha-synuclein. J. Biol. Chem. 2002, 277, 16116–16123. [Google Scholar] [CrossRef]
- Andre, C.; Truong, T.T.; Robert, J.F.; Guillaume, Y.C. Effect of metals on herbicides-alpha-synuclein association: a possible factor in neurodegenerative disease studied by capillary electrophoresis. Electrophoresis 2005, 26, 3256–3264. [Google Scholar] [CrossRef] [PubMed]
- Breydo, L.; Wu, J.W.; Uversky, V.N. Alpha-synuclein misfolding and Parkinson’s disease. Biochim. Biophys. Acta 2012, 1822, 261–285. [Google Scholar] [CrossRef] [PubMed]
- Menzies, F.M.; Fleming, A.; Rubinsztein, D.C. Compromised autophagy and neurodegenerative diseases. Nat. Rev. Neurosci. 2015, 16, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.; Cuervo, A.M. Autophagy gone awry in neurodegenerative diseases. Nat. Neurosci. 2010, 13, 805–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wills, J.; Credle, J.; Oaks, A.W.; Duka, V.; Lee, J.-H.; Jones, J.; Sidhu, A. Paraquat, but not maneb, induces synucleinopathy and tauopathy in striata of mice through inhibition of proteasomal and autophagic pathways. PLoS ONE 2012, 7, e30745. [Google Scholar] [CrossRef]
- Sato, S.; Uchihara, T.; Fukuda, T.; Noda, S.; Kondo, H.; Saiki, S.; Komatsu, M.; Uchiyama, Y.; Tanaka, K.; Hattori, N. Loss of autophagy in dopaminergic neurons causes Lewy pathology and motor dysfunction in aged mice. Sci. Rep. 2018, 8, 2813. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Yang, C.; Iyaswamy, A.; Krishnamoorthi, S.; Sreenivasmurthy, S.G.; Liu, J.; Wang, Z.; Tong, B.C.-K.; Song, J.; Lu, J.; et al. Balancing mTOR Signaling and Autophagy in the Treatment of Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 728. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bockaert, J.; Marin, P. mTOR in Brain Physiology and Pathologies. Physiol. Rev. 2015, 95, 1157–1187. [Google Scholar] [CrossRef] [PubMed]
- Lan, A.-P.; Chen, J.; Zhao, Y.; Chai, Z.; Hu, Y. mTOR Signaling in Parkinson’s Disease. Neuromolecular Med. 2017, 19, 1–10. [Google Scholar] [CrossRef]
- Kametani, F.; Hasegawa, M. Reconsideration of Amyloid Hypothesis and Tau Hypothesis in Alzheimer’s Disease. Front. Neurosci. 2018, 12, 25. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.H.; Oliver, D.M. Amyloid Beta and Phosphorylated Tau-Induced Defective Autophagy and Mitophagy in Alzheimer’s Disease. Cells 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Zurlo, A.; Solmi, M.; Luchini, C.; Trevisan, C.; Bano, G.; Manzato, E.; Sergi, G.; Rylander, R. Magnesium Status in Alzheimer’s Disease: A Systematic Review. Am. J. Alzheimers. Dis. Other Demen. 2016, 31, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Andrasi, E.; Igaz, S.; Molnar, Z.; Mako, S. Disturbances of magnesium concentrations in various brain areas in Alzheimer’s disease. Magnes. Res. 2000, 13, 189–196. [Google Scholar] [PubMed]
- Glick, J.L. Dementias: the role of magnesium deficiency and an hypothesis concerning the pathogenesis of Alzheimer’s disease. Med. Hypotheses 1990, 31, 211–225. [Google Scholar] [CrossRef]
- Cilliler, A.E.; Ozturk, S.; Ozbakir, S. Serum magnesium level and clinical deterioration in Alzheimer’s disease. Gerontology 2007, 53, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Bardgett, M.E.; Schultheis, P.J.; McGill, D.L.; Richmond, R.E.; Wagge, J.R. Magnesium deficiency impairs fear conditioning in mice. Brain Res. 2005, 1038, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Bardgett, M.E.; Schultheis, P.J.; Muzny, A.; Riddle, M.D.; Wagge, J.R. Magnesium deficiency reduces fear-induced conditional lick suppression in mice. Magnes. Res. 2007, 20, 58–65. [Google Scholar] [PubMed]
- Landfield, P.W.; Morgan, G.A. Chronically elevating plasma Mg2+ improves hippocampal frequency potentiation and reversal learning in aged and young rats. Brain Res. 1984, 322, 167–171. [Google Scholar] [CrossRef]
- Enomoto, T.; Osugi, T.; Satoh, H.; McIntosh, T.K.; Nabeshima, T. Pre-Injury magnesium treatment prevents traumatic brain injury-induced hippocampal ERK activation, neuronal loss, and cognitive dysfunction in the radial-arm maze test. J. Neurotrauma 2005, 22, 783–792. [Google Scholar] [CrossRef]
- Hoane, M.R. Treatment with magnesium improves reference memory but not working memory while reducing GFAP expression following traumatic brain injury. Restor. Neurol. Neurosci. 2005, 23, 67–77. [Google Scholar] [PubMed]
- Yu, J.; Sun, M.; Chen, Z.; Lu, J.; Liu, Y.; Zhou, L.; Xu, X.; Fan, D.; Chui, D. Magnesium modulates amyloid-beta protein precursor trafficking and processing. J. Alzheimers. Dis. 2010, 20, 1091–1106. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.-P.; Li, L.; Bao, J.; Wang, Z.-H.; Zeng, J.; Liu, E.-J.; Li, X.-G.; Huang, R.-X.; Gao, D.; Li, M.-Z.; et al. Magnesium protects cognitive functions and synaptic plasticity in streptozotocin-induced sporadic Alzheimer’s model. PLoS One 2014, 9, e108645. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Guan, P.-P.; Zhu, D.; Liang, Y.-Y.; Wang, T.; Wang, Z.-Y.; Wang, P. Magnesium Ions Inhibit the Expression of Tumor Necrosis Factor alpha and the Activity of gamma-Secretase in a beta-Amyloid Protein-Dependent Mechanism in APP/PS1 Transgenic Mice. Front. Mol. Neurosci. 2018, 11, 172. [Google Scholar] [CrossRef] [PubMed]
- Kuramoto, T.; Kuwamura, M.; Tokuda, S.; Izawa, T.; Nakane, Y.; Kitada, K.; Akao, M.; Guénet, J.-L.; Serikawa, T. A mutation in the gene encoding mitochondrial Mg2+ channel MRS2 results in demyelination in the rat. PLoS Genet. 2011, 7, e1001262. [Google Scholar] [CrossRef]
- Kuwamura, M.; Inumaki, K.; Tanaka, M.; Shirai, M.; Izawa, T. Oligodendroglial pathology in the development of myelin breakdown in the dmy mutant rat. Brain Res. 2011, 1389, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Kozin, M.S.; Kulakova, O.G.; Favorova, O.O. Involvement of Mitochondria in Neurodegeneration in Multiple Sclerosis. Biochemistry. (Mosc.) 2018, 83, 813–830. [Google Scholar] [CrossRef]
- Ohno, N.; Ikenaka, K. Axonal and neuronal degeneration in myelin diseases. Neurosci. Res. 2019, 139, 48–57. [Google Scholar] [CrossRef]
- Itoh, K.; Maki, T.; Shindo, A.; Egawa, N.; Liang, A.C.; Itoh, N.; Lo, E.H.; Lok, J.; Arai, K. Magnesium sulfate protects oligodendrocyte lineage cells in a rat cell-culture model of hypoxic-ischemic injury. Neurosci. Res. 2016, 106, 66–69. [Google Scholar] [CrossRef]
- Kuwamura, M.; Tanimura, S.; Hasegawa, Y.; Hoshiai, R.; Moriyama, Y.; Tanaka, M.; Takenaka, S.; Nagayoshi, H.; Izawa, T.; Yamate, J.; et al. Downregulation of aspartoacylase during the progression of myelin breakdown in the dmy mutant rat with mitochondrial magnesium channel MRS2 defect. Brain Res. 2019, 1718, 169–175. [Google Scholar] [CrossRef]
- Madsen, P.M.; Pinto, M.; Patel, S.; McCarthy, S.; Gao, H.; Taherian, M.; Karmally, S.; Pereira, C.V.; Dvoriantchikova, G.; Ivanov, D.; et al. Mitochondrial DNA Double-Strand Breaks in Oligodendrocytes Cause Demyelination, Axonal Injury, and CNS Inflammation. J. Neurosci. 2017, 37, 10185–10199. [Google Scholar] [CrossRef] [PubMed]
- Alexeyev, M.; Shokolenko, I.; Wilson, G.; LeDoux, S. The Maintenance of Mitochondrial DNA Integrity-Critical Analysis and Update. Cold Spring Harb. Perspect. Biol. 2013, 5, a012641. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.M.; George, M.J.; Wilson, A.C. Rapid evolution of animal mitochondrial DNA. Proc. Natl. Acad. Sci. USA 1979, 76, 1967–1971. [Google Scholar] [CrossRef] [PubMed]
- Tatarenkov, A.; Avise, J.C. Rapid concerted evolution in animal mitochondrial DNA. Proc. Biol. Sci. 2007, 274, 1795–1798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCutcheon, J.P. From microbiology to cell biology: when an intracellular bacterium becomes part of its host cell. Curr. Opin. Cell Biol. 2016, 41, 132–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roger, A.J.; Munoz-Gomez, S.A.; Kamikawa, R. The Origin and Diversification of Mitochondria. Curr. Biol. 2017, 27, R1177–R1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, M.A.; Vidoni, S.; Durigon, R.; Pearce, S.F.; Rorbach, J.; He, J.; Brea-Calvo, G.; Minczuk, M.; Reyes, A.; Holt, I.J.; et al. Amino acid starvation has opposite effects on mitochondrial and cytosolic protein synthesis. PLoS ONE 2014, 9, e93597. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamanaka, R.; Shindo, Y.; Oka, K. Magnesium Is a Key Player in Neuronal Maturation and Neuropathology. Int. J. Mol. Sci. 2019, 20, 3439. https://doi.org/10.3390/ijms20143439
Yamanaka R, Shindo Y, Oka K. Magnesium Is a Key Player in Neuronal Maturation and Neuropathology. International Journal of Molecular Sciences. 2019; 20(14):3439. https://doi.org/10.3390/ijms20143439
Chicago/Turabian StyleYamanaka, Ryu, Yutaka Shindo, and Kotaro Oka. 2019. "Magnesium Is a Key Player in Neuronal Maturation and Neuropathology" International Journal of Molecular Sciences 20, no. 14: 3439. https://doi.org/10.3390/ijms20143439
APA StyleYamanaka, R., Shindo, Y., & Oka, K. (2019). Magnesium Is a Key Player in Neuronal Maturation and Neuropathology. International Journal of Molecular Sciences, 20(14), 3439. https://doi.org/10.3390/ijms20143439




