Phenotypic Characterization of Bone Marrow Mononuclear Cells and Derived Stromal Cell Populations from Human Iliac Crest, Vertebral Body and Femoral Head
Abstract
:1. Introduction
2. Results
2.1. BMSC Yield and Growth Kinetics
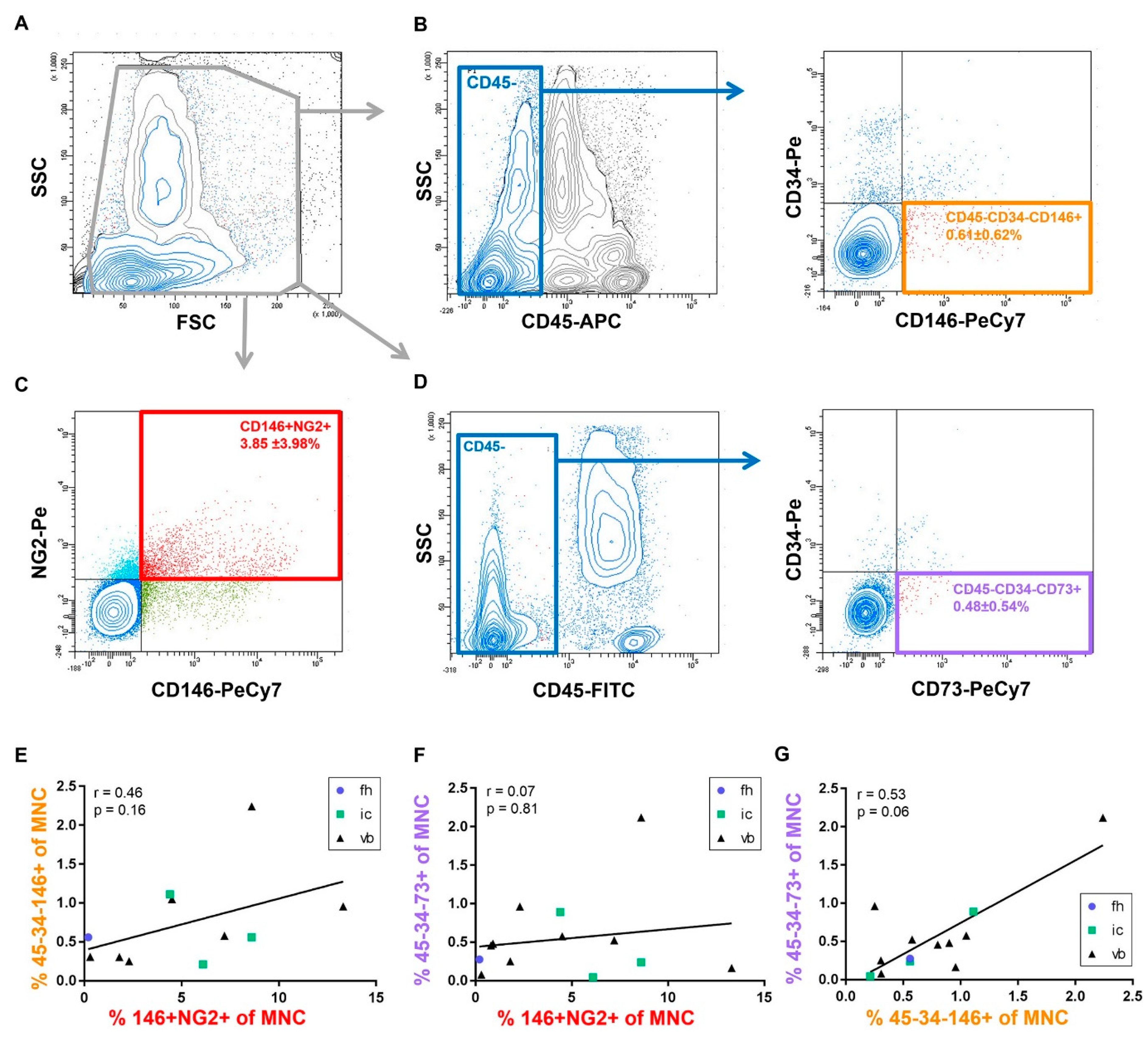
2.2. Identification of Putative Progenitor Cell Subsets within the Freshly Isolated MNC Fraction
2.3. Progenitor Abundance in the Freshly Isolated Mononuclear Cell Fraction Is Not Predictive of the Derived Monolayer Expanded BMSC Population
2.4. Immunophenotyping of BMSCs
2.5. Tri-Lineage In Vitro Differentiation
3. Discussion
4. Materials and Methods
4.1. Bone Marrow Processing and Cell Isolation
4.2. Flow Cytometry Analysis
4.3. In Vitro Differentiation Assays
4.3.1. Chondrogenic Differentiation
4.3.2. Osteogenic Differentiation
4.3.3. Adipogenic Induction
4.4. Statistics
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ARS | alizarin red S |
| BMSC | bone marrow stromal cell |
| CD | cluster of differentiation |
| CFU | colony forming unit |
| DMSO | dimethyl sulfoxide |
| FCS | foetal calf serum |
| FH | femoral head |
| IC | iliac crest |
| LepR | leptin receptor |
| MEM | minimum essential medium |
| MNC | mononuclear cell |
| MSC | mesenchymal stem cell or mesenchymal stromal cell |
| PBS | phosphate buffered saline |
| PDGF | platelet-derived growth factor |
| PDT | population doubling time |
| RT | room temperature |
| sGAG | sulphated glycosaminoglycans |
| TGF | transforming growth factor |
| VB | vertebral body |
References
- Friedenstein, A.; Piatetzky-Shapiro, I.; Petrakova, K. Osteogenesis in transplants of bone marrow cells. Development 1966, 16, 381–390. [Google Scholar]
- Friedenstein, A.J.; Gorskaja, J.F.; Kulagina, N.N. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp. Hematol. 1976, 4, 267–274. [Google Scholar] [PubMed]
- Johnstone, B.; Hering, T.M.; Caplan, A.I.; Goldberg, V.M.; Yoo, J.U. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp. Cell Res. 1998, 238, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Christ, B.; Huang, R.; Scaal, M. Amniote somite derivatives. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2007, 236, 2382–2396. [Google Scholar] [CrossRef] [PubMed]
- Grcevic, D.; Pejda, S.; Matthews, B.G.; Repic, D.; Wang, L.; Li, H.; Kronenberg, M.S.; Jiang, X.; Maye, P.; Adams, D.J.; et al. In vivo fate mapping identifies mesenchymal progenitor cells. Stem Cells 2012, 30, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Isern, J.; Garcia-Garcia, A.; Martin, A.M.; Arranz, L.; Martin-Perez, D.; Torroja, C.; Sanchez-Cabo, F.; Mendez-Ferrer, S. The neural crest is a source of mesenchymal stem cells with specialized hematopoietic stem cell niche function. eLife 2014, 3, e03696. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Boxall, S.A.; Jones, E. Markers for characterization of bone marrow multipotential stromal cells. Stem Cells Int. 2012, 2012, 975871. [Google Scholar] [CrossRef]
- Halfon, S.; Abramov, N.; Grinblat, B.; Ginis, I. Markers distinguishing mesenchymal stem cells from fibroblasts are downregulated with passaging. Stem Cells Dev. 2011, 20, 53–66. [Google Scholar] [CrossRef]
- Jones, E.A.; English, A.; Kinsey, S.E.; Straszynski, L.; Emery, P.; Ponchel, F.; McGonagle, D. Optimization of a flow cytometry-based protocol for detection and phenotypic characterization of multipotent mesenchymal stromal cells from human bone marrow. Cytom. Part B Clin. Cytom. 2006, 70, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Le Blanc, K.; Sigvardsson, M. Primary mesenchymal stem and progenitor cells from bone marrow lack expression of CD44 protein. J. Biol. Chem. 2012, 287, 25795–25807. [Google Scholar] [CrossRef] [PubMed]
- Calvi, L.M.; Adams, G.B.; Weibrecht, K.W.; Weber, J.M.; Olson, D.P.; Knight, M.C.; Martin, R.P.; Schipani, E.; Divieti, P.; Bringhurst, F.R.; et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 2003, 425, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, T.; Kohara, H.; Noda, M.; Nagasawa, T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 2006, 25, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Ferrer, S.; Michurina, T.V.; Ferraro, F.; Mazloom, A.R.; MacArthur, B.D.; Lira, S.A.; Scadden, D.T.; Ma’ayan, A.; Enikolopov, G.N.; Frenette, P.S. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010, 466, 829. [Google Scholar] [CrossRef] [PubMed]
- Rasini, V.; Dominici, M.; Kluba, T.; Siegel, G.; Lusenti, G.; Northoff, H.; Horwitz, E.M.; Schafer, R. Mesenchymal stromal/stem cells markers in the human bone marrow. Cytotherapy 2013, 15, 292–306. [Google Scholar] [CrossRef]
- Tormin, A.; Li, O.; Brune, J.C.; Walsh, S.; Schutz, B.; Ehinger, M.; Ditzel, N.; Kassem, M.; Scheding, S. CD146 expression on primary nonhematopoietic bone marrow stem cells is correlated with in situ localization. Blood 2011, 117, 5067–5077. [Google Scholar] [CrossRef] [Green Version]
- Pacini, S.; Barachini, S.; Montali, M.; Carnicelli, V.; Fazzi, R.; Parchi, P.; Petrini, M. Mesangiogenic Progenitor Cells Derived from One Novel CD64(bright)CD31(bright)CD14(neg) Population in Human Adult Bone Marrow. Stem Cells Dev. 2016, 25, 661–673. [Google Scholar] [CrossRef]
- Muniz, C.; Teodosio, C.; Mayado, A.; Amaral, A.T.; Matarraz, S.; Barcena, P.; Sanchez, M.L.; Alvarez-Twose, I.; Diez-Campelo, M.; Garcia-Montero, A.C.; et al. Ex vivo identification and characterization of a population of CD13(high) CD105(+) CD45(-) mesenchymal stem cells in human bone marrow. Stem Cell Res. Ther. 2015, 6, 169. [Google Scholar] [CrossRef]
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C.-W.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008, 3, 301–313. [Google Scholar] [CrossRef]
- Sacchetti, B.; Funari, A.; Remoli, C.; Giannicola, G.; Kogler, G.; Liedtke, S.; Cossu, G.; Serafini, M.; Sampaolesi, M.; Tagliafico, E. No identical “mesenchymal stem cells” at different times and sites: Human committed progenitors of distinct origin and differentiation potential are incorporated as adventitial cells in microvessels. Stem Cell Rep. 2016, 6, 897–913. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, M.; Bara, J.J.; Sprecher, C.M.; Menzel, U.; Jalowiec, J.M.; Osinga, R.; Scherberich, A.; Alini, M.; Verrier, S. Pericyte plasticity-comparative investigation of the angiogenic and multilineage potential of pericytes from different human tissues. Eur. Cells Mater. 2016, 31, 236–249. [Google Scholar] [CrossRef]
- Chan, C.K.F.; Gulati, G.S.; Sinha, R.; Tompkins, J.V.; Lopez, M.; Carter, A.C.; Ransom, R.C.; Reinisch, A.; Wearda, T.; Murphy, M.; et al. Identification of the Human Skeletal Stem Cell. Cell 2018, 175, 43–56.e21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Ippolito, G.; Schiller, P.C.; Ricordi, C.; Roos, B.A.; Howard, G.A. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 1999, 14, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Muschler, G.F.; Nitto, H.; Boehm, C.A.; Easley, K.A. Age- and gender-related changes in the cellularity of human bone marrow and the prevalence of osteoblastic progenitors. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2001, 19, 117–125. [Google Scholar] [CrossRef]
- Dexheimer, V.; Mueller, S.; Braatz, F.; Richter, W. Reduced reactivation from dormancy but maintained lineage choice of human mesenchymal stem cells with donor age. PLoS ONE 2011, 6, e22980. [Google Scholar] [CrossRef]
- Stenderup, K.; Justesen, J.; Clausen, C.; Kassem, M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone 2003, 33, 919–926. [Google Scholar] [CrossRef]
- Mareschi, K.; Ferrero, I.; Rustichelli, D.; Aschero, S.; Gammaitoni, L.; Aglietta, M.; Madon, E.; Fagioli, F. Expansion of mesenchymal stem cells isolated from pediatric and adult donor bone marrow. J. Cell. Biochem. 2006, 97, 744–754. [Google Scholar] [CrossRef] [Green Version]
- Wagner, W.; Bork, S.; Horn, P.; Krunic, D.; Walenda, T.; Diehlmann, A.; Benes, V.; Blake, J.; Huber, F.X.; Eckstein, V.; et al. Aging and replicative senescence have related effects on human stem and progenitor cells. PLoS ONE 2009, 4, e5846. [Google Scholar] [CrossRef]
- Siegel, G.; Kluba, T.; Hermanutz-Klein, U.; Bieback, K.; Northoff, H.; Schafer, R. Phenotype, donor age and gender affect function of human bone marrow-derived mesenchymal stromal cells. BMC Med. 2013, 11, 146. [Google Scholar] [CrossRef]
- Scharstuhl, A.; Schewe, B.; Benz, K.; Gaissmaier, C.; Buhring, H.J.; Stoop, R. Chondrogenic potential of human adult mesenchymal stem cells is independent of age or osteoarthritis etiology. Stem Cells 2007, 25, 3244–3251. [Google Scholar] [CrossRef] [PubMed]
- Payne, K.A.; Didiano, D.M.; Chu, C.R. Donor sex and age influence the chondrogenic potential of human femoral bone marrow stem cells. Osteoarthr. Cartil. 2010, 18, 705–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Risbud, M.V.; Shapiro, I.M.; Guttapalli, A.; Di Martino, A.; Danielson, K.G.; Beiner, J.M.; Hillibrand, A.; Albert, T.J.; Anderson, D.G.; Vaccaro, A.R. Osteogenic potential of adult human stem cells of the lumbar vertebral body and the iliac crest. Spine 2006, 31, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Min, W.K.; Bae, J.S.; Park, B.C.; Jeon, I.H.; Jin, H.K.; Son, M.J.; Park, E.K.; Kim, S.Y. Proliferation and osteoblastic differentiation of bone marrow stem cells: Comparison of vertebral body and iliac crest. Eur. Spine J. Off. Publ. Eur. Spine Soc. Eur. Spinal Deform. Soc. Eur. Sect. Cerv. Spine Res. Soc. 2010, 19, 1753–1760. [Google Scholar] [CrossRef] [PubMed]
- Davies, B.M.; Snelling, S.J.B.; Quek, L.; Hakimi, O.; Ye, H.; Carr, A.; Price, A.J. Identifying the optimum source of mesenchymal stem cells for use in knee surgery. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2017, 35, 1868–1875. [Google Scholar] [CrossRef] [PubMed]
- Bara, J.J.; Richards, R.G.; Alini, M.; Stoddart, M.J. Concise review: Bone marrow-derived mesenchymal stem cells change phenotype following in vitro culture: Implications for basic research and the clinic. Stem Cells 2014, 32, 1713–1723. [Google Scholar] [CrossRef] [PubMed]
- Fragkakis, E.M.; El-Jawhari, J.J.; Dunsmuir, R.A.; Millner, P.A.; Rao, A.S.; Henshaw, K.T.; Pountos, I.; Jones, E.; Giannoudis, P.V. Vertebral body versus iliac crest bone marrow as a source of multipotential stromal cells: Comparison of processing techniques, tri-lineage differentiation and application on a scaffold for spine fusion. PLoS ONE 2018, 13, e0197969. [Google Scholar] [CrossRef] [PubMed]
- Bara, J.J.; Herrmann, M.; Menzel, U.; Benneker, L.; Alini, M.; Stoddart, M.J. Three-dimensional culture and characterization of mononuclear cells from human bone marrow. Cytotherapy 2015, 17, 458–472. [Google Scholar] [CrossRef]
- Diaz-Flores, L.; Gutierrez, R.; Madrid, J.F.; Varela, H.; Valladares, F.; Acosta, E.; Martin-Vasallo, P.; Diaz-Flores, L., Jr. Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol. Histopathol. 2009, 24, 909–969. [Google Scholar]
- Bardin, N.; Frances, V.; Lesaule, G.; Horschowski, N.; George, F.; Sampol, J. Identification of the S-Endo 1 endothelial-associated antigen. Biochem. Biophys. Res. Commun. 1996, 218, 210–216. [Google Scholar] [CrossRef]
- Schlingemann, R.O.; Rietveld, F.J.; de Waal, R.M.; Ferrone, S.; Ruiter, D.J. Expression of the high molecular weight melanoma-associated antigen by pericytes during angiogenesis in tumors and in healing wounds. Am. J. Pathol. 1990, 136, 1393–1405. [Google Scholar] [PubMed]
- Ozerdem, U.; Grako, K.A.; Dahlin-Huppe, K.; Monosov, E.; Stallcup, W.B. NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2001, 222, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Tigges, U.; Hyer, E.G.; Scharf, J.; Stallcup, W.B. FGF2-dependent neovascularization of subcutaneous Matrigel plugs is initiated by bone marrow-derived pericytes and macrophages. Development 2008, 135, 523–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murfee, W.L.; Rehorn, M.R.; Peirce, S.M.; Skalak, T.C. Perivascular cells along venules upregulate NG2 expression during microvascular remodeling. Microcirculation 2006, 13, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yan, X. CD146, a multi-functional molecule beyond adhesion. Cancer Lett. 2013, 330, 150–162. [Google Scholar] [CrossRef]
- Crisan, M.; Corselli, M.; Chen, C.W.; Peault, B. Multilineage stem cells in the adult: A perivascular legacy? Organogenesis 2011, 7, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, M.; Binder, A.; Menzel, U.; Zeiter, S.; Alini, M.; Verrier, S. CD34/CD133 enriched bone marrow progenitor cells promote neovascularization of tissue engineered constructs in vivo. Stem Cell Res. 2014, 13, 465–477. [Google Scholar] [CrossRef] [Green Version]
- Armulik, A.; Genove, G.; Betsholtz, C. Pericytes: Developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell 2011, 21, 193–215. [Google Scholar] [CrossRef]
- Robey, P. “Mesenchymal stem cells”: Fact or fiction, and implications in their therapeutic use. F1000Research 2017, 6, 524. [Google Scholar] [CrossRef]
- Prazeres, P.H.D.M.; Sena, I.F.G.; Borges, I.D.; de Azevedo, P.O.; Andreotti, J.P.; de Paiva, A.E.; de Almeida, V.M.; Guerra, D.A.D.; dos Santos, G.S.P.; Mintz, A.; et al. Pericytes are heterogeneous in their origin within the same tissue. Dev. Biol. 2017, 427, 6–11. [Google Scholar] [CrossRef]
- Guimaraes-Camboa, N.; Cattaneo, P.; Sun, Y.; Moore-Morris, T.; Gu, Y.; Dalton, N.D.; Rockenstein, E.; Masliah, E.; Peterson, K.L.; Stallcup, W.B.; et al. Pericytes of Multiple Organs Do Not Behave as Mesenchymal Stem Cells In Vivo. Cell Stem Cell 2017, 20, 345–359.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, K.G.; Johnson, K.R.; McKay, R.D.G.; Robey, P.G. Concise Review: Conceptualizing Paralogous Stem-Cell Niches and Unfolding Bone Marrow Progenitor Cell Identities. Stem Cells 2018, 36, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Banfi, A.; Bianchi, G.; Notaro, R.; Luzzatto, L.; Cancedda, R.; Quarto, R. Replicative aging and gene expression in long-term cultures of human bone marrow stromal cells. Tissue Eng. 2002, 8, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Baxter, M.A.; Wynn, R.F.; Jowitt, S.N.; Wraith, J.E.; Fairbairn, L.J.; Bellantuono, I. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells 2004, 22, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Bork, S.; Pfister, S.; Witt, H.; Horn, P.; Korn, B.; Ho, A.D.; Wagner, W. DNA methylation pattern changes upon long-term culture and aging of human mesenchymal stromal cells. Aging Cell 2010, 9, 54–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruder, S.P.; Jaiswal, N.; Haynesworth, S.E. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J. Cell. Biochem. 1997, 64, 278–294. [Google Scholar] [CrossRef]
- Digirolamo, C.M.; Stokes, D.; Colter, D.; Phinney, D.G.; Class, R.; Prockop, D.J. Propagation and senescence of human marrow stromal cells in culture: A simple colony-forming assay identifies samples with the greatest potential to propagate and differentiate. Br. J. Haematol. 1999, 107, 275–281. [Google Scholar] [CrossRef]
- Muraglia, A.; Cancedda, R.; Quarto, R. Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. J. Cell Sci. 2000, 113 Pt 7, 1161–1166. [Google Scholar]
- Redaelli, S.; Bentivegna, A.; Foudah, D.; Miloso, M.; Redondo, J.; Riva, G.; Baronchelli, S.; Dalpra, L.; Tredici, G. From cytogenomic to epigenomic profiles: Monitoring the biologic behavior of in vitro cultured human bone marrow mesenchymal stem cells. Stem Cell Res. Ther. 2012, 3, 47. [Google Scholar] [CrossRef]
- Braccini, A.; Wendt, D.; Jaquiery, C.; Jakob, M.; Heberer, M.; Kenins, L.; Wodnar-Filipowicz, A.; Quarto, R.; Martin, I. Three-dimensional perfusion culture of human bone marrow cells and generation of osteoinductive grafts. Stem Cells 2005, 23, 1066–1072. [Google Scholar] [CrossRef]
- Claros, S.; Rodriguez-Losada, N.; Cruz, E.; Guerado, E.; Becerra, J.; Andrades, J.A. Characterization of adult stem/progenitor cell populations from bone marrow in a three-dimensional collagen gel culture system. Cell Transplant. 2012, 21, 2021–2032. [Google Scholar] [CrossRef] [PubMed]
- Scaglione, S.; Braccini, A.; Wendt, D.; Jaquiery, C.; Beltrame, F.; Quarto, R.; Martin, I. Engineering of osteoinductive grafts by isolation and expansion of ovine bone marrow stromal cells directly on 3D ceramic scaffolds. Biotechnol. Bioeng. 2006, 93, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Katsara, O.; Mahaira, L.G.; Iliopoulou, E.G.; Moustaki, A.; Antsaklis, A.; Loutradis, D.; Stefanidis, K.; Baxevanis, C.N.; Papamichail, M.; Perez, S.A. Effects of donor age, gender, and in vitro cellular aging on the phenotypic, functional, and molecular characteristics of mouse bone marrow-derived mesenchymal stem cells. Stem Cells Dev. 2011, 20, 1549–1561. [Google Scholar] [CrossRef] [PubMed]
- Gill, T.J.; Asnis, P.D.; Berkson, E.M. The treatment of articular cartilage defects using the microfracture technique. J. Orthop. Sports Phys. Ther. 2006, 36, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Saris, D.B.; Vanlauwe, J.; Victor, J.; Haspl, M.; Bohnsack, M.; Fortems, Y.; Vandekerckhove, B.; Almqvist, K.F.; Claes, T.; Handelberg, F.; et al. Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am. J. Sports Med. 2008, 36, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Chawla, A.; Twycross-Lewis, R.; Maffulli, N. Microfracture produces inferior outcomes to other cartilage repair techniques in chondral injuries in the paediatric knee. Br. Med. Bull. 2015, 116, 93–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brittberg, M.; Recker, D.; Ilgenfritz, J.; Saris, D.B.F.; Group, S.E.S. Matrix-Applied Characterized Autologous Cultured Chondrocytes Versus Microfracture: Five-Year Follow-up of a Prospective Randomized Trial. Am. J. Sports Med. 2018, 46, 1343–1351. [Google Scholar] [CrossRef]







| Donor | Age | Sex | Tissue Source | MNCs Seeded | MSC Yield at p0/MNCs Seeded | MSC PDT (p0–p1) |
|---|---|---|---|---|---|---|
| 1 | 86 | Female | Femoral head | 1.97 × 106 | 0.30 | 0.69 |
| 2 | 67 | Female | Femoral head | 1.91 × 107 | 0.19 | 0.77 |
| 3 | 86 | Female | Femoral head | 2.33 × 107 | 0.39 | 0.77 |
| 4 | 51 | Male | Femoral head | 6.25 × 106 | 0.58 | 1.00 |
| 5 | 91 | Male | Femoral head | 4.00 × 105 | 0.51 | 0.91 |
| 6 | 67 | Male | Femoral head | 3.44 × 106 | 0.06 | 0.73 |
| 7 | 46 | Male | Femoral head | 5.05 × 106 | 0.95 | 0.94 |
| 8 | 83 | Male | Femoral head | 6.70 × 106 | 0.13 | 0.35 |
| 9 | 81 | Male | Femoral head | 2.53 × 106 | 0.38 | 0.65 |
| 10 | 83 | Male | Femoral head | 1.53 × 107 | 0.12 | 0.70 |
| 11 | 75 | Female | Femoral head | 3.75 × 106 | 0.95 | 0.55 |
| 12 | 61 | Male | Femoral head | 1.13 × 107 | 0.46 | 0.57 |
| 13 | 68 | Male | Femoral head | 4.21 × 106 | 0.24 | 0.85 |
| 14 | 83 | Male | Femoral head | 6.33 × 106 | 0.14 | 0.80 |
| 15 | 82 | Male | Femoral head | 5.10 x 107 | 0.14 | 0.24 |
| 16 | 88 | Female | Iliac crest | 4.35 × 107 | 0.12 | 0.59 |
| 17 | 48 | Female | Iliac crest | 4.80 × 107 | 0.25 | 0.86 |
| 18 | 46 | Male | Iliac crest | 3.20 × 107 | 0.17 | 0.55 |
| 19 | 14 | Male | Iliac crest | 5.25 × 107 | 0.16 | 0.61 |
| 20 | 41 | Male | Iliac crest | 7.84 ×106 | 0.34 | 0.44 |
| 21 | 32 | Male | Iliac crest | 8.04 × 107 | 0.39 | 0.73 |
| 22 | 23 | Female | Iliac crest | 1.43 × 107 | 0.22 | 0.95 |
| 23 | 53 | Male | Iliac crest | 6.40 × 107 | 0.09 | 0.57 |
| 24 | 75 | Female | Vertebral body | 6.40 × 107 | 0.10 | 1.00 |
| 25 | 64 | Female | Vertebral body | 2.77 × 107 | 0.73 | 0.65 |
| 26 | 39 | Male | Vertebral body | 1.60 × 107 | 0.26 | 0.71 |
| 27 | 67 | Male | Vertebral body | 1.60 × 107 | 0.12 | 0.44 |
| 28 | 46 | Male | Vertebral body | 3.20 × 107 | 0.33 | 0.85 |
| 29 | 89 | Male | Vertebral body | 3.20 × 107 | 0.14 | 0.70 |
| 30 | 77 | Male | Vertebral body | 1.60 × 107 | 0.75 | 0.16 |
| 31 | 40 | Male | Vertebral body | 8.00 ×106 | 0.32 | 0.78 |
| 32 | 60 | Male | Vertebral body | 3.20 × 107 | 0.66 | 0.21 |
| 33 | 75 | Female | Vertebral body | 3.20 × 107 | 0.13 | 0.52 |
| CD146 | NG2 | CD146/NG2 | CD44 | CD90 | CD105 | CD73 | CD44/CD90 CD105/CD73 | ||
|---|---|---|---|---|---|---|---|---|---|
| P0 | fh | 87.5 ± 11.9 | 86.0 ± 17.8 | 75.1 ± 19.1 | 100 ± 0.0 | 94.5 ± 3.6 | 100 ± 0.1 | 100 ± 0.0 | 94.5 ± 3.6 |
| ic | 98.4 ± 1.3 | 88.7 ± 9.0 | 87.3 ± 9.1 | 100 ± 0.1 | 96.9 ± 1.1 | 99.90 ± 0.0 | 99.9 ± 0.1 | 96.9 ± 1.1 | |
| vb | 97.7 ± 1.0 | 65.7 ± 18.5 | 64.3 ± 17.8 | 100 ± 0.0 | 93.7 ± 4.8 | 99.98 ± 0.0 | 100 ± 0.0 | 93.7 ± 4.8 | |
| P1 | fh | 66.4 ± 22.7 | 74.7 ± 16.6 | 47.0 ± 9.5 | 100 ± 0.0 | 97.2 ± 1.5 | 99. 8 ± 0.3 | 100 ± 0.0 | 97.1 ± 1.7 |
| ic | 97.4 ± 2.4 | 87.6 ± 13.0 | 85.2 ± 12.0 | 100 ± 0.1 | 96.6 ± 0.5 | 99.6 ± 0.5 | 99.7 ± 0.5 | 96.6 ± 0.4 | |
| vb | 95.1 ± 2.9 | 73.8 ± 13.6 | 72.7 ± 13.4 | 100 ± 0.1 | 93.7 ± 4.8 | 99.9 ± 0.3 | 100 ± 0.0 | 97.8 ± 2.2 |
| Chondrogenic Differentiation of Bone Marrow Stromal Cells | |||||
|---|---|---|---|---|---|
| Source | Age | Sex | Size | Safranin-O Staining | Score |
| Iliac crest | 23 | Female | +++ | +++ | 6 |
| Iliac crest | 53 | Male | +++ | + | 4 |
| Iliac crest | 45 | Female | ++ | + | 3 |
| Femoral head | 83 | Male | ++ | + | 3 |
| Femoral head | 75 | Female | ++ | + | 3 |
| Femoral head | 51 | Male | + | ++ | 3 |
| Vertebral body | 77 | Male | ++ | + | 3 |
| Vertebral body | 60 | Male | ++ | +++ | 5 |
| Vertebral body | 48 | Male | +++ | +++ | 6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrmann, M.; Hildebrand, M.; Menzel, U.; Fahy, N.; Alini, M.; Lang, S.; Benneker, L.; Verrier, S.; Stoddart, M.J.; Bara, J.J. Phenotypic Characterization of Bone Marrow Mononuclear Cells and Derived Stromal Cell Populations from Human Iliac Crest, Vertebral Body and Femoral Head. Int. J. Mol. Sci. 2019, 20, 3454. https://doi.org/10.3390/ijms20143454
Herrmann M, Hildebrand M, Menzel U, Fahy N, Alini M, Lang S, Benneker L, Verrier S, Stoddart MJ, Bara JJ. Phenotypic Characterization of Bone Marrow Mononuclear Cells and Derived Stromal Cell Populations from Human Iliac Crest, Vertebral Body and Femoral Head. International Journal of Molecular Sciences. 2019; 20(14):3454. https://doi.org/10.3390/ijms20143454
Chicago/Turabian StyleHerrmann, Marietta, Maria Hildebrand, Ursula Menzel, Niamh Fahy, Mauro Alini, Siegmund Lang, Lorin Benneker, Sophie Verrier, Martin J. Stoddart, and Jennifer J. Bara. 2019. "Phenotypic Characterization of Bone Marrow Mononuclear Cells and Derived Stromal Cell Populations from Human Iliac Crest, Vertebral Body and Femoral Head" International Journal of Molecular Sciences 20, no. 14: 3454. https://doi.org/10.3390/ijms20143454
APA StyleHerrmann, M., Hildebrand, M., Menzel, U., Fahy, N., Alini, M., Lang, S., Benneker, L., Verrier, S., Stoddart, M. J., & Bara, J. J. (2019). Phenotypic Characterization of Bone Marrow Mononuclear Cells and Derived Stromal Cell Populations from Human Iliac Crest, Vertebral Body and Femoral Head. International Journal of Molecular Sciences, 20(14), 3454. https://doi.org/10.3390/ijms20143454








