Acid- and Volume-Sensitive Chloride Currents in Microglial Cells
Abstract
:1. Introduction
2. Results
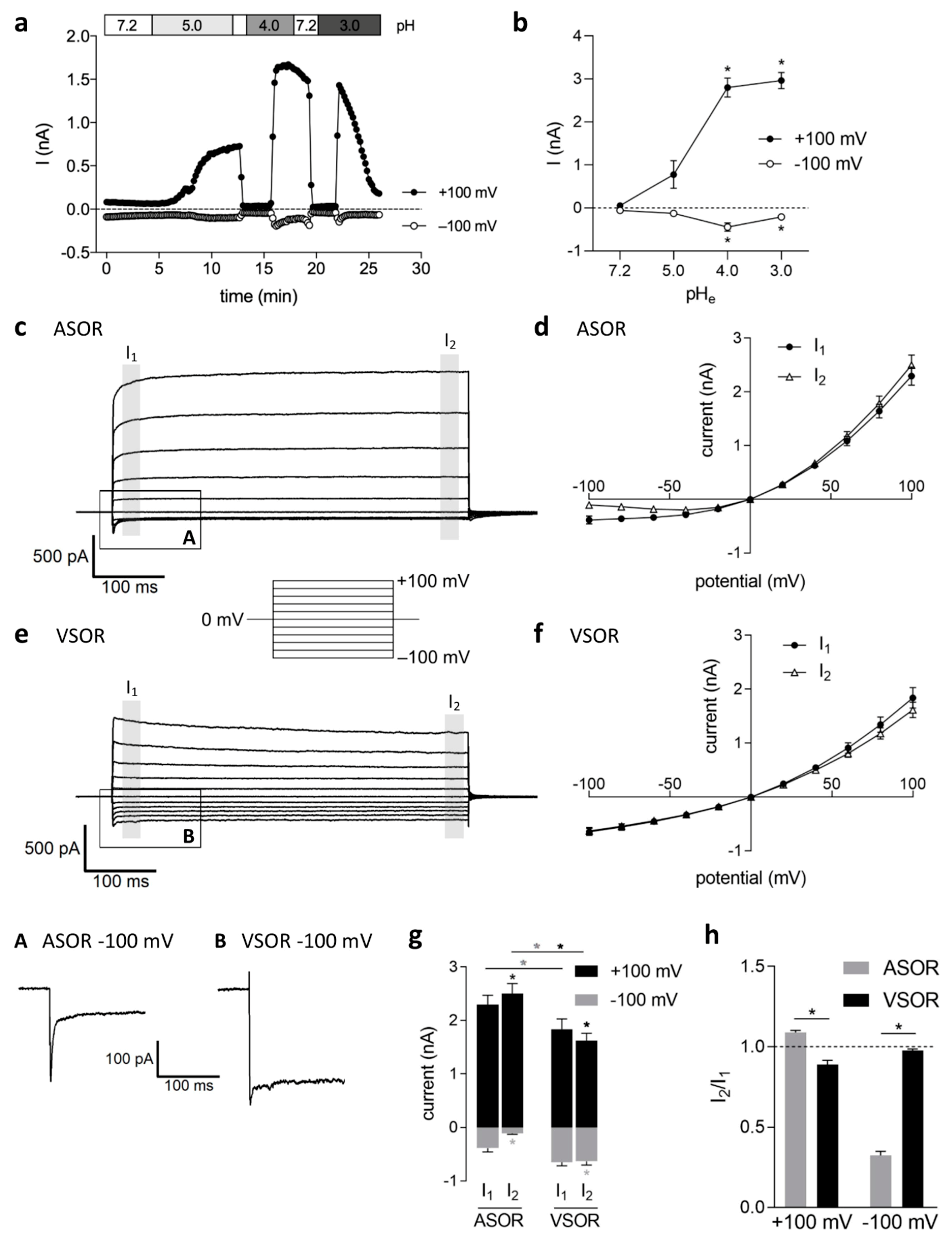
2.1. Current Activation by Extracellular Acidification, Current Amplitudes and Phenotypes
2.2. Relative Ion Permeability of the ASOR Current and Blocker Sensitivity
2.3. ASOR Current Activation Requires Extracellular, but not Intracellular Acidification
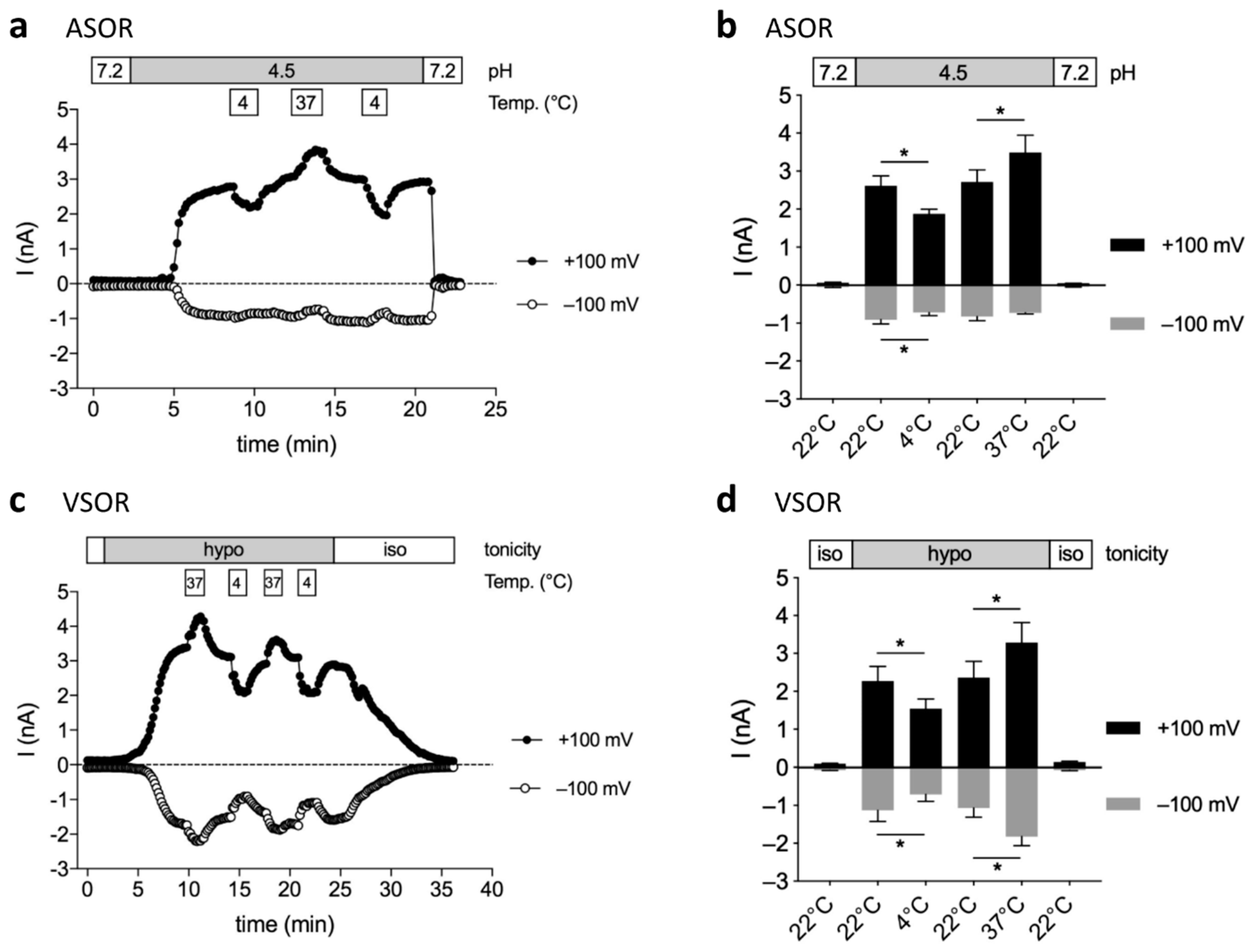
2.4. Temperature-Sensitivity of ASOR and VSOR Currents
2.5. Transient Coactivation of ASOR and VSOR Currents in BV-2 cells
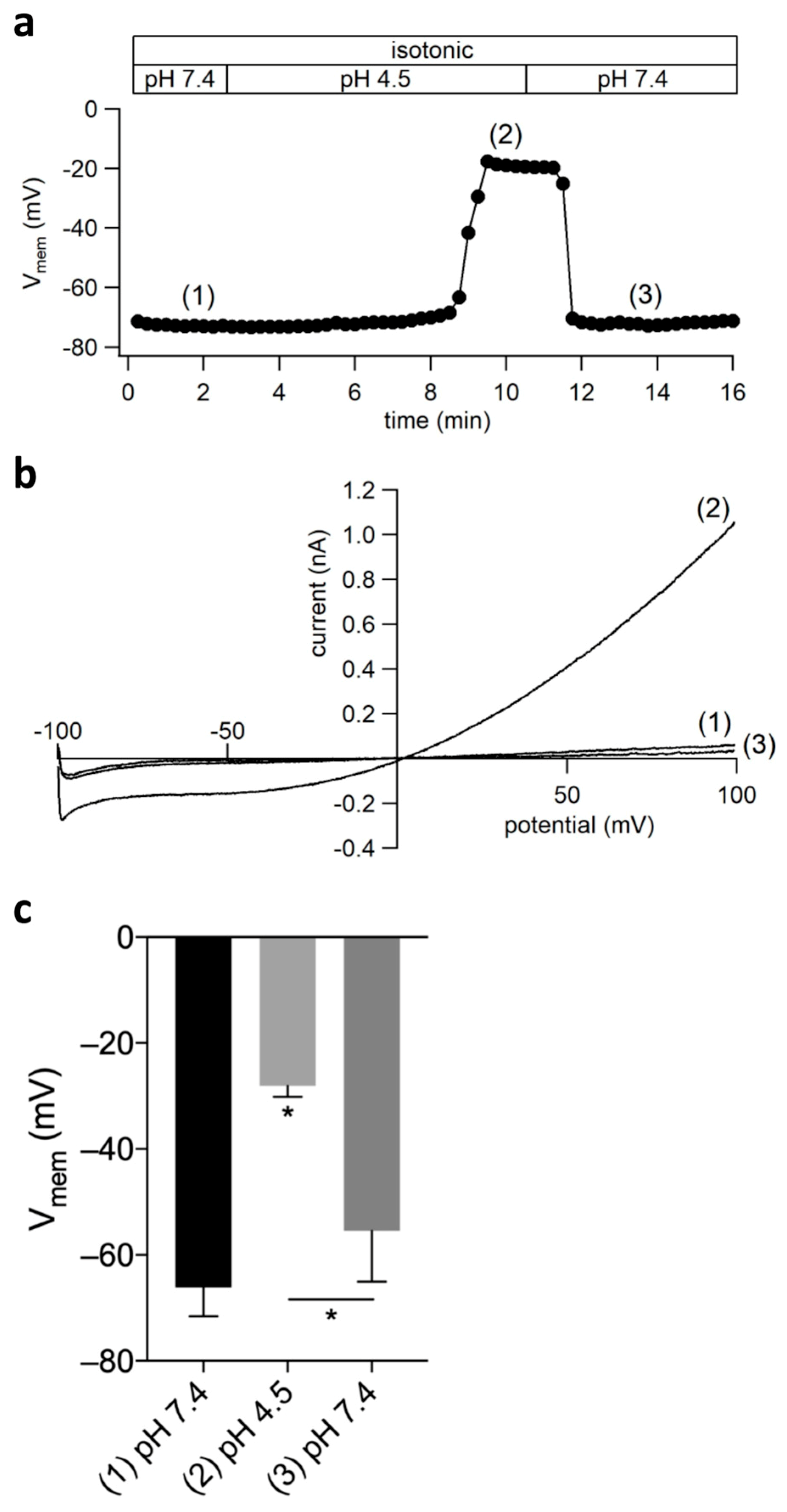
2.6. ASOR Current Activation Depolarizes the Cell Membrane Potential
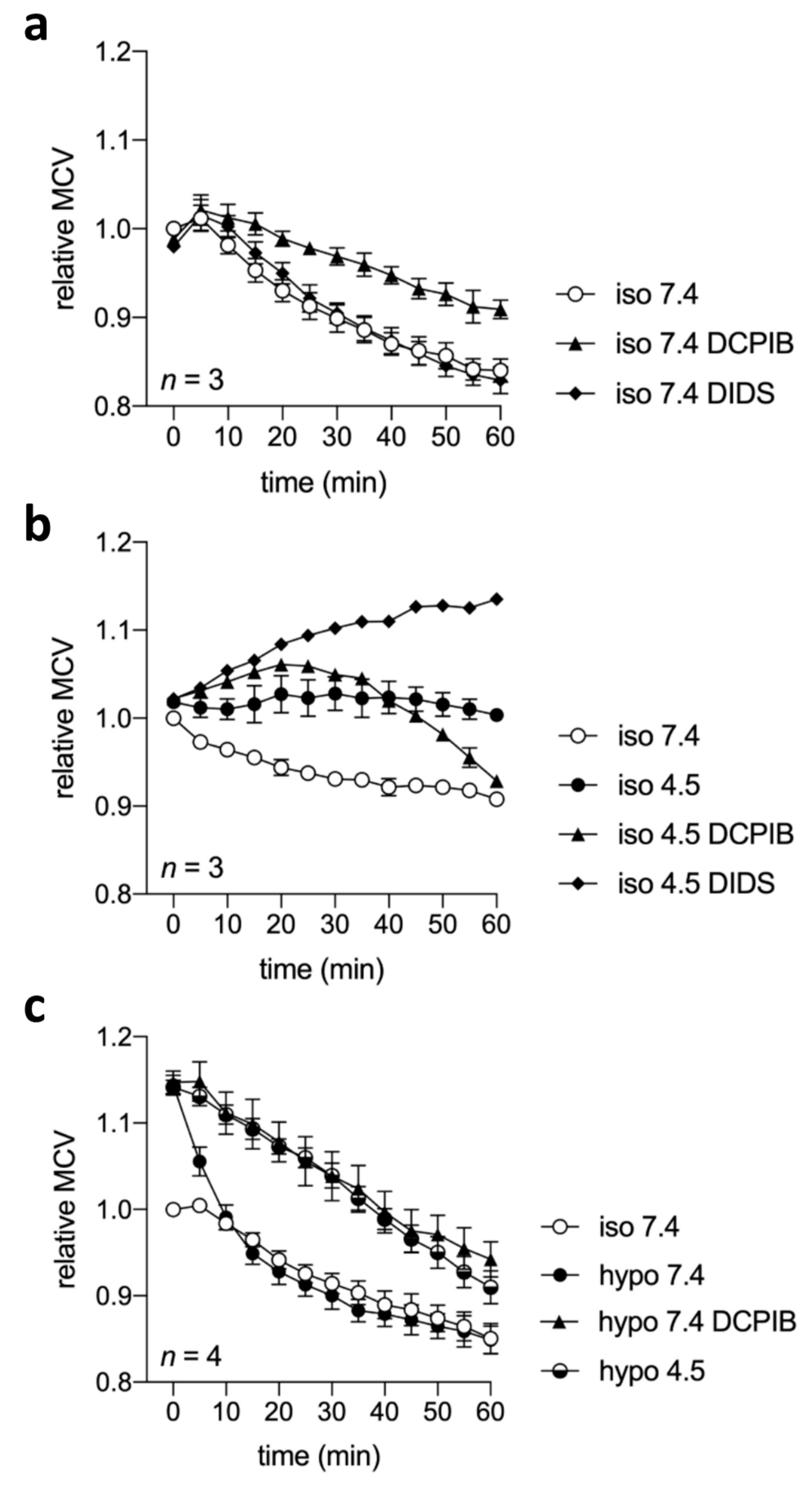
2.7. Cell Volume Regulation under Acidic and/or Isotonic or Hypotonic Conditions
3. Discussion
4. Materials and Methods
4.1. Salts, Chemicals, Drugs
4.2. Cell Culture
4.3. Patch Clamp
4.4. Cell Volume Measurements
4.5. Intracellular pH (pHi) Measurements
4.6. Statistics
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ASOR | Acid-sensitive outwardly rectifying |
| AVD | Apoptotic volume decrease |
| BAPTA-AM | 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis, acetoxymethyl ester |
| BCECF-AM | (2’,7’-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein, acetoxymethyl ester) |
| DCPIB | 4-[(2-butyl-6,7-dichloro-2-cyclopentyl-2,3-dihydro-1-oxo-1H-inden-5-yl)oxy]butanoic acid |
| DIDS | 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid |
| MCV | Mean cell volume |
| NPPB | 5-nitro-2-(3-phenylpropylamino)-benzoic acid |
| pHi | Intracellular pH |
| RVD | Regulatory volume decrease |
| Vmem | Cell membrane potential |
| VSOR | Volume-sensitive outwardly rectifying |
References
- Auzanneau, C.; Thoreau, V.; Kitzis, A.; Becq, F. A Novel Voltage-dependent Chloride Current Activated by Extracellular Acidic pH in Cultured Rat Sertoli Cells. J. Biol. Chem. 2003, 278, 19230–19236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nobles, M.; Higgins, C.F.; Sardini, A. Extracellular acidification elicits a chloride current that shares characteristics with ICl(swell). Am. J. Physiol. Cell Physiol. 2004, 287, C1426–C1435. [Google Scholar] [CrossRef] [PubMed]
- Lambert, S.; Oberwinkler, J. Characterization of a proton-activated, outwardly rectifying anion channel. J. Physiol. 2005, 567, 191–213. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Ehara, T. Acidic extracellular pH-activated outwardly rectifying chloride current in mammalian cardiac myocytes. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H1905–H1914. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Shimizu, T.; Numata, T.; Okada, Y. Role of acid-sensitive outwardly rectifying anion channels in acidosis-induced cell death in human epithelial cells. Pflugers Arch. 2007, 454, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Kucherenko, Y.V.; Morsdorf, D.; Lang, F. Acid-Sensitive Outwardly Rectifying Anion Channels in Human Erythrocytes. J. Membr. Biol. 2009, 230, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kajiya, H.; Okamoto, F.; Ohgi, K.; Nakao, A.; Fukushima, H.; Okabe, K. Characteristics of ClC7 Cl− channels and their inhibition in mutant (G215R) associated with autosomal dominant osteopetrosis type II in native osteoclasts and hClcn7 gene-expressing cells. Pflugers Arch. 2009, 458, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Ohgi, K.; Okamoto, F.; Kajiya, H.; Sakagami, R.; Okabe, K. Antibodies against ClC7 inhibit extracellular acidification-induced Cl− currents and bone resorption activity in mouse osteoclasts. Naunyn Schmiedebergs Arch. Pharmacol. 2011, 383, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Sato-Numata, K.; Numata, T.; Okada, T.; Okada, Y. Acid-sensitive outwardly rectifying (ASOR) anion channels in human epithelial cells are highly sensitive to temperature and independent of ClC-3. Pflugers Arch. 2013, 465, 1535–1543. [Google Scholar] [CrossRef]
- Sato-Numata, K.; Numata, T.; Okada, Y. Temperature sensitivity of acid-sensitive outwardly rectifying (ASOR) anion channels in cortical neurons is involved in hypothermic neuroprotection against acidotoxic necrosis. Channels (Austin) 2014, 8, 278–283. [Google Scholar] [CrossRef] [Green Version]
- Capurro, V.; Gianotti, A.; Caci, E.; Ravazzolo, R.; Galietta, L.J.; Zegarra-Moran, O. Functional analysis of acid-activated Cl− channels: Properties and mechanisms of regulation. Biochim. Biophys. Acta 2015, 1848, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Sato-Numata, K.; Numata, T.; Inoue, R.; Okada, Y. Distinct pharmacological and molecular properties of the acid-sensitive outwardly rectifying (ASOR) anion channel from those of the volume-sensitive outwardly rectifying (VSOR) anion channel. Pflugers Arch. 2016, 468, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Sato-Numata, K.; Numata, T.; Inoue, R.; Sabirov, R.Z.; Okada, Y. Distinct contributions of LRRC8A and its paralogs to the VSOR anion channel from those of the ASOR anion channel. Channels (Austin) 2017, 11, 167–172. [Google Scholar] [CrossRef]
- Valinsky, W.C.; Touyz, R.M.; Shrier, A. Characterization of constitutive and acid-induced outwardly rectifying chloride currents in immortalized mouse distal tubular cells. Biochim. Biophys. Acta 2017, 1861, 2007–2019. [Google Scholar] [CrossRef]
- Lang, F.; Busch, G.L.; Ritter, M.; Volkl, H.; Waldegger, S.; Gulbins, E.; Haussinger, D. Functional Significance of Cell Volume Regulatory Mechanisms. Physiol. Rev. 1998, 78, 247–306. [Google Scholar] [CrossRef]
- Okada, Y.; Maeno, E.; Shimizu, T.; Dezaki, K.; Wang, J.; Morishima, S. Receptor-mediated control of regulatory volume decrease (RVD) and apoptotic volume decrease (AVD). J. Physiol. 2001, 532, 3–16. [Google Scholar] [CrossRef]
- Jentsch, T.J.; Stein, V.; Weinreich, F.; Zdebik, A.A. Molecular Structure and Physiological Function of Chloride Channels. Physiol. Rev. 2002, 82, 503–568. [Google Scholar] [CrossRef] [PubMed]
- Jakab, M.; Ritter, M. Cell Volume Regulatory Ion Transport in the Regulation of Cell Migration. Contrib. Nephrol. 2006, 152, 161–180. [Google Scholar] [CrossRef]
- Stutzin, A.; Hoffmann, E.K. Swelling-activated ion channels: Functional regulation in cell-swelling, proliferation and apoptosis. Acta Physiol. (Oxf.) 2006, 187, 27–42. [Google Scholar] [CrossRef]
- Schwab, A.; Fabian, A.; Hanley, P.J.; Stock, C. Role of Ion Channels and Transporters in Cell Migration. Physiol. Rev. 2012, 92, 1865–1913. [Google Scholar] [CrossRef]
- Harl, B.; Schmolzer, J.; Jakab, M.; Ritter, M.; Kerschbaum, H.H. Chloride Channel Blockers Suppress Formation of Engulfment Pseudopodia in Microglial Cells. Cell. Physiol. Biochem. 2013, 31, 319–337. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, E.K.; Sorensen, B.H.; Sauter, D.P.; Lambert, I.H. Role of volume-regulated and calcium-activated anion channels in cell volume homeostasis, cancer and drug resistance. Channels (Austin) 2015, 9, 380–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedersen, S.F.; Okada, Y.; Nilius, B. Biophysics and Physiology of the Volume-Regulated Anion Channel (VRAC)/Volume-Sensitive Outwardly Rectifying Anion Channel (VSOR). Pflugers Arch. 2016, 468, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Kettenmann, H.; Hanisch, U.K.; Noda, M.; Verkhratsky, A. Physiology of Microglia. Physiol. Rev. 2011, 91, 461–553. [Google Scholar] [CrossRef] [PubMed]
- Furtner, T.; Zierler, S.; Kerschbaum, H.H. Blockade of chloride channels suppresses engulfment of microspheres in the microglial cell line, BV-2. Brain Res. 2007, 1184, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Komm, B.; Beyreis, M.; Kittl, M.; Jakab, M.; Ritter, M.; Kerschbaum, H.H. Glycine modulates membrane potential, cell volume, and phagocytosis in murine microglia. Amino Acids 2014, 46, 1907–1917. [Google Scholar] [CrossRef]
- Kittl, M.; Dobias, H.; Beyreis, M.; Kiesslich, T.; Mayr, C.; Gaisberger, M.; Ritter, M.; Kerschbaum, H.H.; Jakab, M. Glycine Induces Migration of Microglial BV-2 Cells via SNAT-Mediated Cell Swelling. Cell. Physiol. Biochem. 2018, 50, 1460–1473. [Google Scholar] [CrossRef]
- Kittl, M.; Jakab, M.; Steininger, T.S.; Ritter, M.; Kerschbaum, H.H. A Swelling-Activated Chloride Current in Microglial Cells is Suppressed by Epac and Facilitated by PKA-Impact on Phagocytosis. Cell. Physiol. Biochem. 2019, 52, 951–969. [Google Scholar] [CrossRef] [PubMed]
- Warskulat, U.; Zhang, F.; Haussinger, D. Modulation of phagocytosis by anisoosmolarity and betaine in rat liver macrophages (Kupffer cells) and RAW 264.7 mouse macrophages. FEBS Lett. 1996, 391, 287–292. [Google Scholar] [CrossRef] [Green Version]
- Rehncrona, S. Brain acidosis. Ann. Emerg. Med. 1985, 14, 770–776. [Google Scholar] [CrossRef]
- Kempski, O.; Staub, F.; Jansen, M.; Schodel, F.; Baethmann, A. Glial swelling during extracellular acidosis in vitro. Stroke 1988, 19, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Staub, F.; Mackert, B.; Kempski, O.; Peters, J.; Baethmann, A. Swelling and death of neuronal cells by lactic acid. J. Neurol. Sci. 1993, 119, 79–84. [Google Scholar] [CrossRef]
- Staub, F.; Winkler, A.; Haberstok, J.; Plesnila, N.; Peters, J.; Chang, R.C.; Kempski, O.; Baethmann, A. Swelling, intracellular acidosis, and damage of glial cells. Acta Neurochir. Suppl. 1996, 66, 56–62. [Google Scholar] [PubMed]
- Kurita, T.; Yamamura, H.; Suzuki, Y.; Giles, W.R.; Imaizumi, Y. The ClC-7 Chloride Channel Is Downregulated by Hypoosmotic Stress in Human Chondrocytes. Mol. Pharmacol. 2015, 88, 113–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuda, J.J.; Filali, M.S.; Collins, M.M.; Volk, K.A.; Lamb, F.S. The ClC-3 Cl−/H+ Antiporter Becomes Uncoupled at Low Extracellular pH. J. Biol. Chem. 2010, 285, 2569–2579. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.W.; Hu, Z.L.; Ni, M.; Fang, P.; Zhang, P.W.; Shu, Q.; Fan, H.; Zhou, H.Y.; Ni, L.; Zhu, L.Q.; et al. Acid-Sensing Ion Channels Promote the Inflammation and Migration of Cultured Rat Microglia. Glia 2015, 63, 483–496. [Google Scholar] [CrossRef]
- Siddiqui, T.; Lively, S.; Ferreira, R.; Wong, R.; Schlichter, L.C. Expression and Contributions of TRPM7 and KCa2.3/SK3 Channels to the Increased Migration and Invasion of Microglia in Anti-Inflammatory Activation States. PLoS ONE 2014, 9, e106087. [Google Scholar] [CrossRef]
- Ackerman, M.J.; Wickman, K.D.; Clapham, D.E. Hypotonicity Activates a Native Chloride Current in Xenopus Oocytes. J. Gen. Physiol. 1994, 103, 153–179. [Google Scholar] [CrossRef] [PubMed]
- Jackson, P.S.; Strange, K. Characterization of the Voltage-dependent Properties of a Volume-sensitive Anion Conductance. J. Gen. Physiol. 1995, 105, 661–676. [Google Scholar] [CrossRef]
- Voets, T.; Droogmans, G.; Nilius, B. Modulation of Voltage-dependent Properties of a Swelling-activated Cl− Current. J. Gen. Physiol. 1997, 110, 313–325. [Google Scholar] [CrossRef]
- Gerard, V.; Rouzaire-Dubois, B.; Dilda, P.; Dubois, J.M. Alterations of Ionic Membrane Permeabilities in Multidrug-Resistant Neuroblastoma x Glioma Hybrid Cells. J. Exp. Biol. 1998, 201, 21–31. [Google Scholar] [PubMed]
- Nilius, B.; Prenen, J.; Droogmans, G. Modulation of volume-regulated anion channels by extra- and intracellular pH. Pflugers Arch. 1998, 436, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Sabirov, R.Z.; Prenen, J.; Droogmans, G.; Nilius, B. Extra- and intracellular proton-binding sites of volume-regulated anion channels. J. Membr. Biol. 2000, 177, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Voss, F.K.; Ullrich, F.; Munch, J.; Lazarow, K.; Lutter, D.; Mah, N.; Andrade-Navarro, M.A.; von Kries, J.P.; Stauber, T.; Jentsch, T.J. Identification of LRRC8 heteromers as an essential component of the volume-regulated anion channel VRAC. Science 2014, 344, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Dubin, A.E.; Mathur, J.; Tu, B.; Reddy, K.; Miraglia, L.J.; Reinhardt, J.; Orth, A.P.; Patapoutian, A. SWELL1, a plasma membrane protein, is an essential component of volume-regulated anion channel. Cell 2014, 157, 447–458. [Google Scholar] [CrossRef]
- Caputo, A.; Caci, E.; Ferrera, L.; Pedemonte, N.; Barsanti, C.; Sondo, E.; Pfeffer, U.; Ravazzolo, R.; Zegarra-Moran, O.; Galietta, L. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science 2008, 322, 590–594. [Google Scholar] [CrossRef]
- Wang, L.; Ma, W.; Zhu, L.; Ye, D.; Li, Y.; Liu, S.; Li, H.; Zuo, W.; Li, B.; Ye, W.; et al. ClC-3 is a candidate of the channel proteins mediating acid-activated chloride currents in nasopharyngeal carcinoma cells. Am. J. Physiol. Cell Physiol. 2012, 303, C14–C23. [Google Scholar] [CrossRef] [Green Version]
- Pillay, C.S.; Elliott, E.; Dennison, C. Endolysosomal proteolysis and its regulation. Biochem. J. 2002, 363, 417–429. [Google Scholar] [CrossRef]
- Yang, J.; Chen, J.; Del Carmen Vitery, M.; Osei-Owusu, J.; Chu, J.; Yu, H.; Sun, S.; Qiu, Z. PAC, an evolutionarily conserved membrane protein, is a proton-activated chloride channel. Science 2019, 364, 395–399. [Google Scholar] [CrossRef]
- Ullrich, F.; Blin, S.; Lazarow, K.; Daubitz, T.; Kries, J.-P.v.; Jentsch, T.J. Identification of TMEM206 proteins as pore of ASOR acid-sensitive chloride channels. bioRxiv 2019. [Google Scholar] [CrossRef]
- Morihata, H.; Nakamura, F.; Tsutada, T.; Kuno, M. Potentiation of a Voltage-Gated Proton Current in Acidosis-Induced Swelling of Rat Microglia. J. Neurosci. 2000, 20, 7220–7227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkinson, B.L.; Landreth, G.E. The microglial NADPH oxidase complex as a source of oxidative stress in Alzheimer’s disease. J. Neuroinflammation 2006, 3, 30. [Google Scholar] [CrossRef] [PubMed]
- Jakab, M.; Grundbichler, M.; Benicky, J.; Ravasio, A.; Chwatal, S.; Schmidt, S.; Strbak, V.; Furst, J.; Paulmichl, M.; Ritter, M. Glucose Induces Anion Conductance and Cytosol-To-Membrane Transposition of ICln in INS-1E Rat Insulinoma Cells. Cell. Physiol. Biochem. 2006, 18, 21–34. [Google Scholar] [CrossRef] [PubMed]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kittl, M.; Helm, K.; Beyreis, M.; Mayr, C.; Gaisberger, M.; Winklmayr, M.; Ritter, M.; Jakab, M. Acid- and Volume-Sensitive Chloride Currents in Microglial Cells. Int. J. Mol. Sci. 2019, 20, 3475. https://doi.org/10.3390/ijms20143475
Kittl M, Helm K, Beyreis M, Mayr C, Gaisberger M, Winklmayr M, Ritter M, Jakab M. Acid- and Volume-Sensitive Chloride Currents in Microglial Cells. International Journal of Molecular Sciences. 2019; 20(14):3475. https://doi.org/10.3390/ijms20143475
Chicago/Turabian StyleKittl, Michael, Katharina Helm, Marlena Beyreis, Christian Mayr, Martin Gaisberger, Martina Winklmayr, Markus Ritter, and Martin Jakab. 2019. "Acid- and Volume-Sensitive Chloride Currents in Microglial Cells" International Journal of Molecular Sciences 20, no. 14: 3475. https://doi.org/10.3390/ijms20143475
APA StyleKittl, M., Helm, K., Beyreis, M., Mayr, C., Gaisberger, M., Winklmayr, M., Ritter, M., & Jakab, M. (2019). Acid- and Volume-Sensitive Chloride Currents in Microglial Cells. International Journal of Molecular Sciences, 20(14), 3475. https://doi.org/10.3390/ijms20143475





