Repression of the Glucocorticoid Receptor Increases Hypoxic-Ischemic Brain Injury in the Male Neonatal Rat
Abstract
1. Introduction
2. Results
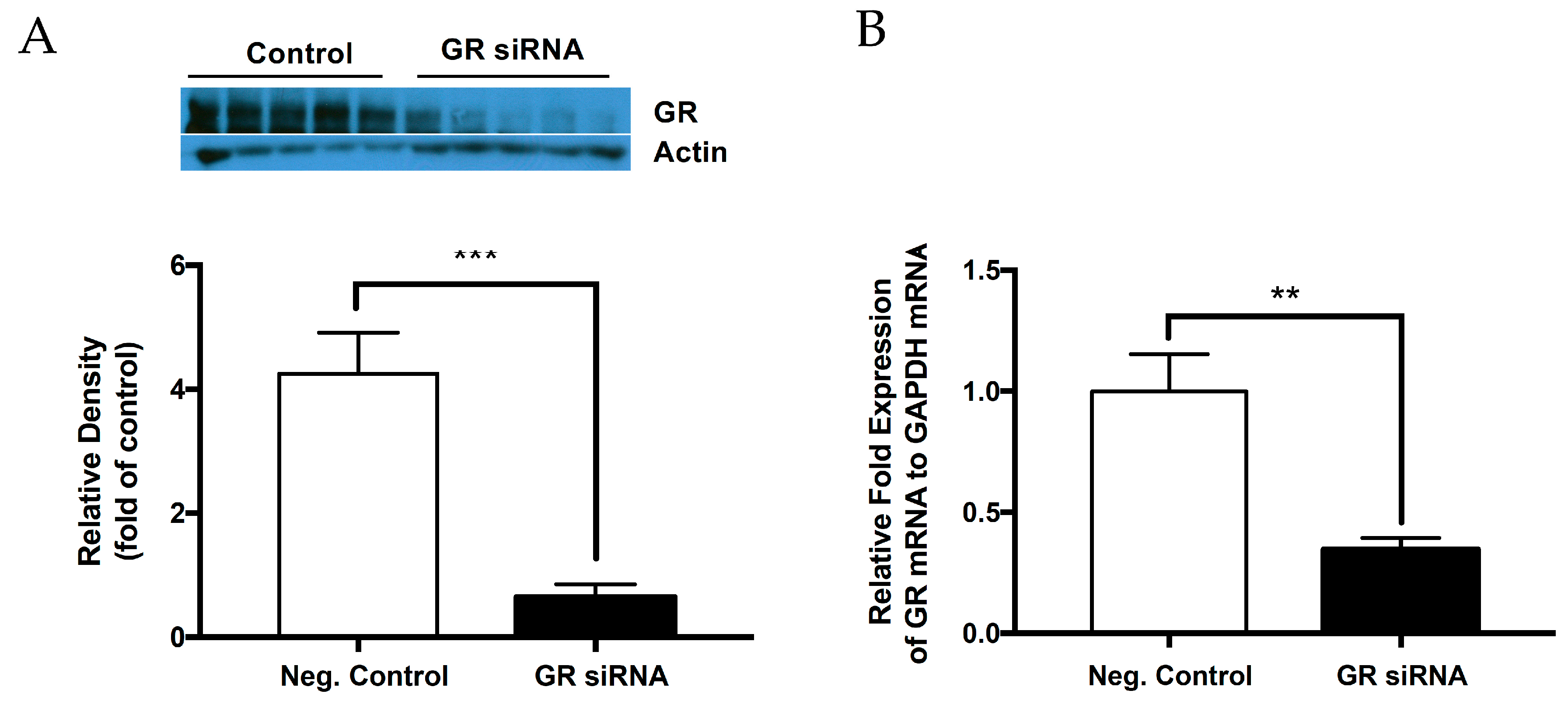
2.1. Knockdown of Glucocorticoid Receptor in the Neonatal Brain
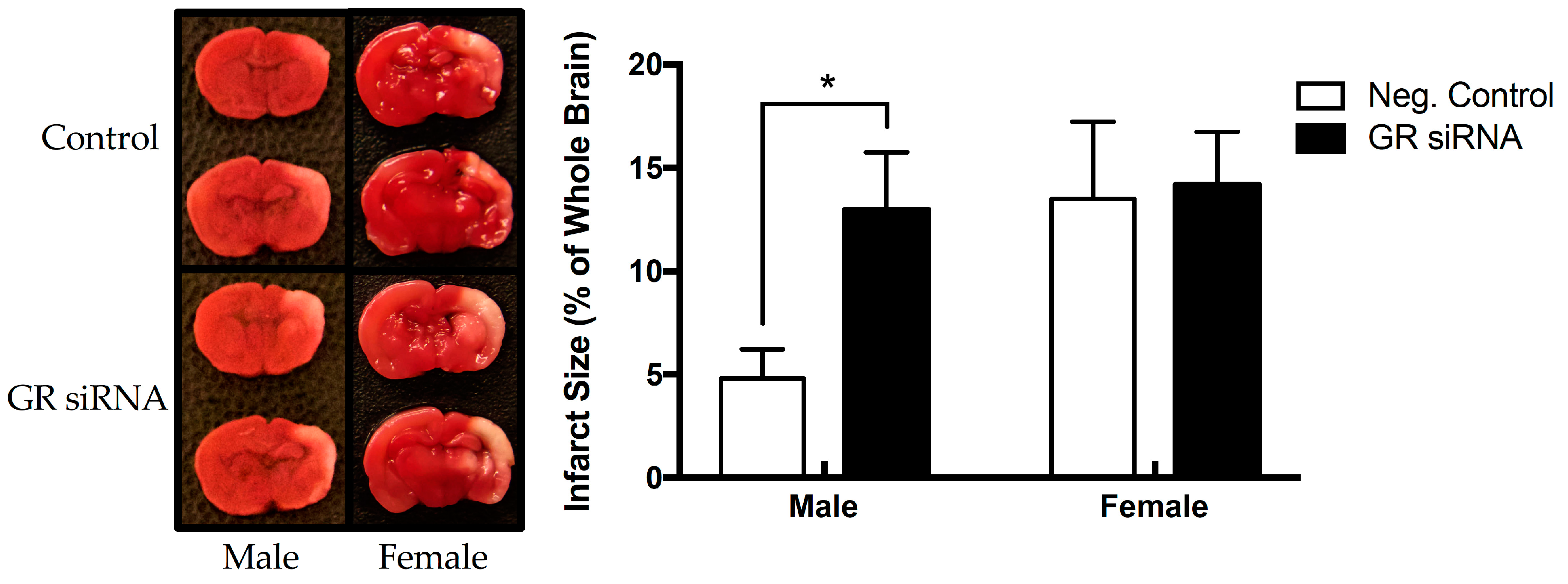
2.2. Effect of GR Repression on HI-Induced Infarction Size in the Neonatal Brain
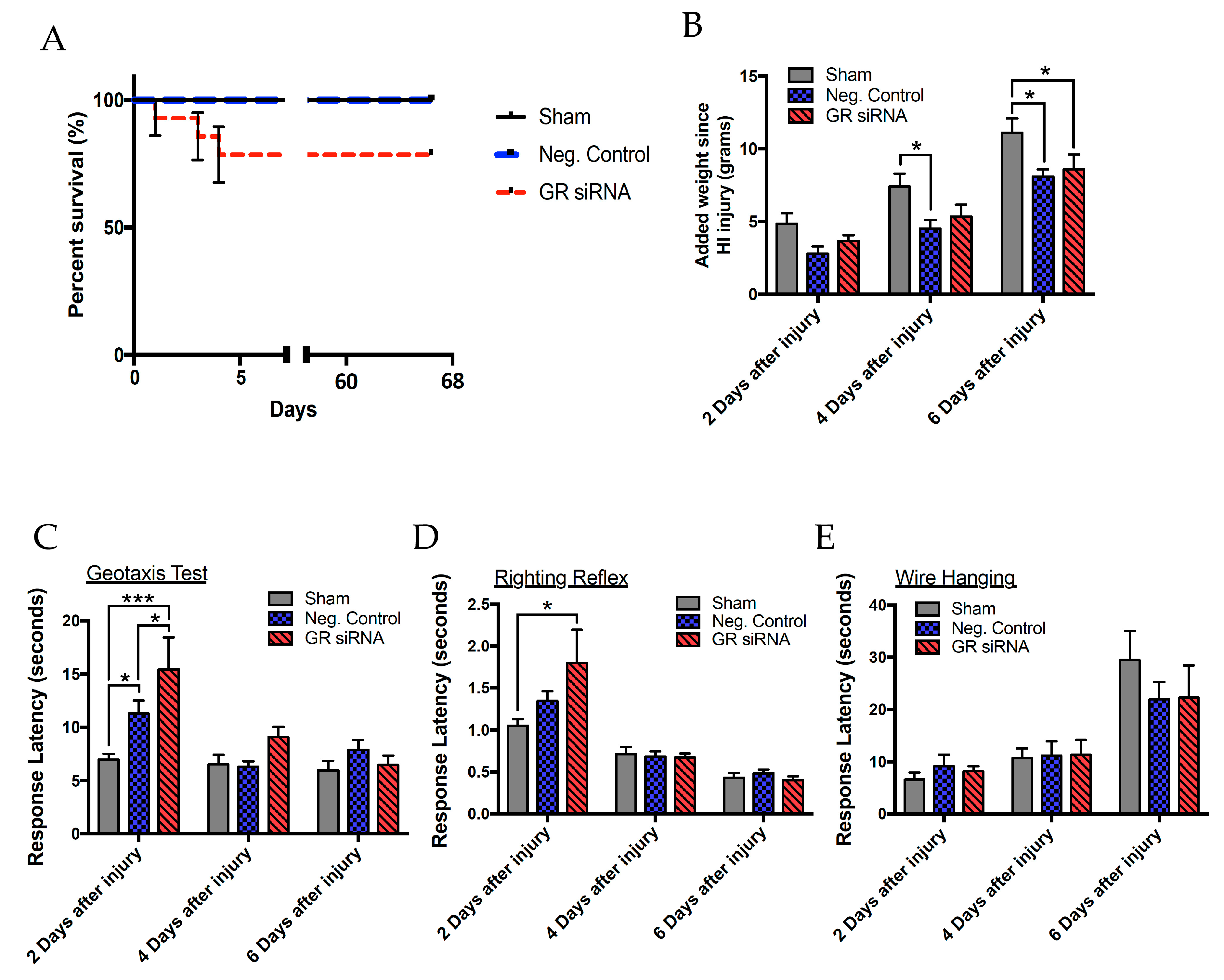
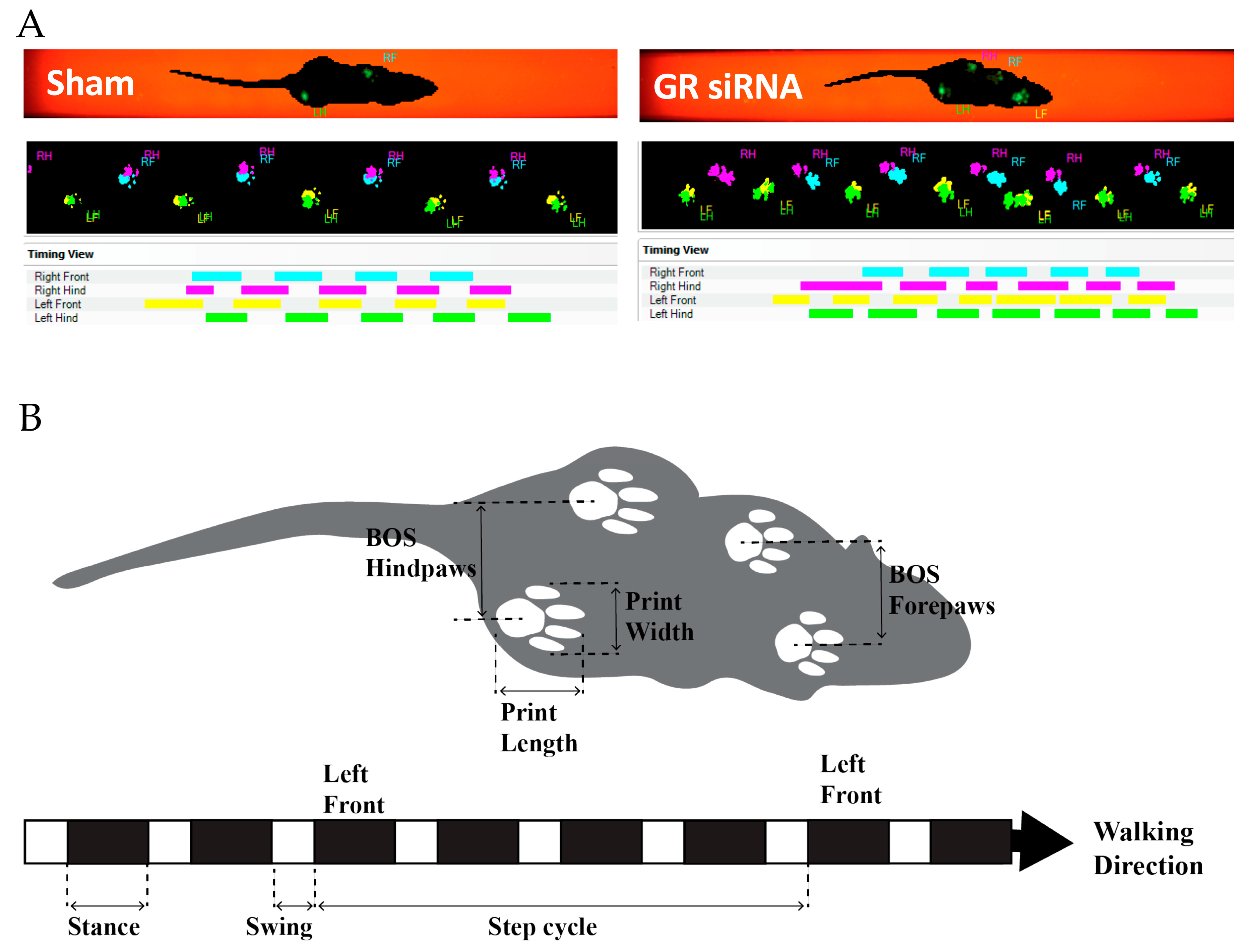
2.3. Effect of GR Knockdown on HI-Induced Changes in Short- and Long-Term Neurobehavioral Function in Male Pups
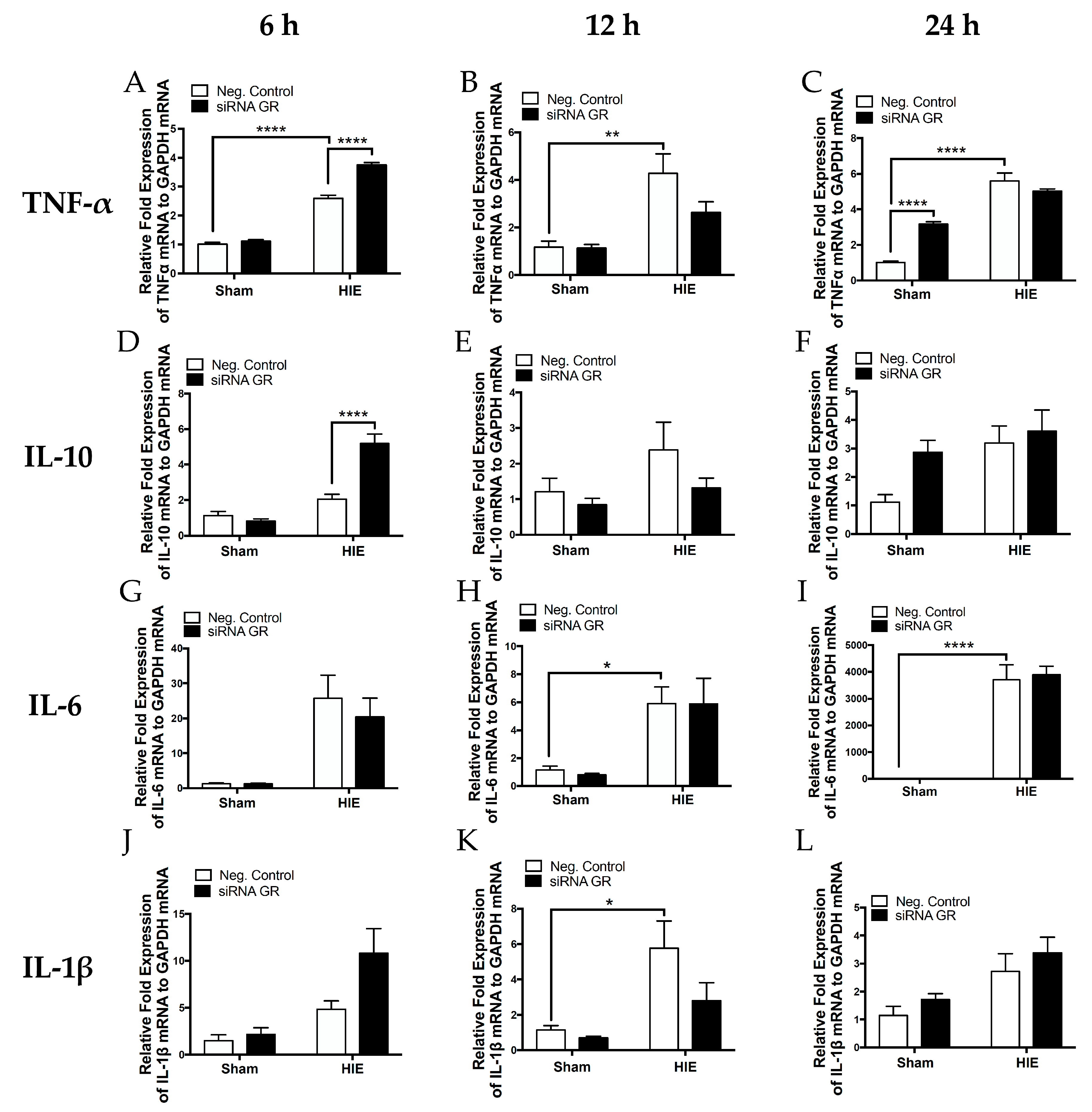
2.4. Effect of GR Knockdown on HI-Induced Changes in Inflammatory Cytokine Production in the Neonatal Brain
3. Discussion
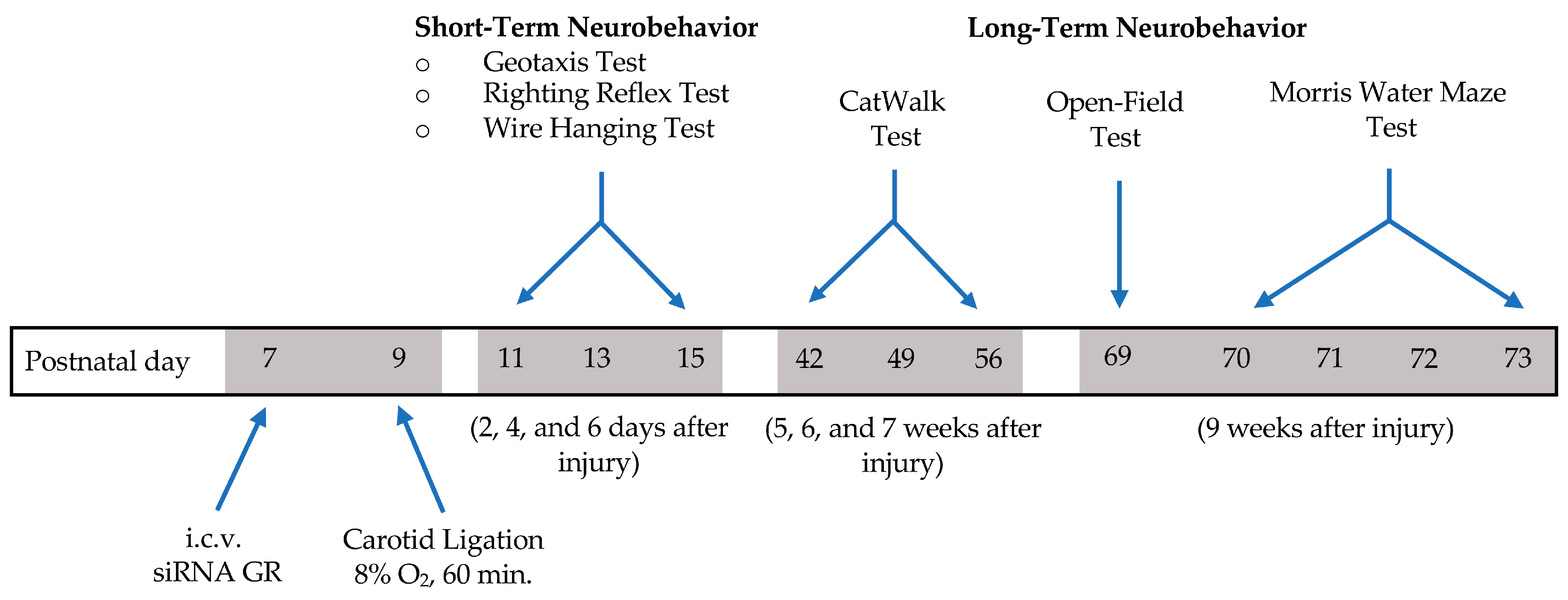
4. Materials and Methods
4.1. Experimental Animals
4.2. Intracerebroventricular (ICV) Injection
4.3. Neonatal Hypoxic–Ischemic Encephalopathy (HIE) Rat Model
4.4. Measurement of Infarction Size
4.5. Neurobehavioral Tests
4.6. Real time RT-qPCR
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Abraham, I.M.; Harkany, T.; Horvath, K.M.; Luiten, P.G. Action of glucocorticoids on survival of nerve cells: Promoting neurodegeneration or neuroprotection? J. Neuroendocrinol. 2001, 13, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Rhodes, P.G.; Bhatt, A.J. Dexamethasone pre-treatment protects brain against hypoxic-ischemic injury partially through up-regulation of vascular endothelial growth factor A in neonatal rats. Neuroscience 2011, 179, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Lear, C.A.; Koome, M.E.; Davidson, J.O.; Drury, P.P.; Quaedackers, J.S.; Galinsky, R.; Gunn, A.J.; Bennet, L. The effects of dexamethasone on post-asphyxial cerebral oxygenation in the preterm fetal sheep. J. Physiol. 2014, 592, 5493–5505. [Google Scholar] [CrossRef] [PubMed]
- Black, R.E.; Cousens, S.; Johnson, H.L.; Lawn, J.E.; Rudan, I.; Bassani, D.G.; Jha, P.; Campbell, H.; Walker, C.F.; Cibulskis, R.; et al. Global, regional, and national causes of child mortality in 2008: A systematic analysis. Lancet 2010, 375, 1969–1987. [Google Scholar] [CrossRef]
- Ahearne, C.E.; Boylan, G.B.; Murray, D.M. Short and long term prognosis in perinatal asphyxia: An update. World J. Clin. Pediatr. 2016, 5, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Paquette, A.G.; Lester, B.M.; Lesseur, C.; Armstrong, D.A.; Guerin, D.J.; Appleton, A.A.; Marsit, C.J. Placental epigenetic patterning of glucocorticoid response genes is associated with infant neurodevelopment. Epigenomics 2015, 7, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Concepcion, K.R.; Zhang, L. Corticosteroids and perinatal hypoxic-ischemic brain injury. Drug Discov. Today 2018, 23, 1718–1732. [Google Scholar] [CrossRef] [PubMed]
- Bruder, E.D.; Kamer, K.J.; Guenther, M.A.; Raff, H. Adrenocorticotropic hormone and corticosterone responses to acute hypoxia in the neonatal rat: Effects of body temperature maintenance. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R708–R715. [Google Scholar] [CrossRef][Green Version]
- Chintamaneni, K.; Bruder, E.D.; Raff, H. Programming of the hypothalamic-pituitary-adrenal axis by neonatal intermittent hypoxia: Effects on adult male ACTH and corticosterone responses are stress specific. Endocrinology 2014, 155, 1763–1770. [Google Scholar] [CrossRef]
- Gonzalez-Rodriguez, P.J.; Xiong, F.; Li, Y.; Zhou, J.; Zhang, L. Fetal hypoxia increases vulnerability of hypoxic-ischemic brain injury in neonatal rats: Role of glucocorticoid receptors. Neurobiol. Dis. 2014, 65, 172–179. [Google Scholar] [CrossRef]
- Ma, Q.; Dasgupta, C.; Li, Y.; Bajwa, N.M.; Xiong, F.; Harding, B.; Hartman, R.; Zhang, L. Inhibition of microRNA-210 provides neuroprotection in hypoxic-ischemic brain injury in neonatal rats. Neurobiol. Dis. 2016, 89, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gonzalez, P.; Zhang, L. Fetal stress and programming of hypoxic/ischemic-sensitive phenotype in the neonatal brain: Mechanisms and possible interventions. Prog. Neurobiol. 2012, 98, 145–165. [Google Scholar] [CrossRef] [PubMed]
- Harding, B.; Conception, K.; Li, Y.; Zhang, L. Glucocorticoids protect neonatal rat brain in model of hypoxic-ischemic encephalopathy (HIE). Int. J. Mol. Sci. 2017, 18, 17. [Google Scholar] [CrossRef] [PubMed]
- Charriaut-Marlangue, C.; Besson, V.C.; Baud, O. Sexually dimorphic outcomes after neonatal stroke and hypoxia-ischemia. Int. J. Mol. Sci. 2018, 19, 61. [Google Scholar] [CrossRef]
- Netto, C.A.; Sanches, E.; Odorcyk, F.K.; Duran-Carabali, L.E.; Weis, S.N. Sex-dependent consequences of neonatal brain hypoxia-ischemia in the rat. J. Neurosci. Res. 2017, 95, 409–421. [Google Scholar] [CrossRef]
- Smith, A.L.; Garbus, H.; Rosenkrantz, T.S.; Fitch, R.H. Sex differences in behavioral outcomes following temperature modulation during induced neonatal hypoxic ischemic injury in rats. Brain Sci. 2015, 5, 220–240. [Google Scholar] [CrossRef]
- Rodriguez-Fanjul, J.; Duran Fernandez-Feijoo, C.; Lopez-Abad, M.; Lopez Ramos, M.G.; Balada Caballe, R.; Alcantara-Horillo, S.; Camprubi Camprubi, M. Neuroprotection with hypothermia and allopurinol in an animal model of hypoxic-ischemic injury: Is it a gender question? PLoS ONE 2017, 12, e0184643. [Google Scholar] [CrossRef]
- Mirza, M.A.; Ritzel, R.; Xu, Y.; McCullough, L.D.; Liu, F. Sexually dimorphic outcomes and inflammatory responses in hypoxic-ischemic encephalopathy. J. Neuroinflamm. 2015, 12, 32. [Google Scholar] [CrossRef]
- Adams, J.; Buelke-Sam, J.; Kimmel, C.A.; Nelson, C.J.; Reiter, L.W.; Sobotka, T.J.; Tilson, H.A.; Nelson, B.K. Collaborative behavioral teratology study: Protocol design and testing procedures. Neurobehav. Toxicol. Teratol. 1985, 7, 579–586. [Google Scholar]
- Rice, J.E., 3rd; Vannucci, R.C.; Brierley, J.B. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann. Neurol. 1981, 9, 131–141. [Google Scholar] [CrossRef]
- Towfighi, J.; Yager, J.Y.; Housman, C.; Vannucci, R.C. Neuropathology of remote hypoxic-ischemic damage in the immature rat. Acta Neuropathol. 1991, 81, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.D.; Pierce, L.; Ciardiello, A.; Hutton, A.; Paskewitz, S.; Aronowitz, E.; Voss, H.U.; Moore, H.; Vannucci, S.J. Therapeutic hypothermia and hypoxia-ischemia in the term-equivalent neonatal rat: Characterization of a translational preclinical model. Pediatr. Res. 2015, 78, 264–271. [Google Scholar] [CrossRef] [PubMed]
- De Kloet, E.R.; Claessens, S.E.; Kentrop, J. Context modulates outcome of perinatal glucocorticoid action in the brain. Front. Endocrinol. (Lausanne) 2014, 5, 100. [Google Scholar] [CrossRef] [PubMed]
- Dalm, S.; Karssen, A.M.; Meijer, O.C.; Belanoff, J.K.; de Kloet, E.R. Resetting the stress system with a mifepristone challenge. Cell. Mol. Neurobiol. 2019, 39, 503–522. [Google Scholar] [CrossRef] [PubMed]
- Sarvilinna, N.; Unkila-Kallio, L.; Harkki, P.; Tiitinen, A.; Heikinheimo, O. Selective progesterone receptor modulators: New possibilities for gynecologic hormone therapy. Duodecim 2017, 133, 27–33. [Google Scholar] [PubMed]
- Hagberg, H.; Mallard, C.; Ferriero, D.M.; Vannucci, S.J.; Levison, S.W.; Vexler, Z.S.; Gressens, P. The role of inflammation in perinatal brain injury. Nat. Rev. Neurol. 2015, 11, 192–208. [Google Scholar] [CrossRef] [PubMed]
- Felszeghy, K.; Banisadr, G.; Rostene, W.; Nyakas, C.; Haour, F. Dexamethasone downregulates chemokine receptor CXCR4 and exerts neuroprotection against hypoxia/ischemia-induced brain injury in neonatal rats. Neuroimmunomodulation 2004, 11, 404–413. [Google Scholar] [CrossRef]
- Bennet, L.; Davidson, J.O.; Koome, M.; Gunn, A.J. Glucocorticoids and preterm hypoxic-ischemic brain injury: The good and the bad. J. Pregnancy 2012, 2012, 751694. [Google Scholar] [CrossRef]
- Roberts, D.; Dalziel, S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst. Rev. 2007, 4, CD004454. [Google Scholar]
- Rumajogee, P.; Bregman, T.; Miller, S.P.; Yager, J.Y.; Fehlings, M.G. Rodent hypoxia-ischemia models for cerebral palsy research: A systematic review. Front. Neurol. 2016, 7, 57. [Google Scholar] [CrossRef]
- Ten, V.S.; Bradley-Moore, M.; Gingrich, J.A.; Stark, R.I.; Pinsky, D.J. Brain injury and neurofunctional deficit in neonatal mice with hypoxic-ischemic encephalopathy. Behav. Brain Res. 2003, 145, 209–219. [Google Scholar] [CrossRef]
- Weitzdoerfer, R.; Pollak, A.; Lubec, B. Perinatal asphyxia in the rat has lifelong effects on morphology, cognitive functions, and behavior. Semin. Perinatol. 2004, 28, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Jansen, E.M.; Low, W.C. Quantitative analysis of contralateral hemisphere hypertrophy and sensorimotor performance in adult rats following unilateral neonatal ischemic-hypoxic brain injury. Brain Res. 1996, 708, 93–99. [Google Scholar] [CrossRef]
- Bona, E.; Johansson, B.B.; Hagberg, H. Sensorimotor function and neuropathology five to six weeks after hypoxia-ischemia in seven-day-old rats. Pediatr. Res. 1997, 42, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, L.; Ma, Q.; Concepcion, K.R.; Song, M.A.; Zhang, P.; Fu, Y.; Xiao, D.; Zhang, L. Repression of the glucocorticoid receptor aggravates acute ischemic brain injuries in adult mice. Int. J. Mol. Sci. 2018, 19, 2428. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.; Yeh, C.M.; Yu, T.H.; Chang, K.H.; Huang, C.C.; Hsu, K.S. Neonatal dexamethasone treatment exacerbates hypoxia/ischemia-induced white matter injury. Mol. Neurobiol. 2017, 54, 7083–7095. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Mishima, K.; Aoo, N.; Liu, A.X.; Egashira, N.; Iwasaki, K.; Fujiwara, M.; Ikenoue, T. Dexamethasone prevents long-lasting learning impairment following a combination of lipopolysaccharide and hypoxia-ischemia in neonatal rats. Am. J. Obstet. Gynecol. 2005, 192, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Mishima, K.; Yoshikawa, T.; Iwasaki, K.; Fujiwara, M.; Xia, Y.X.; Ikenoue, T. Dexamethasone prevents long-lasting learning impairment following neonatal hypoxic-ischemic brain insult in rats. Behav. Brain Res. 2002, 136, 161–170. [Google Scholar] [CrossRef]
- Ng, S.S.; Hui-Chan, C.W. The timed up & go test: Its reliability and association with lower-limb impairments and locomotor capacities in people with chronic stroke. Arch. Phys. Med. Rehabil. 2005, 86, 1641–1647. [Google Scholar] [PubMed]
- Liu, Y.; Ao, L.J.; Lu, G.; Leong, E.; Liu, Q.; Wang, X.H.; Zhu, X.L.; Sun, T.F.; Fei, Z.; Jiu, T.; et al. Quantitative gait analysis of long-term locomotion deficits in classical unilateral striatal intracerebral hemorrhage rat model. Behav. Brain Res. 2013, 257, 166–177. [Google Scholar] [CrossRef]
- Webb, A.A.; Muir, G.D. Compensatory locomotor adjustments of rats with cervical or thoracic spinal cord hemisections. J. Neurotrauma 2002, 19, 239–256. [Google Scholar] [CrossRef] [PubMed]
- Parkkinen, S.; Ortega, F.J.; Kuptsova, K.; Huttunen, J.; Tarkka, I.; Jolkkonen, J. Gait impairment in a rat model of focal cerebral ischemia. Stroke Res. Treat. 2013, 2013, 410972. [Google Scholar] [CrossRef] [PubMed]
- Vorhees, C.V.; Williams, M.T. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006, 1, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, L.C.B.; Pittenger, C. Cued and spatial learning in the water maze: Equivalent learning in male and female mice. Neurosci. Lett. 2010, 483, 148–151. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tian, S.F.; Yang, H.H.; Xiao, D.P.; Huang, Y.J.; He, G.Y.; Ma, H.R.; Xia, F.; Shi, X.C. Mechanisms of neuroprotection from hypoxia-ischemia (HI) brain injury by up-regulation of cytoglobin (CYGB) in a neonatal rat model. J. Biol. Chem. 2013, 288, 15988–16003. [Google Scholar] [CrossRef] [PubMed]
- Goren, B.; Cakir, A.; Ocalan, B.; Serter Kocoglu, S.; Alkan, T.; Cansev, M.; Kahveci, N. Long-term cognitive effects of uridine treatment in a neonatal rat model of hypoxic-ischemic encephalopathy. Brain Res. 2017, 1659, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Concepcion, K.; Meng, X.; Zhang, L. Brain-immune interactions in perinatal hypoxic-ischemic brain injury. Prog. Neurobiol. 2017, 159, 50–68. [Google Scholar] [CrossRef]
- Aly, H.; Khashaba, M.T.; El-Ayouty, M.; El-Sayed, O.; Hasanein, B.M. IL-1beta, IL-6 and TNF-alpha and outcomes of neonatal hypoxic ischemic encephalopathy. Brain Dev. 2006, 28, 178–182. [Google Scholar] [CrossRef]
- Silveira, R.C.; Procianoy, R.S. Interleukin-6 and tumor necrosis factor-alpha levels in plasma and cerebrospinal fluid of term newborn infants with hypoxic-ischemic encephalopathy. J. Pediatr. 2003, 143, 625–629. [Google Scholar] [CrossRef]
- Carrillo-de Sauvage, M.A.; Maatouk, L.; Arnoux, I.; Pasco, M.; Sanz Diez, A.; Delahaye, M.; Herrero, M.T.; Newman, T.A.; Calvo, C.F.; Audinat, E.; et al. Potent and multiple regulatory actions of microglial glucocorticoid receptors during CNS inflammation. Cell Death Differ. 2013, 20, 1546–1557. [Google Scholar] [CrossRef]
- Liu, H.S.; Shi, H.L.; Huang, F.; Peterson, K.E.; Wu, H.; Lan, Y.Y.; Zhang, B.B.; He, Y.X.; Woods, T.; Du, M.; et al. Astragaloside IV inhibits microglia activation via glucocorticoid receptor mediated signaling pathway. Sci. Rep. 2016, 6, 19137. [Google Scholar] [CrossRef] [PubMed]
- Sierra, A.; Gottfried-Blackmore, A.; Milner, T.A.; McEwen, B.S.; Bulloch, K. Steroid hormone receptor expression and function in microglia. Glia 2008, 56, 659–674. [Google Scholar] [CrossRef] [PubMed]
- Saijo, K.; Collier, J.G.; Li, A.C.; Katzenellenbogen, J.A.; Glass, C.K. An ADIOL-ERbeta-CtBP transrepression pathway negatively regulates microglia-mediated inflammation. Cell 2011, 145, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Ros-Bernal, F.; Hunot, S.; Herrero, M.T.; Parnadeau, S.; Corvol, J.C.; Lu, L.; Alvarez-Fischer, D.; Carrillo-de Sauvage, M.A.; Saurini, F.; Coussieu, C.; et al. Microglial glucocorticoid receptors play a pivotal role in regulating dopaminergic neurodegeneration in parkinsonism. Proc. Natl. Acad. Sci. USA 2011, 108, 6632–6637. [Google Scholar] [CrossRef] [PubMed]
- Bertorelli, R.; Adami, M.; Di Santo, E.; Ghezzi, P. MK 801 and dexamethasone reduce both tumor necrosis factor levels and infarct volume after focal cerebral ischemia in the rat brain. Neurosci. Lett. 1998, 246, 41–44. [Google Scholar] [CrossRef]
- Jing, Y.; Hou, Y.; Song, Y.; Yin, J. Methylprednisolone improves the survival of new neurons following transient cerebral ischemia in rats. Acta Neurobiol. Exp. (Wars) 2012, 72, 240–252. [Google Scholar]
- McNairn, A.J.; Chuang, C.H.; Bloom, J.C.; Wallace, M.D.; Schimenti, J.C. Female-biased embryonic death from inflammation induced by genomic instability. Nature 2019, 567, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Kosten, T.A.; Nielsen, D.A. Litter and sex effects on maternal behavior and DNA methylation of the Nr3c1 exon 17 promoter gene in hippocampus and cerebellum. Int. J. Dev. Neurosci. 2014, 36, 5–12. [Google Scholar] [CrossRef]
- Vannucci, R.C.; Vannucci, S.J. A model of perinatal hypoxic-ischemic brain damage. Ann. N. Y. Acad. Sci. 1997, 835, 234–249. [Google Scholar] [CrossRef]
- Semple, B.D.; Blomgren, K.; Gimlin, K.; Ferriero, D.M.; Noble-Haeusslein, L.J. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol. 2013, 106–107, 1–16. [Google Scholar] [CrossRef]
- Kamper, J.E.; Pop, V.; Fukuda, A.M.; Ajao, D.O.; Hartman, R.E.; Badaut, J. Juvenile traumatic brain injury evolves into a chronic brain disorder: Behavioral and histological changes over 6months. Exp. Neurol. 2013, 250, 8–19. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knox-Concepcion, K.R.; Figueroa, J.D.; Hartman, R.E.; Li, Y.; Zhang, L. Repression of the Glucocorticoid Receptor Increases Hypoxic-Ischemic Brain Injury in the Male Neonatal Rat. Int. J. Mol. Sci. 2019, 20, 3493. https://doi.org/10.3390/ijms20143493
Knox-Concepcion KR, Figueroa JD, Hartman RE, Li Y, Zhang L. Repression of the Glucocorticoid Receptor Increases Hypoxic-Ischemic Brain Injury in the Male Neonatal Rat. International Journal of Molecular Sciences. 2019; 20(14):3493. https://doi.org/10.3390/ijms20143493
Chicago/Turabian StyleKnox-Concepcion, Katherine R., Johnny D. Figueroa, Richard E. Hartman, Yong Li, and Lubo Zhang. 2019. "Repression of the Glucocorticoid Receptor Increases Hypoxic-Ischemic Brain Injury in the Male Neonatal Rat" International Journal of Molecular Sciences 20, no. 14: 3493. https://doi.org/10.3390/ijms20143493
APA StyleKnox-Concepcion, K. R., Figueroa, J. D., Hartman, R. E., Li, Y., & Zhang, L. (2019). Repression of the Glucocorticoid Receptor Increases Hypoxic-Ischemic Brain Injury in the Male Neonatal Rat. International Journal of Molecular Sciences, 20(14), 3493. https://doi.org/10.3390/ijms20143493






