Reactions in the Radiosensitizer Misonidazole Induced by Low-Energy (0–10 eV) Electrons
Abstract
:1. Introduction
2. Results and Discussion
2.1. General Features of Associative (AA) and Dissociative Electron Attachment (DEA) and Characterization of the Involved Resonances
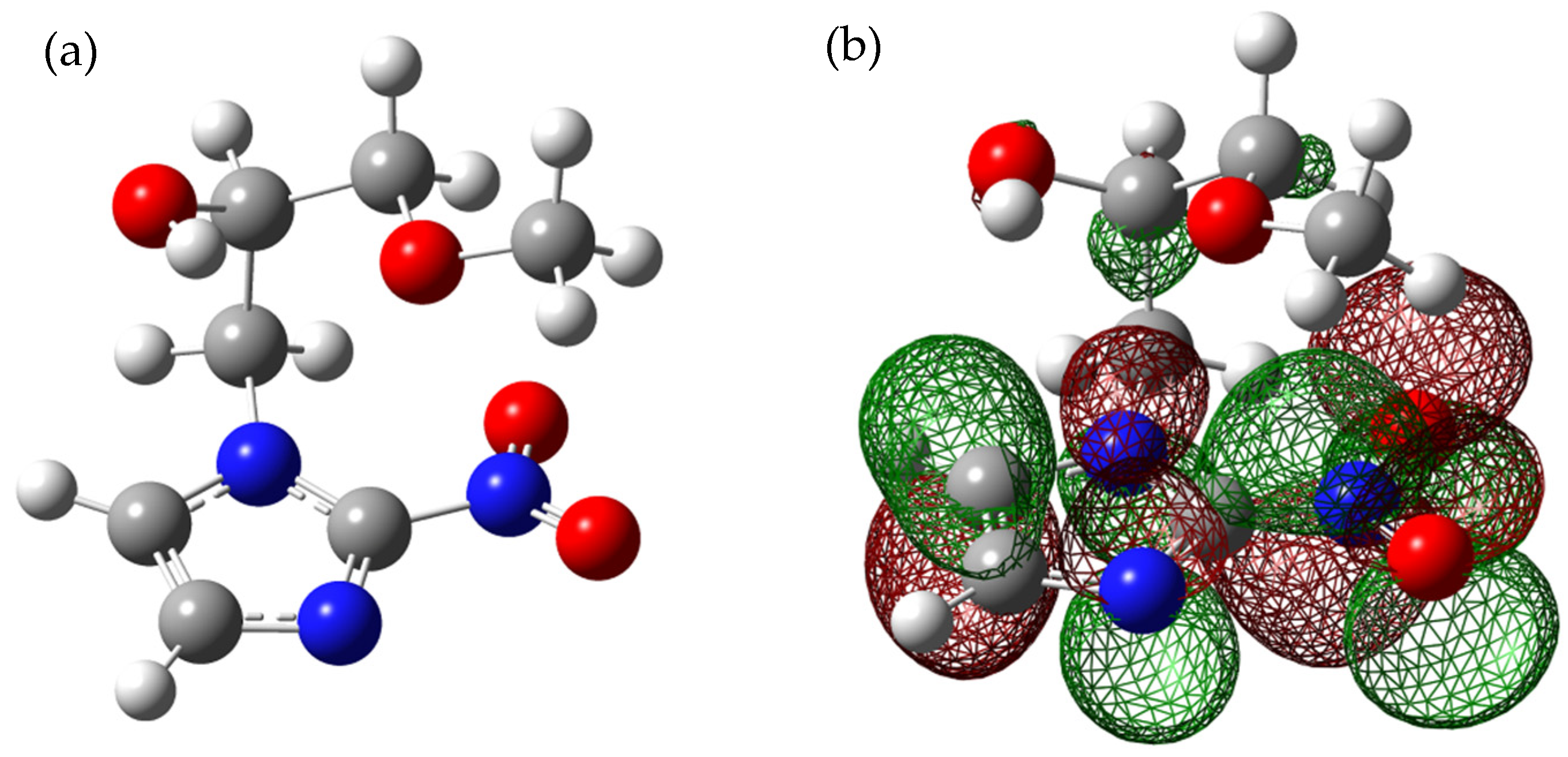
2.1.1. Formation of the Non-Decomposed Parent Anion
2.1.2. Dissociative Electron Attachment (DEA)
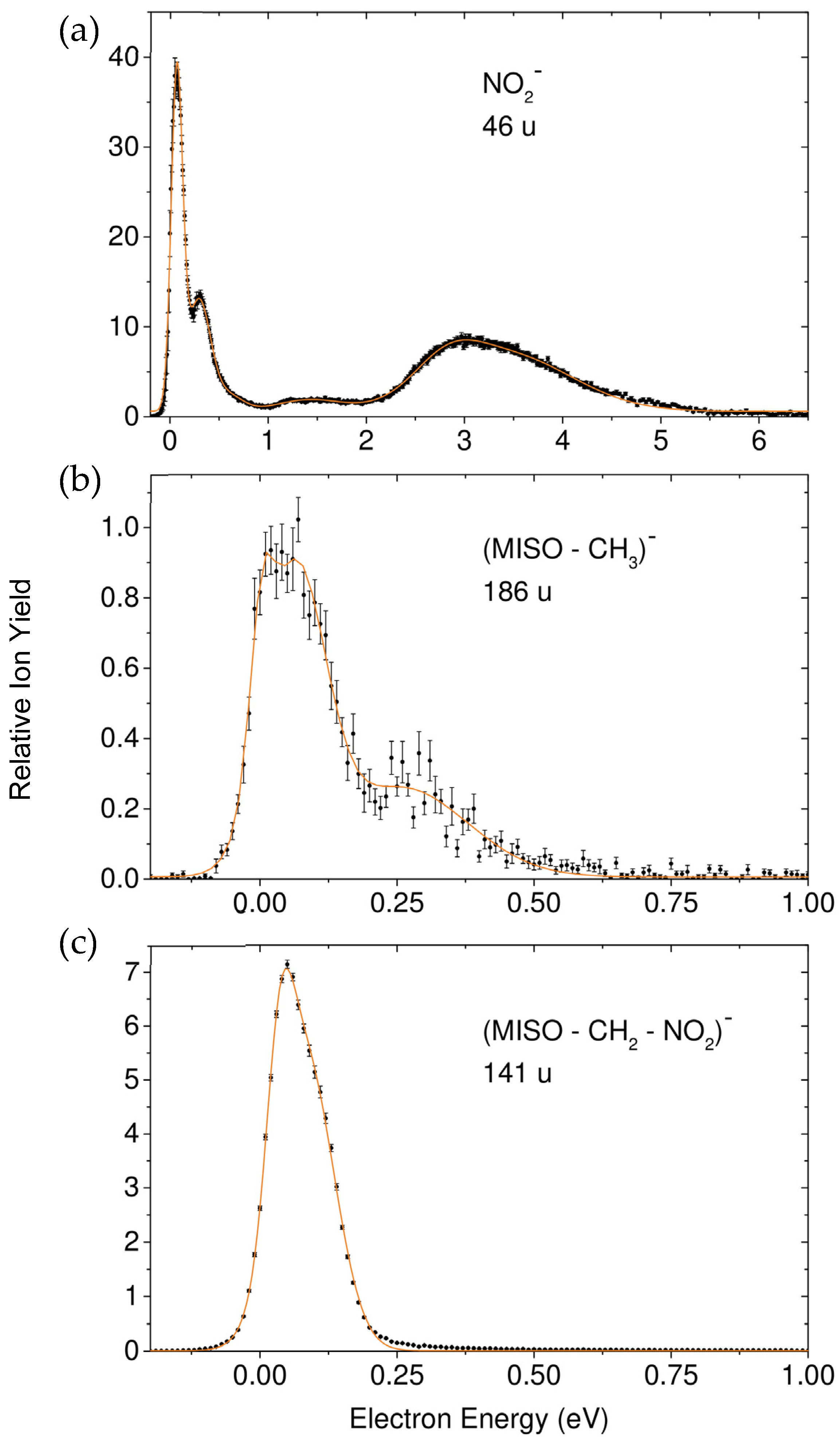
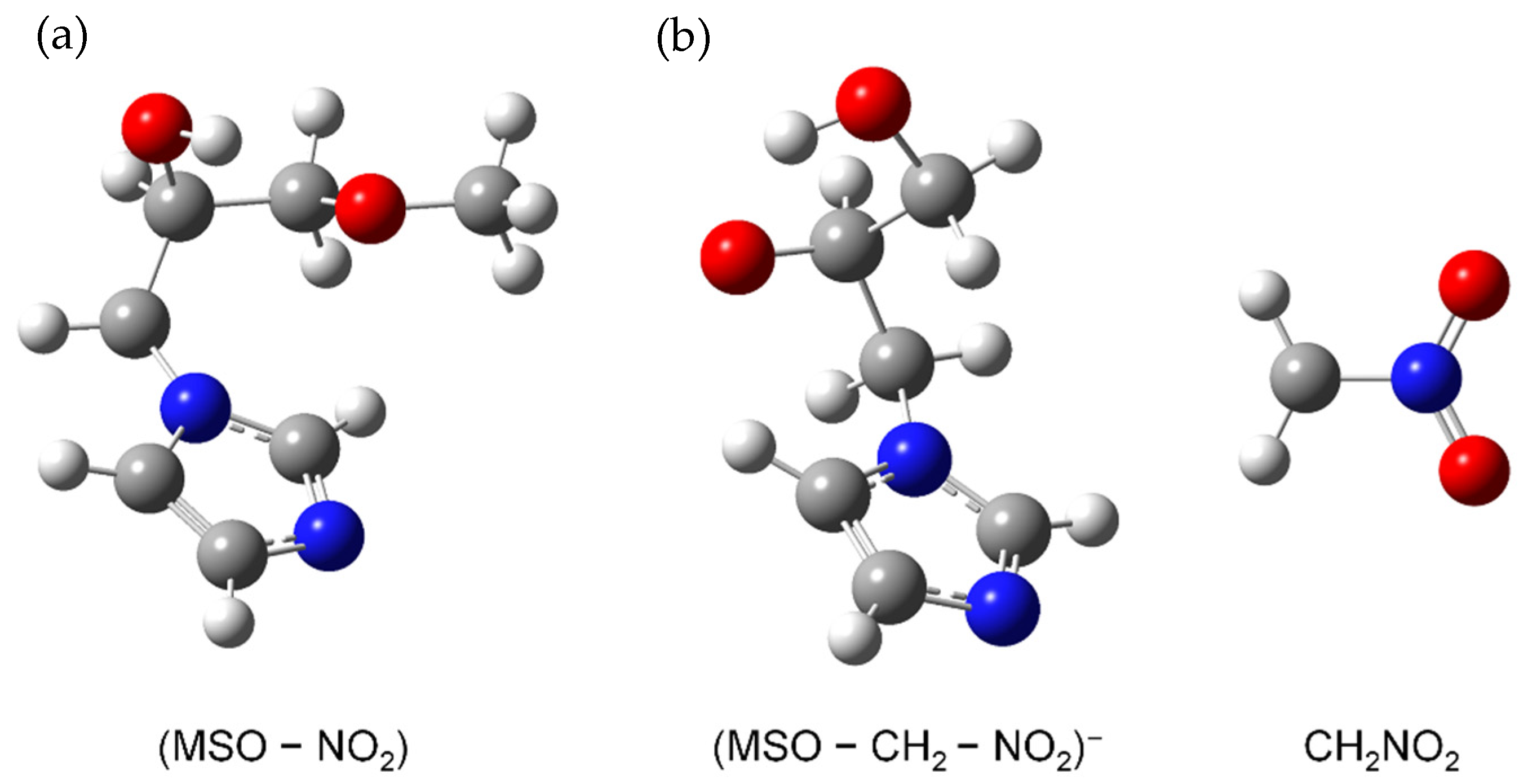
Formation of NO2−, Loss of the Neutral Unit CH3, and Loss of the Two Neutral Units NO2 + CH2
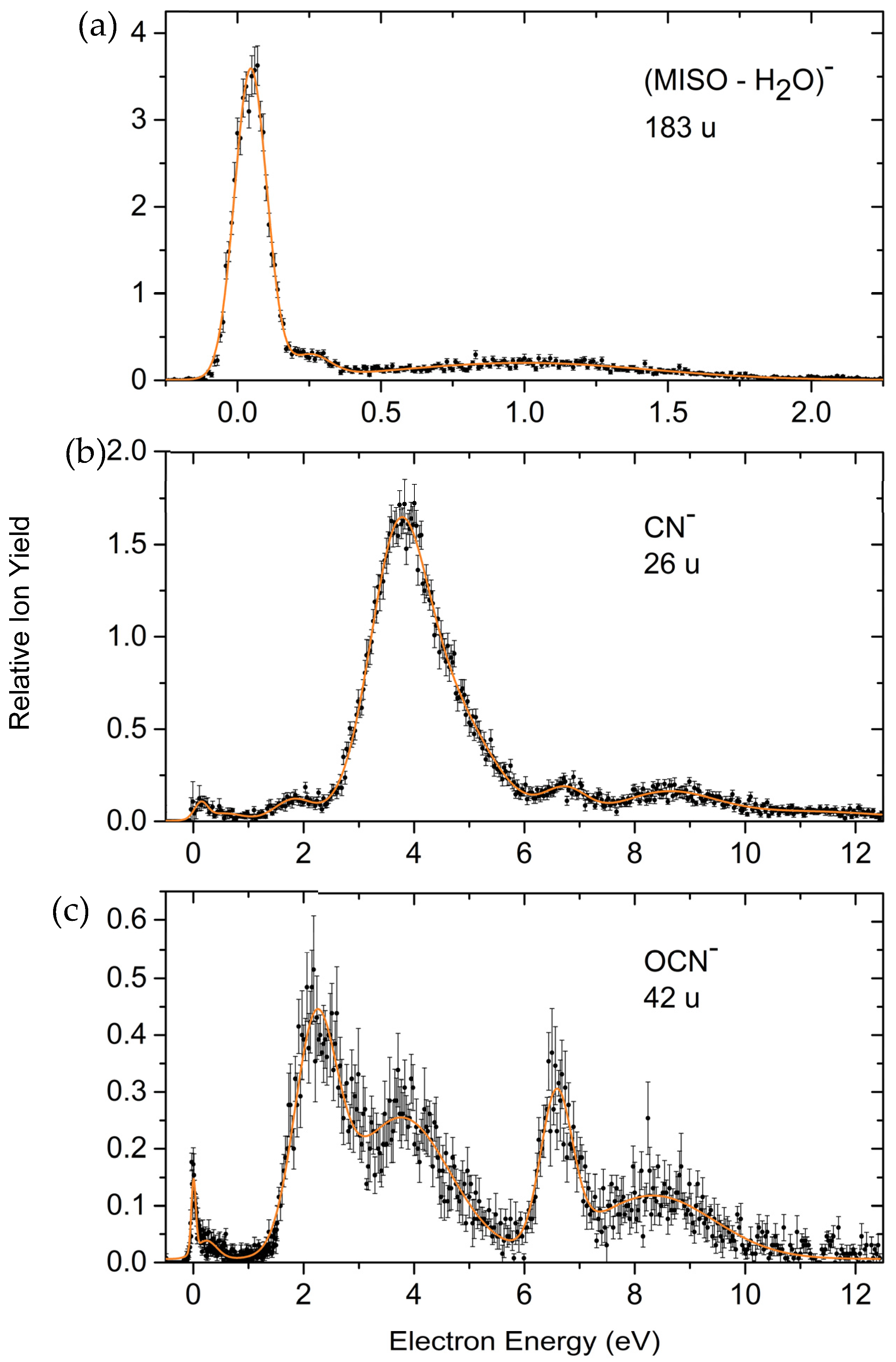
Loss of Neutral H2O and Excision of the Pseudohalogens CN− and OCN−
Formation of Nitroimidazolic Anions
3. Materials and Methods
3.1. Experiment
3.2. Calculations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MISO | Misonidazole |
| NI | Nitroimidazole |
| AA | Associative attachment |
| DEA | Dissociative electron attachment |
| DNA | Deoxyribonucleic acid |
| SOMO | Singly occupied molecular orbital |
| MO | Molecular orbital |
| BO | Born–Oppenheimer |
| TNI | Transitory negative ion |
| VFR | Vibrational Feshbach resonance |
| EA | Electron affinity |
| HEM | Hemispherical electron monochromator |
References
- Overgaard, J.; Hansen, H.S.; Overgaard, M.; Bastholt, L.; Berthelsen, A.; Specht, L.; Lindeløv, B.; Jørgensen, K. ARandomized Double-Blind Phase IIIStudy of Nimorazole as a Hypoxic Radiosensitizer of Primary Radiotherapy in Supraglottic Larynx Pharynx, C.a.r.c.i.n.o.m.a. Results of the Danish Head Neck Cancer Study (DAHANCA) Protocol, 5.-8.5. Radiother. Oncol. 1998, 46, 135–146. [Google Scholar] [CrossRef]
- Overgaard, J. Hypoxic Modification of Radiotherapy in Squamous Cell Carcinoma of the Head and Neck—A Systematic Review and Meta-Analysis. Radiother. Oncol. 2011, 100, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.I. Nitroimidazole drugs—Action and Resistance Mechanisms I. Mechanisms of Action. J. Antimicrob. Chemother. 1993, 31, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Polska, K.; Rak, J.; Wagner, J.R.; Sanche, L. Fundamental Mechanisms of DNA Radiosensitization: Damage Induced by Low-Energy Electrons in Brominated Oligonucleotide Trimers. J. Phys. Chem. B 2012, 116, 9676–9682. [Google Scholar] [CrossRef] [PubMed]
- Baccarelli, I.; Bald, I.; Gianturco, F.A.; Illenberger, E.; Kopyra, J. Electron-Induced Damage of DNA and Its Components: Experiments and Theoretical Models. J. Phys. Rep. 2011, 508, 1–44. [Google Scholar] [CrossRef]
- Abdoul-Carime, H.; Huels, M.A.; Brüning, F.; Illenberger, E.; Sanche, L. Dissociative Electron Attachment to Gas-Phase 5-Bromouracil. J. Chem. Phys. 2000, 113, 2517–2521. [Google Scholar] [CrossRef]
- Boudaïfffa, B.; Cloutier, P.; Hunting, D.; Huels, M.A.; Sanche, L. Resonant Formation of DNA Strand Breaks by Low-Energy (3 to 20 eV) Electrons. Science 2000, 287, 1658–1660. [Google Scholar]
- Ren, X.G.; Al Maalouf, E.J.; Dorn, A.; Denifl, S. Direct Evidence of two Interatomic Relaxation Mechanisms in Argon Dimers Ionized by Electron Impact. Nat. Commun. 2016, 7, 11093. [Google Scholar] [CrossRef]
- Zimbrick, J.D. Radiation Chemistry and the Radiation Research Society: A History from the Beginning. Radiat. Res. 2002, 158, 127–140. [Google Scholar] [CrossRef]
- Illenberger, E. Electron Attachment Processes in Molecules and Molecular Aggregates. In Gaseous Molecular Ions. An Introduction to Elementary Processes Induced by Ionization; Baumgärtel, H., Franck, E.U., Grünbein, W., Eds.; Steinkopff Verlag, Darmstadt/Springer-Verlag: New York, NY, USA, 1992; Volume 2, pp. 291–337. [Google Scholar]
- Hotop, H.; Ruf, M.-W.; Allan, M.; Fabrikant, I. Resonance and Threshold Phenomena in Low-Energy Electron Collisions with Molecules and Clusters. Adv. At. Mol. Opt. Phys. 2003, 49, 85–216. [Google Scholar]
- Christophorou, L.G.; Olthoff, J.K. Fundamental Electron-Molecule Interactions and Their Technological Significance. In Fundamental Electron Interactions with Plasma Processing Gases; Kluwer Academic: New York, NY, USA, 2004; pp. 1–59. [Google Scholar]
- Langer, J.; Balog, R.; Stano, M.; Abdoul-Carime, H.; Illenberger, E. Low Energy Electron Driven Reactions in Free and Bound Molecules: From Unimolecular Processes in the Gas Phase to Complex Reactions in a Condensed Environment. Int. J. Mass Spectrom. 2004, 233, 267–291. [Google Scholar]
- Gorfinkiel, J.; Ptasinska, S. Electron Scattering from Molecules and Molecular Aggregates of Biological Relevance. J. Phys. B At. Mol. Opt. Phys. 2017, 50, 182001. [Google Scholar] [CrossRef]
- Michael, B.D.; O´Neill, P.A. A Sting in the Tail of Electron Tracks. Science 2002, 287, 1603–1606. [Google Scholar] [CrossRef] [PubMed]
- von Sonntag, C. Formation of Reactive Free Radicals in an Aqueous Environment. In Free Radical Induced DNA Damage and Its Repair; Springer-Verlag: Berlin, Germany, 2006; pp. 7–46. [Google Scholar]
- Ferreira da Silva, F.; do, N.; Varella, M.T.; Jones, N.C.; Vrønnin Hoffmann, S.; Denifl, S.; Bald, I.; Kopyra, J. Electron-Induced Reactions in 3-Bromopyruvic Acid. Chem. Eur. J. 2019, 25, 5498–5506. [Google Scholar] [CrossRef] [PubMed]
- Schürmann, R.; Tanzer, K.; Dąbkowska, I.; Denifl, S.; Bald, I. Stability of the Parent Anion of the Potential Radiosensitizer 8-Bromoadenine Formed by Low-Energy (<3 eV) Electron Attachment. J. Phys. Chem. B 2017, 121, 5730–5734. [Google Scholar]
- Meißner, R.; Makurat, S.; Kozak, W.; Limão-Vieira, P.; Rak, J.; Denifl, S. Electron-Induced Dissociation of the Potential Radiosensitizer 5-Selenocyanato-2′-deoxyuridine. J. Phys. Chem. B 2019, 123, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Ameixa, J.; Arthur-Baidoo, E.; Meißner, R.; Makurat, S.; Kozak, W.; Butowska, K.; Ferreira da Silva, F.; Rak, J.; Denifl, S. Low-energy Electron-Induced Decomposition of 5-trifluoromethanesulfonyl-uracil: A Potential Radiosensitizer. J. Chem. Phys. 2018, 149, 164307. [Google Scholar] [CrossRef]
- Overgaard, J. Hypoxic Radiosensitization: Adored and Ignored. J. Clin. Oncol. 2007, 25, 4066–4074. [Google Scholar] [CrossRef]
- Feketeová, L.; Albright, A.L.; Sørensen, B.S.; Horsman, M.R.; White, J.; O’Hair, R.A.J.; Bassler, N. Formation of Radical Anions of Radiosensitizers and Related Model Compounds via Electrospray Ionization. Int. J. Mass Spectrom. 2014, 365–366, 56–63. [Google Scholar] [CrossRef]
- Klar, D.; Ruf, M.-W.; Hotop, H. Attachment of Electrons to Molecules at meV Resolution. Aust. J. Phys. 1992, 45, 263–291. [Google Scholar] [CrossRef]
- Klar, D.; Ruf, M.-W.; Hotop, H. Dissociative Electron Attachment to CCl4 Molecules at Low Electron Energies with meV Resolution. Int. J. Mass Spectrom. 2001, 205, 93–110. [Google Scholar] [CrossRef]
- Sulzer, P.; Rondino, F.; Ptasinska, S.; Illenberger, E.; Märk, T.D.; Scheier, P. Probing Trinitrotoluene (TNT) by Low-Energy Electrons: Strong Fragmentation Following Attachment of Electrons Near 0 eV. Int. J. Mass Spectrom. 2008, 271, 149–153. [Google Scholar] [CrossRef]
- Sulzer, P.; Mauracher, A.; Denifl, S.; Probst, M.; Märk, T.D.; Scheier, P.; Illenberger, E. Probing Di-Nitrobenzene by Low Energy Electrons Identification of Isomers via Resonances in Dissociative Electron Attachment. Int. J. Mass Spectrom. 2007, 266, 138–148. [Google Scholar] [CrossRef]
- Pshenichnyuk, S.A.; Vorob’ev, A.S.; Asfandiarov, N.L.; Modelli, A. Molecular Anion Formation in 9,10-anthraquinone: Dependence of the Electron Detachment Rate on Temperature and Incident Electron Energy. J. Chem. Phys. 2010, 132, 244313. [Google Scholar] [CrossRef] [PubMed]
- Khvostenko, O.G.; Shchukin, P.V.; Tuimedov, G.M.; Muftakhov, M.V.; Tseplin, E.E.; Tseplina, S.N.; Mazunov, V.A. Negative Ion Mass Spectrum of the Resonance Electron Capture by Molecules of p-Benzoquinone. Int. J. Mass Spectrom. 2008, 273, 69–77. [Google Scholar] [CrossRef]
- Ómarsson, B.; Ingólfsson, O. Stabilization, Fragmentation and Rearrangement Reactions in Low-Energy Electron Interaction with Tetrafluoro-Para-Benzoquinone: A Combined Theoretical and Experimental Study. Phys. Chem. Chem. Phys. 2013, 15, 16758. [Google Scholar] [CrossRef]
- Matejcik, S.; Märk, T.D.; Spanel, P.; Smith, D.; Jaffke, T.; Illenberger, E. Formation and Decay of C60− Following Free-Electron Capture by C60. J. Chem. Phys. 1995, 102, 2516–2521. [Google Scholar] [CrossRef]
- Ptasińska, S.; Echt, O.; Denifl, S.; Stano, M.; Sulzer, P.; Zappa, F.; Scheier, P.; Märk, T.D. Electron Attachment to Higher Fullerenes and to Sc3N@C80. J. Phys. Chem. A 2006, 110, 8451–8456. [Google Scholar] [CrossRef]
- Meißner, R.; Kočišek, J.; Feketeová, L.; Fedor, J.; Fárník, M.; Limão-Vieira, P.; Illenberger, E.; Denifl, S. Low-Energy Electrons Transform the Nimorazole Molecule into a Radiosensitiser. Nat. Commun. 2019, 10, 2388. [Google Scholar] [CrossRef]
- Tanzer, K.; Feketeová, L.; Puschnigg, B.; Scheier, P.; Illenberger, E.; Denifl, S. Reactions in Nitroimidazole Triggered by Low-Energy (0–2 eV) Electrons: Methylation at N1-H Completely Blocks Reactivity. Angew. Chem. Int. Ed. Engl. 2014, 53, 12240–12243. [Google Scholar] [CrossRef]
- Tanzer, K.; Feketeová, L.; Puschnigg, B.; Scheier, P.; Illenberger, E.; Denifl, S. Reactions in Nitroimidazole and Methylnitroimidazole Triggerd by Low Energy (0–8 eV) Electrons. J. Phys. Chem. A 2015, 119, 6668–6675. [Google Scholar] [CrossRef] [PubMed]
- Modelli, A.; Venuti, M. Empty Level Structure and Dissociative Electron Attachment in Gas-Phase Nitro Derivatives. Int. J. Mass Spectrom. 2001, 205, 7–16. [Google Scholar] [CrossRef]
- Sommerfeld, T. Coupling Between Dipole-Bound and Valence States: The Nitromethane Anion. Phys. Chem. Chem. Phys. 2002, 4, 2511–2516. [Google Scholar] [CrossRef]
- Kossoski, F.; Varella, M.T.D.N. How Does Methylation Suppress the Electron-Induced Decomposition of 1-Methyl-Nitroimidazoles? J. Chem. Phys. 2017, 147, 164310. [Google Scholar] [CrossRef] [PubMed]
- Hahndorf, I.; Lehr, L.; Illenberger, E.; Manz, J. Temperature Effects in Dissociative Electron Attachment to CF3Cl. Chem. Phys. Lett. 1994, 231, 460–466. [Google Scholar] [CrossRef]
- Hahndorf, I.; Illenberger, E. Temperature Effects in Dissociative Electron Attachment. Int. J. Mass Spectrom. Ion Proc. 1997, 167/168, 87–101. [Google Scholar] [CrossRef]
- Ribar, A.; Fink, K.; Probst, M.; Huber, S.E.; Feketeová, L.; Denifl, S. Isomer Selectivity in Low-Energy Electron Attachment to Nitroimidazoles. Chem. Eur. J. 2017, 23, 12892–12899. [Google Scholar] [CrossRef]
- NIST Chemistry WebBook. Available online: http://webbook.nist.gov/chemistry (accessed on 10 November 2019).
- Pelc, A.; Huber, S.E.; Matias, C.; Czupyt, Z.; Denifl, S. Formation of Negative Ions upon Dissociative Electron Attachment to the Astrochemically Relevant Molecule Aminoacetonitrile. J. Phys. Chem. A 2016, 120, 903–910. [Google Scholar] [CrossRef]
- Lentz, D.; Heni, M.; Illenberger, E. The Isomers CF3CN and CF3NC: Formation and Dissociation of the Anions Formed on Electron Attachment. Int. J. Mass Spectrom. Ion Proc. 1986, 71, 199–210. [Google Scholar]
- Heni, M.; Illenberger, E. Electron Attachment by Saturated Nitriles, Acrylonitrile (C2H3CN and Benzonitrile (C6H5CN). Int. J. Mass Spectrom. Ion Proc. 1986, 73, 127–144. [Google Scholar] [CrossRef]
- König-Lehmann, C.; Kopyra, J.; Dabkowska, I.; Kosicek, J.; Illenberger, E. Excision of CN− and OCN− from Acetamide and Some Amide Derivatives Triggered by Low Energy Electrons. Phys. Chem. Chem. Phys. 2008, 10, 6954–6961. [Google Scholar] [CrossRef] [PubMed]
- da Silva, F.F.; Matias, C.; Almeida, D.; García, G.; Ingólfsson, O.; Flosadóttir, H.D.; Omarsson, B.; Ptasinska, S.; Puschnigg, B.; Scheier, P.; et al. NCO−, a Key Fragment Upon Dissociative Electron Attachment and Electron Transfer to Pyrimidine Bases: Site Selectivity for a Slow Decay Process. J. Am. Soc. Mass Spectrom. 2013, 24, 1787–1797. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-class Functionals and 12 Other Functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. Density Functionals with Broad Applicability in Chemistry. Acc. Chem. Res. 2008, 41, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Gaussian 09, Revision D.01; Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. (Eds.) Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Illenberger, E. Electron Attachment Reactions in Molecular Clusters. Chem. Rev. 1992, 92, 1589–1610. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meißner, R.; Feketeová, L.; Illenberger, E.; Denifl, S. Reactions in the Radiosensitizer Misonidazole Induced by Low-Energy (0–10 eV) Electrons. Int. J. Mol. Sci. 2019, 20, 3496. https://doi.org/10.3390/ijms20143496
Meißner R, Feketeová L, Illenberger E, Denifl S. Reactions in the Radiosensitizer Misonidazole Induced by Low-Energy (0–10 eV) Electrons. International Journal of Molecular Sciences. 2019; 20(14):3496. https://doi.org/10.3390/ijms20143496
Chicago/Turabian StyleMeißner, Rebecca, Linda Feketeová, Eugen Illenberger, and Stephan Denifl. 2019. "Reactions in the Radiosensitizer Misonidazole Induced by Low-Energy (0–10 eV) Electrons" International Journal of Molecular Sciences 20, no. 14: 3496. https://doi.org/10.3390/ijms20143496
APA StyleMeißner, R., Feketeová, L., Illenberger, E., & Denifl, S. (2019). Reactions in the Radiosensitizer Misonidazole Induced by Low-Energy (0–10 eV) Electrons. International Journal of Molecular Sciences, 20(14), 3496. https://doi.org/10.3390/ijms20143496




