Dyslipidemia and Meibomian Gland Dysfunction: Utility of Lipidomics and Experimental Prospects with a Diet-Induced Obesity Mouse Model
Abstract
1. Introduction
2. Methods
2.1. Literature Search Strategy
2.2. Inclusion and Exclusion Criteria
- (a)
- Were original research papers published in English
- (b)
- Involved human subjects
- (c)
- Described study design
- (d)
- Contained quantitative information on the clinically accepted definition and/or diagnosis of dyslipidemia and meibomian gland dysfunction
- (e)
- Attempted to determine if there is a relationship between dyslipidemia and meibomian gland dysfunction
2.3. Appraisal of Included Studies
2.4. Data Extraction and Analysis
3. Results
4. Discussion
4.1. Blood Lipid Profiles and MGD; Study Limitations and Confounders
4.2. The Diet-Induced Obesity Mouse Model
4.3. Application of Lipidomics to Investigate the Link between Dyslipidemia and MGD in the Experimental Situation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MG | Meibomian gland |
| MGD | Meibomian gland dysfunction |
| LDL | Low-density lipoproteins |
| HDL | High-density lipoproteins |
| TC | Total cholesterol |
| TG | Triglycerides |
| HD | High-fat diet |
| ND | Normal diet |
| DIO | Diet-induced obesity |
| PPAR | Proliferator-activated receptor |
Appendix A
| Study | Diagnosis/Staging of MGD |
| Guliani et al. [31], (2018) | Evaluation of eight glands of the central third of the lower eyelid only for meibum quality Meibum quality score 0 = clear meibum 1 = cloudy meibum 2 = cloudy with debris 3 = thick like toothpaste Evaluation of the glandular expressibility of five glands of the central third of the lower eyelid Expressibility score 1 = 3–4 glands expressible 2 = 1–2 glands expressible 3 = no glands expressible |
| Braich et al. [22] (2016) | Slit lamp with digital pressure assessment of the central third of upper and lower eyelid meibomian glands for glandular obstruction and meibum quality. Glandular obstruction grades 0 = no obstruction, meibum easily expressed 1 = mild expression, meibum expressible with mild pressure 2 = moderate obstruction, meibum expressible with moderate pressure 3 = complete obstruction Meibum quality scores 0 = clear fluid 1 = cloudy fluid 2 = cloudy particulate fluid 3 = toothpaste-like * Scores ≥ 2 = moderate to severe MGD |
| Pinna et al. [32] (2013) | Slit lamp evaluation for glandular obstruction and meibum quality using firm digital pressure over the central third of the upper and lower eyelids Glandular obstruction scores 0 = no obstruction, meibum easily expressed 1 = mild obstruction, meibum expressible with mild pressure 2 = moderate obstruction, meibum expressible with moderate pressure 3 = complete obstruction, no glands expressible even with hard pressure Meibum quality scores 0 = clear fluid 1 = cloudy fluid 2 = cloudy particulate fluid 3 = toothpaste-like * Scores ≥ 2 = moderate to severe MGD |
| Bukhari [23] (2013) | Slit lamp with digital pressure evaluation of meibum expressivity of a fixed number of the glands MGD staging 0 = patent meibomian gland orifices expressing clear fluid and mild digital pressure 1 = presence of ductal plugging and the expression of clear fluid when firm digital pressure was applied to the glands. 2 = presence of ductal plugging and the expression of cloudy fluid when firm pressure was applied to the glands 3 = ductal plugging and the presence of inspissated materials, lack of expression when digital pressure was applied to the glands or glandular loss. |
| Dao et al. [24] (2010) | Clinical examination for glandular obstruction and meibum quality Glandular obstruction score range 0 = no obstruction to 4 = complete obstruction Meibum quality score range 0 = clear to 4 = toothpaste-like * Scores ≥ 3 = moderate to severe MGD |
References
- Knop, E.; Knop, N.; Millar, T.; Obata, H.; Sullivan, D.A. The international workshop on meibomian gland dysfunction: Report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1938–1978. [Google Scholar] [CrossRef] [PubMed]
- Millar, T.J.; Schuett, B.S. The real reason for having a meibomian lipid layer covering the outer surface of the tear film–A review. Exp. Eye Res. 2015, 137, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Mudgil, P. Antimicrobial role of human meibomian lipids at the ocular surface. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7272–7277. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.M.; Nichols, J.J. The neurobiology of the meibomian glands. Ocul. Surf. 2014, 12, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Seifert, P.; Spitznas, M. Vasoactive intestinal polypeptide (VIP) innervation of the human eyelid glands. Exp. Eye Res. 1999, 68, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Schirra, F.; Richards, S.M.; Liu, M.; Suzuki, T.; Yamagami, H.; Sullivan, D.A. Androgen regulation of lipogenic pathways in the mouse meibomian gland. Exp. Eye Res. 2006, 83, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Stern, M.E.; Beuerman, R.W.; Fox, R.I.; Gao, J.; Mircheff, A.K.; Pflugfelder, S.C. A unified theory of the role of the ocular surface in dry eye. In Lacrimal Gland, Tear Film, and Dry Eye Syndromes 2; Springer: Boston, MA, USA, 1998; pp. 643–651. [Google Scholar]
- Bron, A.; Tiffany, J. The contribution of meibomian disease to dry eye. Ocul. Surf. 2004, 2, 149–164. [Google Scholar] [CrossRef]
- The definition and classification of dry eye disease: Report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul. Surf. 2007, 5, 75–92. [CrossRef]
- Bron, A.J.; De Paiva, C.S.; Chauhan, S.K.; Bonini, S.; Gabison, E.E.; Jain, S.; Uchino, Y. Tfos dews II pathophysiology report. Ocul. Surf. 2017, 15, 438–510. [Google Scholar] [CrossRef]
- Nelson, J.D.; Shimazaki, J.; Benitez-del-Castillo, J.M.; Craig, J.P.; McCulley, J.P.; Den, S.; Foulks, G.N. The international workshop on meibomian gland dysfunction: Report of the definition and classification subcommittee. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1930–1937. [Google Scholar] [CrossRef]
- Schaumberg, D.A.; Nichols, J.J.; Papas, E.B.; Tong, L.; Uchino, M.; Nichols, K.K. The international workshop on meibomian gland dysfunction: Report of the subcommittee on the epidemiology of, and associated risk factors for, MGD. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1994–2005. [Google Scholar] [CrossRef] [PubMed]
- Arita, R.; Itoh, K.; Maeda, S.; Maeda, K.; Furuta, A.; Tomidokoro, A.; Amano, S. Proposed diagnostic criteria for seborrheic meibomian gland dysfunction. Cornea 2010, 29, 980–984. [Google Scholar] [CrossRef] [PubMed]
- Shine, W.E.; McCulley, J.P. Association of meibum oleic acid with meibomian seborrhea. Cornea 2000, 19, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Tamer, C.; Oksuz, H.; Sogut, S. Androgen status of the nonautoimmune dry eye subtypes. Ophthalmic Res. 2006, 38, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Suhalim, J.L.; Parfitt, G.J.; Xie, Y.; De Paiva, C.S.; Pflugfelder, S.C.; Shah, T.N.; Jester, J.V. Effect of desiccating stress on mouse meibomian gland function. Ocul. Surf. 2014, 12, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Arita, R.; Itoh, K.; Inoue, K.; Kuchiba, A.; Yamaguchi, T.; Amano, S. Contact lens wear is associated with decrease of meibomian glands. Ophthalmology 2009, 116, 379–384. [Google Scholar] [CrossRef]
- Moy, A.; McNamara, N.A.; Lin, M.C. Effects of isotretinoin on meibomian glands. Optom. Vis. Sci. 2015, 92, 925–930. [Google Scholar] [CrossRef]
- Eom, Y.; Baek, S.; Kim, H.M.; Song, J.S. Meibomian gland dysfunction in patients with chemotherapy-induced lacrimal drainage obstruction. Cornea 2017, 36, 572–577. [Google Scholar] [CrossRef]
- Woo, Y.J.; Ko, J.; Ji, Y.W.; Kim, T.I.; Yoon, J.S. Meibomian gland dysfunction associated with periocular radiotherapy. Cornea 2017, 36, 1486–1491. [Google Scholar] [CrossRef]
- Westekemper, H.; Anastassiou, G.; Sauerwien, W.; Chauvel, P.; Bornfeld, N.; Steuhl, K.P.; Meller, D. Analysis of ocular surface altertions following proton beam radiation in eyes with conjuctival malignant melanoma. Ophthamologe 2006, 103, 858–895. [Google Scholar]
- Braich, P.S.; Howard, M.K.; Singh, J.S. Dyslipidemia and its association with meibomian gland dysfunction. Int. Ophthalmol. 2016, 36, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, A.A. Associations between the grade of meibomian gland dysfunction and dyslipidemia. Ophthalmic Plast. Reconstr. Surg. 2013, 29, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Dao, A.H.; Spindle, J.D.; Harp, B.A.; Jacob, A.; Chuang, A.Z.; Yee, R.W. Association of dyslipidemia in moderate to severe meibomian gland dysfunction. Am. J. Ophthalmol. 2010, 150, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Kuriakose, R.K.; Braich, P.S. Dyslipidemia and its Association with Meibomian Gland Dysfunction: A Systematic Review. Int. Ophthalmol. 2018, 38, 1809–1816. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Brewer, H.B.; Cleeman, J.I.; Smith, S.C.; Lenfant, C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 2004, 109, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.; Dziura, J.; Burgert, T.S.; Tamborlane, W.V.; Taksali, S.E.; Yeckel, C.W.; Sherwin, R.S. Obesity and the metabolic syndrome in children and adolescents. N. Engl. J. Med. 2004, 350, 2362–2374. [Google Scholar] [CrossRef]
- Isomaa, B.; Almgren, P.; Tuomi, T.; Forsén, B.; Lahti, K.; Nissén, M.; Groop, L. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care 2001, 24, 683–689. [Google Scholar] [CrossRef]
- Tietge, U.J. Hyperlipidemia and cardiovascular disease: Inflammation, dyslipidemia, and atherosclerosis. Curr. Opin. Lipidol. 2014, 25, 94–95. [Google Scholar] [CrossRef]
- Van Haeringen, N.; Glasius, E. Cholesterol in human tear fluid. Exp. Eye Res. 1975, 20, 271–274. [Google Scholar] [CrossRef]
- Guliani, B.P.; Bhalla, A.; Naik, M.P. Association of the severity of meibomian gland dysfunction with dyslipidemia in Indian population. Indian J. Ophthalmol. 2018, 66, 1411–1416. [Google Scholar]
- Pinna, A.; Blasetti, F.; Zinellu, A.; Carru, C.; Solinas, G. Meibomian gland dysfunction and hypercholesterolemia. Ophthalmology 2013, 120, 2385–2389. [Google Scholar] [CrossRef] [PubMed]
- Shine, W.E.; McCulley, J.P. The role of cholesterol in chronic blepharitis. Investig. Ophthalmol. Vis. Sci. 1991, 32, 2272–2280. [Google Scholar]
- Butovich, I.A. Fatty acid composition of cholesteryl esters of human meibomian gland secretions. Steroids 2010, 75, 726–733. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Butovich, I.A.; Lu, H.; McMahon, A.; Eule, J.C. Toward an animal model of the human tear film: Biochemical comparison of the mouse, canine, rabbit, and human meibomian lipidomes. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6881–6896. [Google Scholar] [CrossRef] [PubMed]
- Wojtowicz, J.C.; Butovich, I.A.; McCulley, J.P. Historical brief on composition of human meibum lipids. Ocul. Surf. 2009, 7, 145–153. [Google Scholar] [CrossRef]
- Gorgas, K.; Völkl, A. Peroxisomes in sebaceous glands. IV. Aggregates of tubular peroxisomes in the mouse Meibomian gland. Histochem. J. 1984, 16, 1079–1098. [Google Scholar] [CrossRef] [PubMed]
- Reins, R.Y.; Lema, C.; Courson, J.; Kunnen, C.M.; Redfern, R.L. MyD88 Deficiency Protects Against Dry Eye–Induced Damage. Investig. Ophthalmol. Vis. Sci. 2018, 59, 2967–2976. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Potma, E.O.; Suhalim, J.L.; Nien, C.L.; Jester, J.V.; Miljkovic, M.D.; Diem, M. Picosecond spectral coherent anti-Stokes Raman scattering imaging with principal component analysis of meibomian glands. J. Biomed. Opt. 2011, 16, 021104. [Google Scholar] [CrossRef]
- Hargrave, A.; Mehta, P.; Landry, P.; Amanda, H.; Dupre, M.; Magadi, S.; Burns, A.R. Metabolic syndrome affects mouse corneal epithelium and nerve morphology. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3076. [Google Scholar]
- Yang, K.; Han, X. Lipidomics: Techniques, applications, and outcomes related to biomedical sciences. Trends Biochem. Sci. 2016, 41, 954–969. [Google Scholar] [CrossRef]
- Narducci, E.; Waltz, A.; Gorski, K.; Leppla, L.; Donaldson, M. The clinical utility of functional performance tests within one-year post-acl reconstruction: A systematic review. Int. J. Sports Phys. Ther. 2011, 6, 333. [Google Scholar] [PubMed]
- Lo, C.K.L.; Mertz, D.; Loeb, M. Newcastle-Ottawa Scale: Comparing reviewers’ to authors’ assessments. BMC Med. Res. Methodol. 2014, 14, 45. [Google Scholar] [CrossRef] [PubMed]
- Khera, A.V.; Cuchel, M.; De la Llera-Moya, M.; Rodrigues, A.; Burke, M.F.; Jafri, K.; Mohler, E.R. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N. Engl. J. Med. 2011, 364, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Barter, P.; Rye, K.A. High density lipoproteins and coronary heart disease. Atherosclerosis 1996, 121, 1–12. [Google Scholar] [CrossRef]
- Modulo, C.M.; Filho, E.B.M.; Malki, L.T.; Dias, A.C.; De Souza, J.C.; Oliveira, H.C.; Rocha, E.M. The role of dyslipidemia on ocular surface, lacrimal and meibomian gland structure and function. Curr. Eye Res. 2012, 37, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Bowe, B.; Xie, Y.; Xian, H.; Balasubramanian, S.; Al-Aly, Z. Low levels of high-density lipoprotein cholesterol increase the risk of incident kidney disease and its progression. Kidney Int. 2016, 89, 886–896. [Google Scholar] [CrossRef] [PubMed]
- Bowe, B.; Xie, Y.; Xian, H.; Balasubramanian, S.; Zayed, M.A.; Al-Aly, Z. High density lipoprotein cholesterol and the risk of all-cause mortality among US veterans. Clin. J. Am. Soc. Nephrol. 2016, 11, 1784–1793. [Google Scholar] [CrossRef] [PubMed]
- Ko, D.T.; Alter, D.A.; Guo, H.; Koh, M.; Lau, G.; Austin, P.C.; Wijeysundera, H.C. High-density lipoprotein cholesterol and cause-specific mortality in individuals without previous cardiovascular conditions: The CANHEART study. J. Am. Coll. Cardiol. 2016, 68, 2073–2083. [Google Scholar] [CrossRef]
- Sullivan, D.A.; Sullivan, B.D.; Evans, J.E.; Schirra, F.; Yamagami, H.; Liu, M.; Dana, M.R. Androgen deficiency, Meibomian gland dysfunction, and evaporative dry eye. Ann. N. Y. Acad. Sci. 2002, 966, 211–222. [Google Scholar] [CrossRef]
- Sullivan, B.; Cermak, J.; Sullivan, R.; Papas, A.; Evans, J.; Dana, M.R.; Sullivan, D.A. Correlations between nutrient intake and the polar lipid profiles of meibomian gland secretions in women with Sjögren’s syndrome. In Lacrimal Gland, Tear Film, and Dry Eye Syndromes 3; Springer: Boston, MA, USA, 2002; pp. 441–447. [Google Scholar]
- Engel, L.; Wittig, S.; Bock, F.; Sauerbier, L.; Scheid, C.; Holtick, U.; Steven, P. Meibography and meibomian gland measurements in ocular graft-versus-host disease. Bone Marrow Transplant. 2015, 50, 961–967. [Google Scholar] [CrossRef]
- Nien, C.J.; Massei, S.; Lin, G.; Nabavi, C.; Tao, J.; Brown, D.J.; Jester, J.V. Effects of age and dysfunction on human meibomian glands. Arch. Ophthalmol. 2011, 129, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Le Couteur, D.G.; Cogger, V.C.; McCUSKEY, R.S.; De Cabo, R.; SmedsrØd, B.; Sorensen, K.K.; Fraser, R. Age-related changes in the liver sinusoidal endothelium: A mechanism for dyslipidemia. Ann. N. Y. Acad. Sci. 2007, 1114, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.H.; Li, J.J. Aging and dyslipidemia: A review of potential mechanisms. Ageing Res. Rev. 2015, 19, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Green-Church, K.B.; Butovich, I.; Willcox, M.; Borchman, D.; Paulsen, F.; Barabino, S.; Glasgow, B.J. The international workshop on meibomian gland dysfunction: Report of the subcommittee on tear film lipids and lipid–protein interactions in health and disease. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1979–1993. [Google Scholar] [CrossRef] [PubMed]
- Butovich, I.A.; Wojtowicz, J.C.; Molai, M. Human tear film and meibum. Very long chain wax esters and (O-acyl)-omega-hydroxy fatty acids of meibum. J. Lipid Res. 2009, 50, 2471–2485. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.H.; Kunnen, C.M.; Duchoslav, E.; Dolla, N.K.; Kelso, M.J.; Papas, E.B.; Mitchell, T.W. A comparison of patient matched meibum and tear lipidomes. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7417–7423. [Google Scholar] [CrossRef]
- Wenk, M.R. The emerging field of lipidomics. Nat. Rev. Drug. Discov. 2005, 4, 594–610. [Google Scholar] [CrossRef]
- Wenk, M.R. Lipidomics: New tools and applications. Cell 2010, 143, 888–895. [Google Scholar] [CrossRef]
- Shevchenko, A.; Simons, K. Lipidomics: Coming to grips with lipid diversity. Nat. Rev. Mol. Cell Biol. 2010, 11, 593–598. [Google Scholar] [CrossRef]
- Brügger, B. Lipidomics: Analysis of the lipid composition of cells and subcellular organelles by electrospray ionization mass spectrometry. Annu. Rev. Biochem. 2014, 83, 79–98. [Google Scholar] [CrossRef]
- Yetukuri, L.; Ekroos, K.; Vidal-Puig, A.; Orešič, M. Informatics and computational strategies for the study of lipids. Mol. Biosyst. 2008, 4, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Stegemann, C.; Drozdov, I.; Shalhoub, J.; Humphries, J.; Ladroue, C.; Didangelos, A.; Smith, A. Comparative lipidomics profiling of human atherosclerotic plaques. Circ. Cardiovasc. Genet. 2011, 4, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Meikle, P.J.; Wong, G.; Barlow, C.K.; Kingwell, B.A. Lipidomics: Potential role in risk prediction and therapeutic monitoring for diabetes and cardiovascular disease. Pharmacol. Ther. 2014, 143, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Prüss, H.; Rosche, B.; Sullivan, A.B.; Brommer, B.; Wengert, O.; Gronert, K.; Schwab, J.M. Proresolution lipid mediators in multiple sclerosis—differential, disease severity-dependent synthesis—A clinical pilot trial. PLoS ONE 2013, 8, e55859. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ren, S.; Piao, H.L.; Wang, F.; Yin, P.; Xu, C.; Zhao, X. Integration of lipidomics and transcriptomics unravels aberrant lipid metabolism and defines cholesteryl oleate as potential biomarker of prostate cancer. Sci. Rep. 2016, 6, 20984. [Google Scholar] [CrossRef] [PubMed]
- Butovich, I.A. Tear film lipids. Exp. Eye Res. 2013, 117, 4–27. [Google Scholar] [CrossRef]
- Zhou, L.; Beuerman, R.W. Tear analysis in ocular surface diseases. Prog. Retin. Eye Res. 2012, 31, 527–550. [Google Scholar] [CrossRef]
- Harvey, D.; Tiffany, J. Identification of meibomian gland lipids by gas chromatography—mass spectrometry: Application to the meibomian lipids of the mouse. J. Chromatogr. A 1984, 301, 173–187. [Google Scholar] [CrossRef]
- Butovich, I.A. On the lipid composition of human meibum and tears: Comparative analysis of nonpolar lipids. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3779–3789. [Google Scholar] [CrossRef]
- Butovich, I.A.; Uchiyama, E.; Di Pascuale, M.A.; McCulley, J.P. Liquid chromatography–mass spectrometric analysis of lipids present in human meibomian gland secretions. Lipids 2007, 42, 765–776. [Google Scholar] [CrossRef]
- Butovich, I.A. Lipidomics of human meibomian gland secretions: Chemistry, biophysics, and physiological role of meibomian lipids. Prog. Lipid Res. 2011, 50, 278–301. [Google Scholar] [CrossRef] [PubMed]
- Hammad, S.M. Blood sphingolipids in homeostasis and pathobiology. In Sphingolipids and Metabolic Disease; Springer: Boston, MA, USA, 2011; pp. 57–66. [Google Scholar]
- Sharrett, A.R.; Ballantyne, C.; Coady, S.; Heiss, G.; Sorlie, P.; Catellier, D.; Patsch, W. Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein (a), apolipoproteins AI and B, and HDL density subfractions: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation 2001, 104, 1108–1113. [Google Scholar] [CrossRef] [PubMed]
- Borchman, D.; Foulks, G.N.; Yappert, M.C.; Milliner, S.E. Changes in human meibum lipid composition with age using nuclear magnetic resonance spectroscopy. Investig. Ophthalmol. Vis. Sci. 2012, 53, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Borchman, D.; Ramasubramanian, A.; Foulks, G.N. Human Meibum Cholesteryl and Wax Ester Variability with Age, Sex, and Meibomian Gland Dysfunction. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2286–2293. [Google Scholar] [CrossRef] [PubMed]
- Jester, J.; Nicolaides, N.; Kiss-Palvolgyi, I.; Smith, R. Meibomian gland dysfunction. II. The role of keratinization in a rabbit model of MGD. Investig. Ophthalmol. Vis. Sci. 1989, 30, 936–945. [Google Scholar]
- Parfitt, G.J.; Xie, Y.; Geyfman, M.; Brown, D.J.; Jester, J.V. Absence of ductal hyper-keratinization in mouse age-related meibomian gland dysfunction (ARMGD). Aging 2013, 5, 825–834. [Google Scholar] [CrossRef]
- Borchman, D.; Foulks, G.N.; Yappert, M.C.; Bell, J.; Wells, E.; Neravetla, S.; Greenstone, V. Human meibum lipid conformation and thermodynamic changes with meibomian-gland dysfunction. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3805–3817. [Google Scholar] [CrossRef]
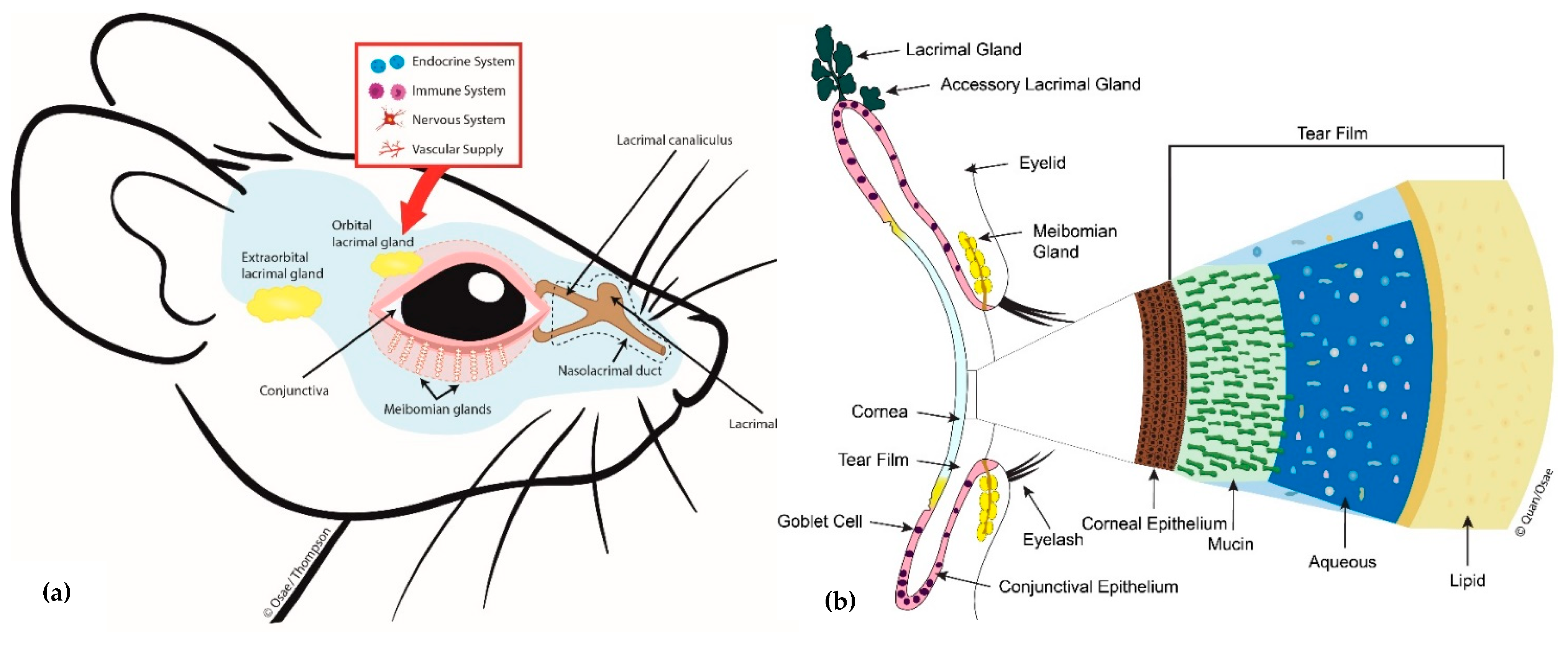
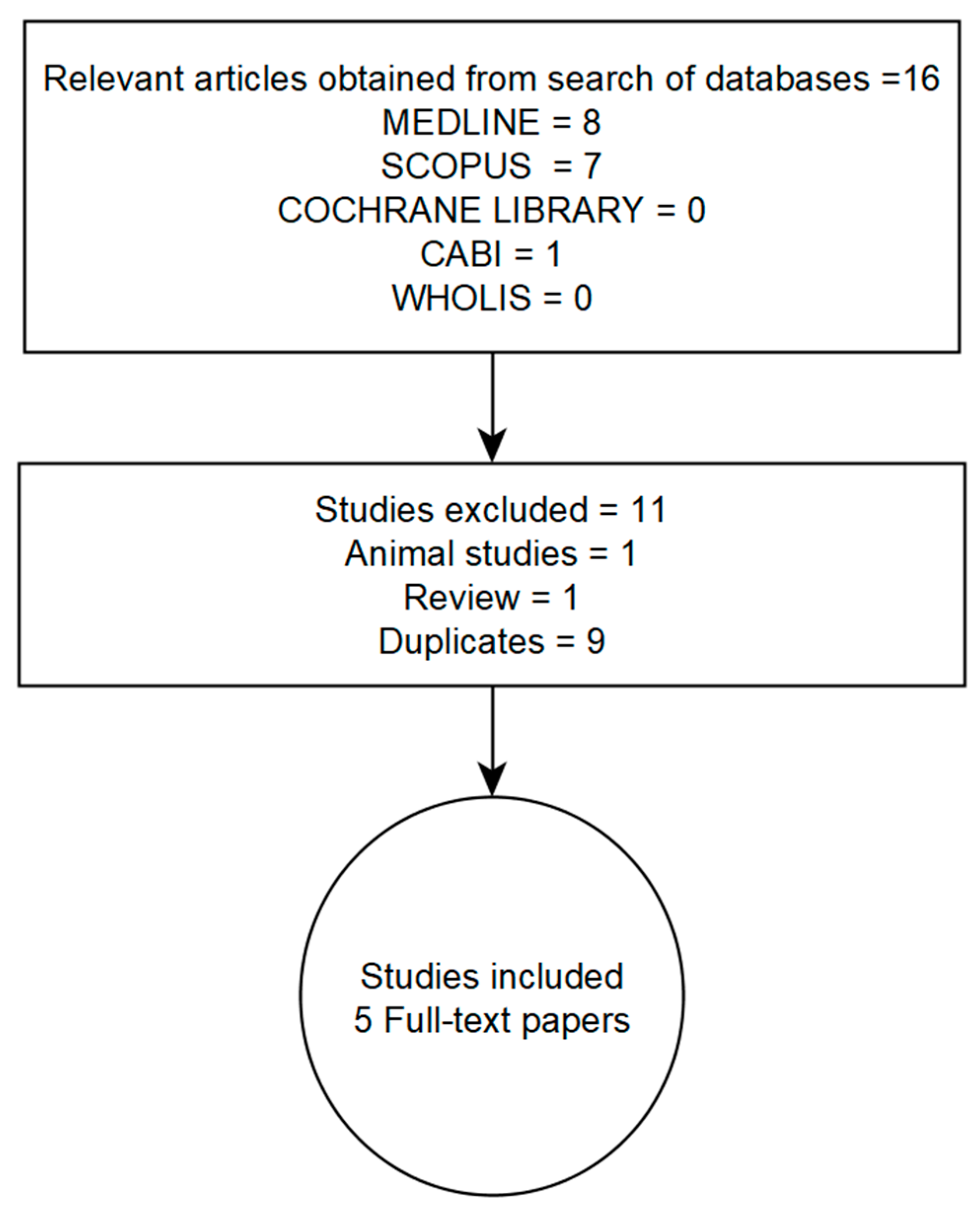

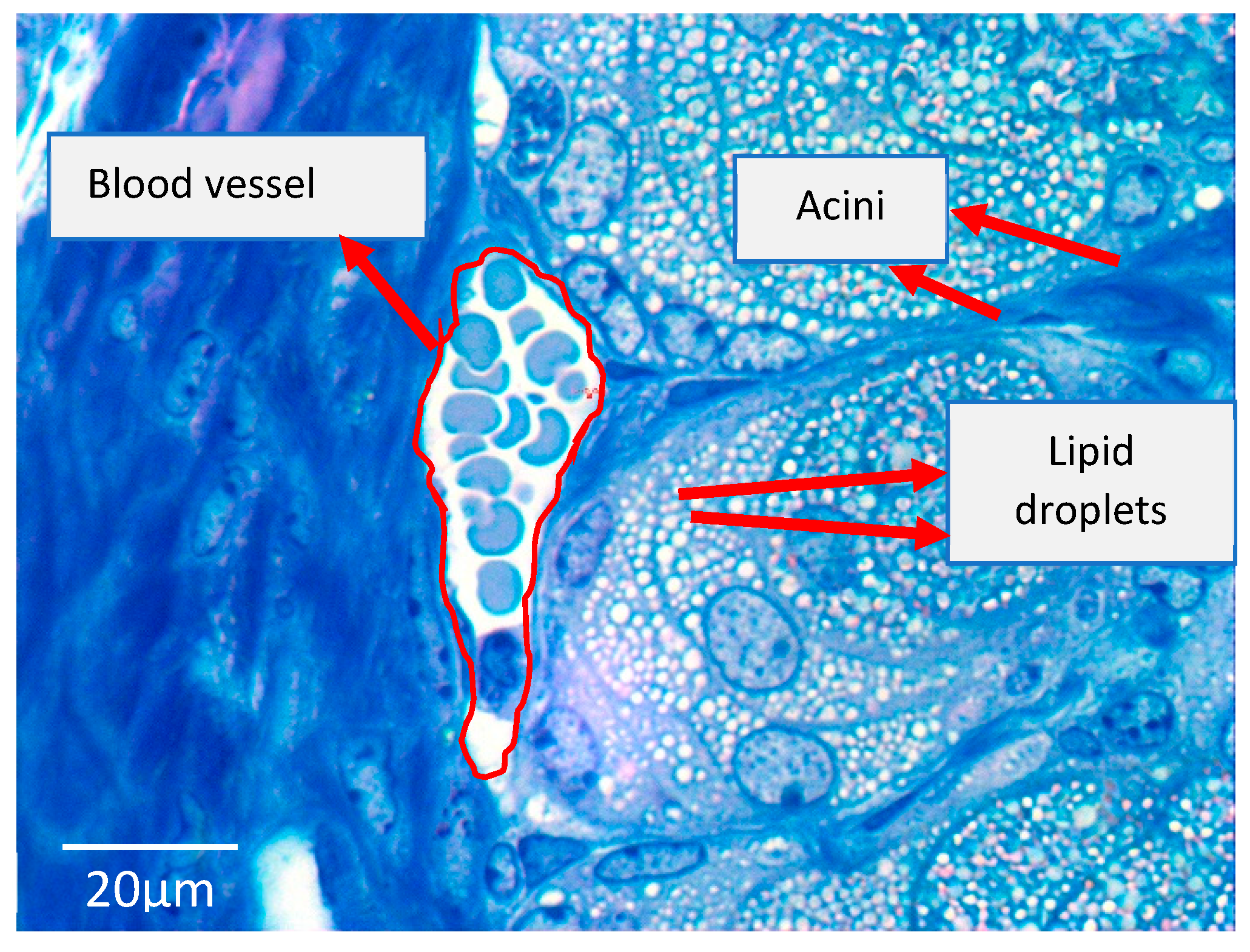
| Study | Country/Ethnicity | Study Description | No. of Participants | Age Range (yrs) | No. of Meibomian Gland Dysfunction (MGD) Cases vs. Non-MGD | † Prevalence Ratio of Dyslipidemia MGD: non-MGD | Named Serum Lipids Showing Significant Associations with MGD Status |
|---|---|---|---|---|---|---|---|
| Guliani et al. [31] (2018) | India/Indian | Prospective observational case-control hospital-based study | 180 | 18–54 | 90 90 | 1.6 | TC > 200 mg/dL TGs >150 mg/dL LDL > 130 mg/dL |
| Braich et al. [22] (2016) | Indian/Indian | Case-control hospital-based study | 224 | 19–75 | 109 115 | 8.7 | HDL < 40 mg/dL TC > 200 mg/dL TGs >150 mg/dL LDL > 130 mg/dL |
| Pinna et al. [32] (2013) | Italy/Italian | Observational, case-control pilot study | 123 | 18–54 | 60 63 | 9.2 | TC > 200 mg/dL TGs >150 mg/dL LDL > 130 mg/dL HDL > 40 mg/dL |
| Bukhari [23] (2013) | Saudi Arabia/N.S. | Prospective cohort study | 236 | 15–78 | 132 104 | ~1.0 | None |
| Dao et al. [24] (2010) | United States/N.S. | Retrospective case-control study | 46 (cases only) | 27–82 | * 46 * Unknown no. of controls | ** 1.5 ** Assuming n = 46 for controls | No specific tests of associations performed |
| Quality Assessment Criteria | Acceptable Criteria | Guliani et al. [31] (2018) | Braich et al. [22] (2016) | Pinna et al. [32] (2013) | Bukhari [23] (2013) | Dao et al. [24] (2010) |
|---|---|---|---|---|---|---|
| Selection | ||||||
| Definition of case or exposure (i.e., dyslipidemia and MGD status) | Adequate i. For dyslipidemia if based on at least an assessment of fasting triglycerides, total cholesterol, HDL or LDL levels | ✓ | ✓ | ✓ | ✓ | ✓ |
| ii. For MGD if based on at least a symptom assessment, meibography, meibum expressibility or quality or slit lamp examination of morphologic eye lid features | ||||||
| Representativeness of cases or exposed cohort? | Representative of average adult in community (age/sex/being at risk) | ✓ | ✓ | ✓ | ✓ | ✓ |
| Selection of controls or non-exposed cohorts | Specified as drawn from same the same community as cases or exposed cohort | - | ✓ | ✓ | ✓ | - |
| Comparability | ||||||
| Study has sufficiently controlled for age/sex | Yes | ✓ | ✓ | ✓ | ✓ | ✓ |
| Study considered at least three additional risk factors for MGD | Aging, prolonged contact lens wear, recurrent eyelid infections, autoimmune disease e.g., Sjogren’s syndrome, Stevens–Johnson syndrome, use of certain drugs like isotretinoin, antihistamines, hormone replacement therapy | ✓ | ✓ | ✓ | - | ✓ |
| Outcome | ||||||
| Assessment of outcome | Independent blind assessment, record linkage or self-report | - | - | - | - | ✓ |
| Response rate | Similar across groups | ✓ | ✓ | ✓ | ✓ | N/A |
| Same ascertainment method for cases and controls | Yes | ✓ | ✓ | ✓ | ✓ | - |
| Conclusively reports a direct link between MGD and dyslipidemia | Yes | - | - | - | - | - |
| Overall quality score (Maximum 9) | 6 | 7 | 7 | 6 | 5 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osae, E.A.; Steven, P.; Redfern, R.; Hanlon, S.; Smith, C.W.; Rumbaut, R.E.; Burns, A.R. Dyslipidemia and Meibomian Gland Dysfunction: Utility of Lipidomics and Experimental Prospects with a Diet-Induced Obesity Mouse Model. Int. J. Mol. Sci. 2019, 20, 3505. https://doi.org/10.3390/ijms20143505
Osae EA, Steven P, Redfern R, Hanlon S, Smith CW, Rumbaut RE, Burns AR. Dyslipidemia and Meibomian Gland Dysfunction: Utility of Lipidomics and Experimental Prospects with a Diet-Induced Obesity Mouse Model. International Journal of Molecular Sciences. 2019; 20(14):3505. https://doi.org/10.3390/ijms20143505
Chicago/Turabian StyleOsae, Eugene A., Philipp Steven, Rachel Redfern, Samuel Hanlon, C. Wayne Smith, Rolando E. Rumbaut, and Alan R. Burns. 2019. "Dyslipidemia and Meibomian Gland Dysfunction: Utility of Lipidomics and Experimental Prospects with a Diet-Induced Obesity Mouse Model" International Journal of Molecular Sciences 20, no. 14: 3505. https://doi.org/10.3390/ijms20143505
APA StyleOsae, E. A., Steven, P., Redfern, R., Hanlon, S., Smith, C. W., Rumbaut, R. E., & Burns, A. R. (2019). Dyslipidemia and Meibomian Gland Dysfunction: Utility of Lipidomics and Experimental Prospects with a Diet-Induced Obesity Mouse Model. International Journal of Molecular Sciences, 20(14), 3505. https://doi.org/10.3390/ijms20143505





