Gene Delivery Therapeutics in the Treatment of Periodontitis and Peri-Implantitis: A State of the Art Review
Abstract
1. Introduction
1.1. Periodontitis and Peri-Implantitis
1.2. Periodontal/Peri-Implant Tissue Regeneration
1.3. Tissue Engineering/Regenerative Medicine (TE/RM)
1.4. Rationale of Gene Therapy for Periodontal Tissue Engineering
2. Gene Therapy
2.1. Definition and History
2.2. General Principles of Gene Therapy
2.3. DNA Versus RNA Delivery
3. Delivery Vehicles: Viral Versus Non-Viral Vectors
3.1. Viral Vectors
3.2. Non-Viral Vectors
3.3. Inducible Systems for Viral Delivery
3.4. Exosomes
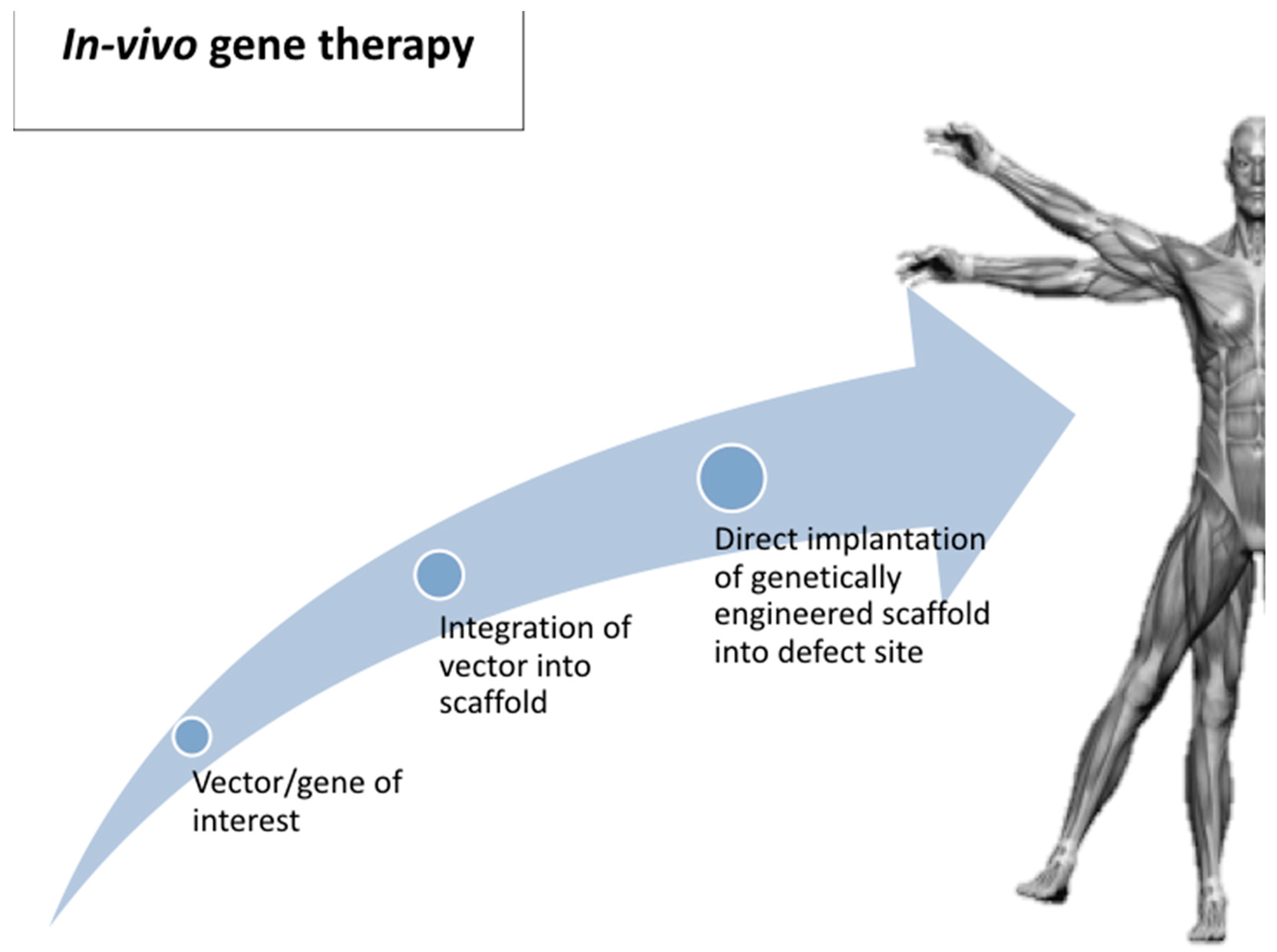
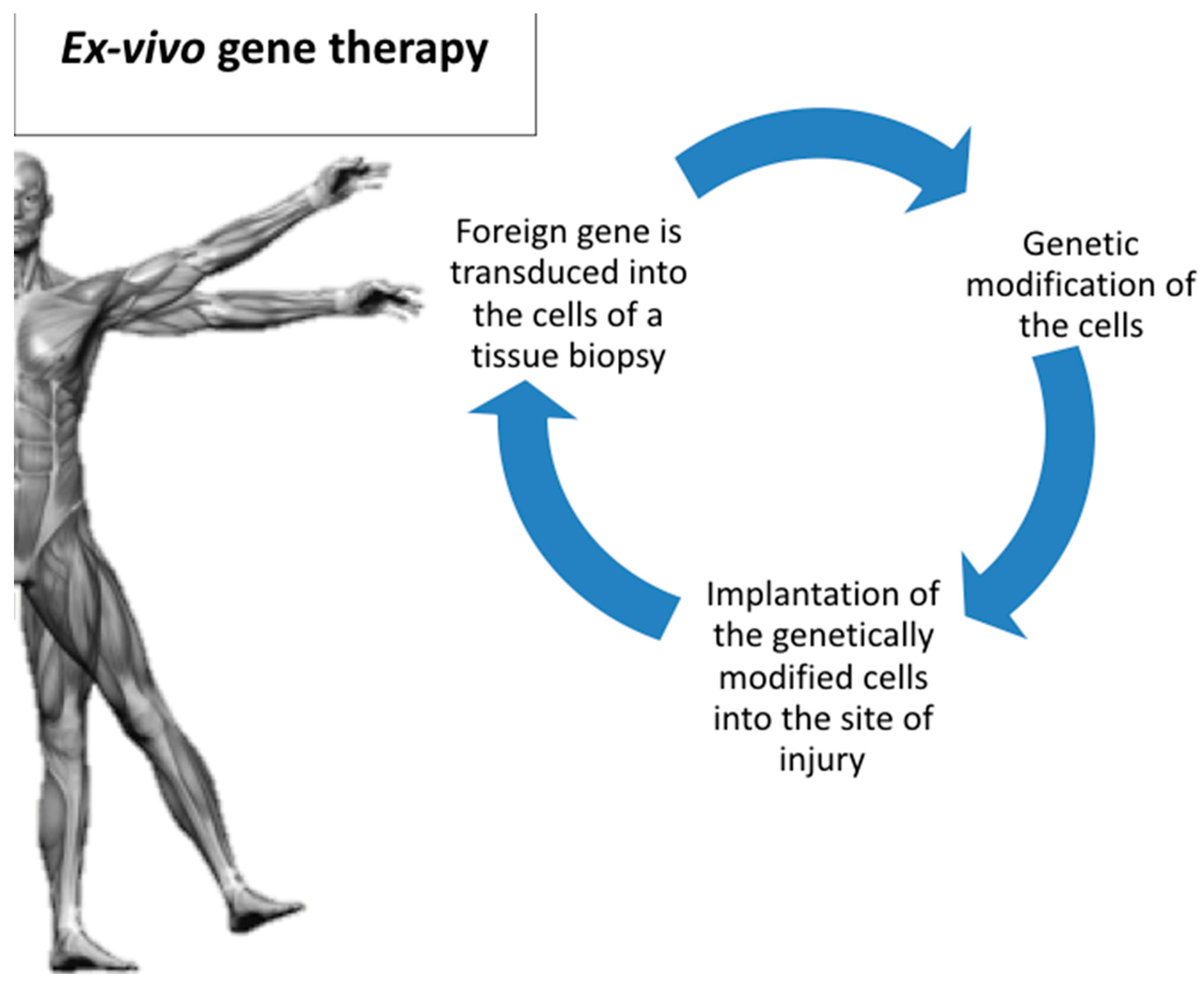
4. Route of Gene Delivery: Ex Vivo Versus In Vivo
5. Gene Therapy Applications for Periodontal Tissue Engineering
5.1. Adenovirus Vectors (AV)
5.2. AAV (Adeno-Associated Viral Vectors)
5.3. rAds (Recombinant Adeno-Viral) Vectors
5.4. Lentiviral Vectors
5.5. Non-Viral Vectors
5.6. The Gene-Activated Matrix (GAM)
5.7. Bubble Liposomes and Ultrasound for Gene Delivery
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, Z.; Chen, X.; Zhang, Z.; Zhang, X.; Saunders, L.; Zhou, Y.; Ma, P.X. Nanofibrous Spongy Microspheres to Distinctly Release miRNA and Growth Factors to Enrich Regulatory T Cells and Rescue Periodontal Bone Loss. ACS. Nano. 2018, 12, 9785–9799. [Google Scholar] [CrossRef] [PubMed]
- Ramseier, C.A.; Abramson, Z.R.; Jin, Q.; Giannobile, W.V. Gene Therapeutics for Periodontal Regenerative Medicine. Dent. Clin. N. Am. 2006, 50, 245–263. [Google Scholar] [CrossRef]
- Elangovan, S.; Karimbux, N. Review Paper: DNA Delivery Strategies to Promote Periodontal Regeneration. J. Biomater. Appl. 2010, 25, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L. Current Concepts of Epigenetics and Its Role in Periodontitis. Curr. Oral Health Rep. 2017, 4, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Colombo, A.P.V.; Tanner, A.C.R. The Role of Bacterial Biofilms in Dental Caries and Periodontal and Peri-implant Diseases: A Historical Perspective. J. Dent. Res. 2019, 98, 373–385. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, K.H.; Gwak, E.H.; Rhee, S.H.; Lee, J.C.; Shin, S.Y.; Koo, K.T.; Lee, Y.M.; Seol, Y.J. Ex vivo bone morphogenetic protein 2 gene delivery using periodontal ligament stem cells for enhanced re-osseointegration in the regenerative treatment of peri-implantitis. J. Biomed. Mater. Res. Part A. 2015, 103, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Taba, M., Jr.; Jin, Q.; Sugai, J.V.; Giannobile, W.V. Current concepts in periodontal bioengineering. Orthod. Craniofac. Res. 2005, 8, 292–302. [Google Scholar] [CrossRef]
- Chen, Y.L.; Chen, P.K.; Jeng, L.B.; Huang, C.S.; Yang, L.C.; Chung, H.Y.; Chang, S.C. Periodontal regeneration using ex vivo autologous stem cells engineered to express the BMP-2 gene: An alternative to alveolaplasty. Gene Ther. 2008, 15, 1469–1477. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, K.M.; Lundvig, D.M.; Middelkoop, E.; Wagener, F.A.; Von den Hoff, J.W. Mechanical cues in orofacial tissue engineering and regenerative medicine. Wound Rep. Reg. 2015, 23, 302–311. [Google Scholar] [CrossRef]
- Mason, C.; Dunnil, P. A brief definition of regenerative medicine. Regen. Med. 2008, 3, 1–5. [Google Scholar] [CrossRef]
- Chen, F.M.; An, Y.; Zhang, R.; Zhang, M. New insights into and novel applications of release technology for periodontal reconstructive therapies. J. Control. Release 2011, 149, 92–110. [Google Scholar] [CrossRef] [PubMed]
- Abbayya, K.; Zope, S.A.; Naduwinmani, S.; Pisal, A.; Puthanakar, N. Cell- and Gene- Based therapeutics for periodontal regeneration. Int. J. Prev. Med. 2015, 6, 110. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.M.; Ma, Z.W.; Wang, Q.T.; Wu, Z.F. Gene Delivery for Periodontal Tissue Engineering: Current Knowledge—Future Possibilities. Curr. Gene Ther. 2009, 9, 248–266. [Google Scholar] [CrossRef] [PubMed]
- Kaigler, D.; Cirelli, J.A.; Giannobile, W.V. Growth factor delivery for oral and periodontal tissue engineering. Expert Opin. Drug Deliv. 2006, 3, 647–662. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Cheng, K.C.; Kruger, L.G.; Larsson, L.; Sugai, J.V.; Lahann, J.; Giannobile, W.V. Multigrowth Factor Delivery via Immobilization of Gene Therapy Vectors. Adv. Mater. 2016, 16, 3145–3151. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Kolk, A.; Wolfart, S.; Pautke, C.; Warnke, P.H.; Plank, C.; Smeets, R. Future of local bone regeneration—Protein versus gene therapy. J. Craniomaxillofac. Surg. 2011, 39, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Bartold, P.M.; Narayanan, A.S. Molecular and cell biology of healthy and diseased periodontal tissues. Periodontol. 2000 2006, 40, 29–49. [Google Scholar] [CrossRef] [PubMed]
- Polimeni, G.; Xiropaidis, A.V.; Wikesjö, U.M. Biology and principles of periodontal wound healing/regeneration. Periodontol. 2000 2006, 41, 30–47. [Google Scholar] [CrossRef]
- Giannobile, W.V.; Lee, C.S.; Tomala, M.P.; Tejeda, K.M.; Zhu, Z. Platelet-Derived Growth Factor (PDGF) Gene Delivery for Application in Periodontal Tissue Engineering. J. Periodontol. 2001, 72, 815–823. [Google Scholar] [CrossRef]
- Chang, P.C.; Seol, Y.J.; Cirelli, J.A.; Pellegrini, G.; Jin, Q.; Franco, L.M.; Goldstein, S.A.; Chandler, L.A.; Barbara Sosnowski, B.; Giannobile, W.V. PDGF-B Gene Therapy Accelerates Bone Engineering and Oral Implant Osseointegration. Gene Ther. 2010, 17, 95–104. [Google Scholar] [CrossRef][Green Version]
- Prabhakar, A.R.; Paul, J.M.; Basappa, N. Gene Therapy and its Implications in Dentistry. Int. J. Clin. Pediatr. Dent. 2011, 4, 85–92. [Google Scholar] [CrossRef]
- Wirth, T.; Parker, N.; Ylä-Herttuala, S. History of gene therapy. Gene 2013, 525, 162–169. [Google Scholar] [CrossRef]
- Wegman, F.; Oner, F.C.; Dhert, W.J.; Alblas, J. Non-viral gene therapy for bone tissue engineering. Biotechnol. Genet. Eng. Rev. 2013, 29, 206–220. [Google Scholar] [CrossRef]
- Ramseier, C.A.; Rasperini, G.; Batia, S.; Giannobile, W.V. Advanced regenerative technologies for periodontal tissue repair. Periodontol. 2000 2012, 59, 185–202. [Google Scholar] [CrossRef]
- Amuk, N.G.; Kurt, G.; Baran, Y.; Seyrantepe, V.; Yandim, M.K.; Adan, A.; Demir, S.A.; Kiraz, Y.; Sonmez, M.F. Effects of cell-mediated osteoprotegerin gene transfer and mesenchymal stem cell applications on orthodontically induced root resorption of rat teeth. Eur. J. Orthod. 2017, 39, 235–242. [Google Scholar] [CrossRef]
- Elangovan, S.; Kormann, M.S.; Khorsand, B.; Salem, A.K. The Oral and Craniofacial Relevance of Chemically Modified RNA Therapeutics. Discov. Med. 2016, 21, 35–39. [Google Scholar]
- Evans, C. Gene therapy for the regeneration of bone. Injury 2011, 42, 599–604. [Google Scholar] [CrossRef][Green Version]
- Evans, C. Gene delivery to bone. Adv. Drug Deliv. Rev. 2012, 64, 1331–1340. [Google Scholar] [CrossRef]
- Phillips, J.E.; Gersbach, C.A.; Garcia, A.J. Virus-based gene therapy strategies for bone regeneration. Biomaterials 2007, 28, 211–229. [Google Scholar] [CrossRef]
- Kaestner, L.; Scholz, A.; Lipp, P. Conceptual and technical aspects of transfection and gene delivery. Bioorg. Med. Chem. Lett. 2015, 25, 1171–1176. [Google Scholar] [CrossRef]
- Elangovan, S.; Jain, S.; Tsai, P.C.; Margolis, H.C.; Amiji, M. Nano-Sized Calcium Phosphate Particles for Periodontal Gene Therapy. J. Periodontol. 2013, 84, 117–125. [Google Scholar] [CrossRef]
- Gossen, M.; Bujard, H. Studying gene function in eukaryotes by conditional gene inactivation. Annu. Rev. Genet. 2002, 36, 153–173. [Google Scholar] [CrossRef]
- Pan, B.T.; Johnstone, R.M. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: Selective externalization of the receptor. Cell 1983, 33, 967–978. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Mathiyalagan, P.; Sahoo, S. Exosomes-Based GeneTherapyfor MicroRNA Delivery. Methods Mol. Biol. 2017, 1521, 139–152. [Google Scholar]
- Thery, C.; Ostrowski, M.; Segura, E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009, 9, 581–593. [Google Scholar] [CrossRef]
- Parolini, I.; Federici, C.; Raggi, C.; Lugini, L.; Palleschi, S.; De Milito, A.; Coscia, C.; Iessi, E.; Logozzi, M.; Molinari, A.; et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J. Biol. Chem. 2009, 284, 34211–34222. [Google Scholar] [CrossRef]
- Jin, Q.M.; Anusaksathien, O.; Webb, S.A.; Rutherford, R.B.; Giannobile, W.V. Gene Therapy of Bone Morphogenetic Protein for Periodontal Tissue Engineering. J. Periodontol. 2003, 74, 202–213. [Google Scholar] [CrossRef]
- Jin, Q.M.; Zhao, M.; Economides, A.N.; Somerman, M.J.; Giannobile, W.V. Noggin Gene Delivery Inhibits Cementoblast-Induced Mineralization. Connect Tissue Res. 2004, 45, 50–59. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, J.; Shi, B.; Wang, Y.; Chen, X.; Huang, C.; Yang, X.; Xu, D.; Cheng, X.; Chen, X. Combination of scaffold and adenovirus vectors expressing bone morphogenetic protein-7 for alveolar bone regeneration at implant defects. Biomaterials 2007, 28, 4635–4642. [Google Scholar] [CrossRef]
- Anusaksathien, O.; Webb, S.A.; Jin, Q.M.; Giannobile, W.V. Platelet-derived growth factor gene delivery stimulates ex vivo gingival repair. Tissue Eng. 2003, 9, 745–756. [Google Scholar] [CrossRef]
- Anusaksathien, O.; Jin, Q.; Zhao, M.; Somerman, M.J.; Giannobile, W.V. Effect of sustained gene delivery of platelet-derived growth factor or its antagonist (PDGF-1308) on tissue-engineered cementum. J. Periodontol. 2004, 75, 429–440. [Google Scholar] [CrossRef]
- Lin, Z.; Sugai, J.V.; Jin, Q.; Chandler, L.A.; Giannobile, W.V. Platelet-derived growth factor-B gene delivery sustains gingival fibroblast signal transduction. J. Periodontal Res. 2008, 43, 440–449. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, Z.; Xie, Y.; Hu, J.; Wang, H.; Fan, Z.; Zhang, C.; Wang, J.; Wu, C.T.; Wang, S. Adenovirus-mediated transfer of hepatocyte growth factor gene to human dental pulp stem cells under good manufacturing practice improves their potential for periodontal regeneration in swine. Stem Cell Res. Ther. 2015, 6, 249. [Google Scholar] [CrossRef]
- Warrington, K.H., Jr.; Herzog, R.W. Treatment of human disease by adeno-associated viral gene transfer. Hum. Genet. 2006, 119, 571–603. [Google Scholar] [CrossRef]
- Cirelli, J.A.; Park, C.H.; MacKool, K.; Taba, M., Jr.; Lustig, K.H.; Burstein, H.; Giannobile, W.V. AAV2/1-TNFR:Fc gene delivery prevents periodontal disease progression. Gene Ther. 2009, 16, 426–436. [Google Scholar] [CrossRef]
- Jiang, H.; Chen, W.; Zhu, G.; Zhang, L.; Tucker, B.; Hao, L.; Feng, S.; Ci, H.; Ma, J.; Wang, L.; et al. RNAi-Mediated Silencing of Atp6i and Atp6i Haplo insufficiency Prevents Both Bone Loss and Inflammation in a Mouse Model of Periodontal Disease. PLoS ONE 2013, 8, e58599. [Google Scholar]
- Chen, W.; Gao, B.; Hao, L.; Zhu, G.; Jules, J.; MacDougall, M.J.; Wang, J.; Han, X.; Zhou, X.; Li, Y.P. The silencing of cathepsin K used in gene therapy for periodontal disease reveals the role of cathepsin K in chronic infection and inflammation. J. Periodontal Res. 2016, 51, 647–660. [Google Scholar] [CrossRef]
- Jiang, S.; Tang, K.; Chen, B.; Yan, F. Regenerative effect of hOPG gene-modified autologous PDLs in combination with cell transplantation on periodontal defection in beagle dogs. Cytotechnology 2016, 68, 2613–2623. [Google Scholar] [CrossRef][Green Version]
- Zhu, Z.; Lee, C.S.; Tejeda, K.M.; Giannobile, W.V. Gene Transfer and Expression of Platelet-derived Growth Factors Modulate Periodontal Cellular Activity. J. Dent. Res. 2001, 80, 892–897. [Google Scholar] [CrossRef]
- Dunn, C.A.; Jin, Q.; Taba, M., Jr.; Franceschi, R.T.; Bruce Rutherford, R.; Giannobile, W.V. BMP gene delivery for alveolar bone engineering at dental implant defects. Mol. Ther. 2005, 11, 294–299. [Google Scholar] [CrossRef]
- Xiang, L.; Ma, L.; He, Y.; Wei, N.; Gong, P. Transfection with follicular dendritic cell secreted protein to affect phenotype expression of human periodontal ligament cells. J. Cell. Biochem. 2014, 115, 940–948. [Google Scholar] [CrossRef]
- Zhu, S.Y.; Wang, P.L.; Liao, C.S.; Yang, Y.Q.; Yuan, C.Y.; Wang, S.; Dissanayaka, W.L.; Heng, B.C.; Zhang, C.F. Transgenic expression of ephrinB2 in periodontal ligament stem cells (PDLSCs) modulates osteogenic differentiation via signaling crosstalk between ephrinB2 and EphB4 in PDLSCs and between PDLSCs and preosteoblasts within co-culture. J. Periodontal Res. 2017, 52, 562–573. [Google Scholar] [CrossRef]
- Logan, A.C.; Lutzko, C.; Kohn, D.B. Advances in lentiviral vector design for gene-modification of hematopoietic stem cells. Curr. Opin. Biotechnol. 2002, 13, 429–436. [Google Scholar] [CrossRef]
- Wang, Y.; Shang, S.; Li, C. Comparison of different kinds of non-viral vectors for gene delivery to human periodontal ligament stem cells. J. Dent. Sci. 2015, 10, 414–422. [Google Scholar] [CrossRef]
- Peng, L.; Cheng, X.; Zhuo, R.; Lan, J.; Wang, Y.; Shi, B.; Li, S. Novel gene-activated matrix with embedded chitosan/plasmid DNA nanoparticles encoding PDGF for periodontal tissue engineering. J. Biomed. Mater. Res. Part A. 2009, 90, 564–576. [Google Scholar] [CrossRef]
- Chang, P.C.; Cirelli, J.A.; Jin, Q.; Seol, Y.J.; Sugai, J.V.; D’Silva, N.J.; Danciu, T.E.; Chandler, L.A.; Sosnowski, B.A.; Giannobile, W.V. Adenovirus encoding human platelet-derived growth factor-B delivered to alveolar bone defects exhibits safety and biodistribution profiles favorable for clinical use. Hum. Gene Ther. 2009, 20, 486–496. [Google Scholar] [CrossRef]
- Yang, C.; Liu, Y.; Li, C.; Zhang, B. Repair of mandibular defects by bone marrow stromal cells expressing the basic fibroblast growth factor transgene combined with multi-pore mineralized Bio-Oss. Mol. Med. Rep. 2013, 7, 99–104. [Google Scholar] [CrossRef]
- Plonka, A.B.; Khorsand, B.; Yu, N.; Sugai, J.V.; Salem, A.K.; Giannobile, W.V.; Elangovan, S. Effect of Sustained PDGF Non-Viral Gene Delivery on Repair of Tooth-Supporting Bone Defects. Gene Ther. 2017, 24, 31–39. [Google Scholar] [CrossRef]
- Sugano, M.; Negishi, Y.; Endo-Takahashi, Y.; Hamano, N.; Usui, M.; Suzuki, R.; Maruyama, K.; Aramaki, Y.; Yamamoto, M. Gene delivery to periodontal tissue using Bubble liposomes and ultrasound. J. Periodonal Res. 2014, 49, 398–404. [Google Scholar] [CrossRef]
- Asa’ad, F.; Pagni, G.; Pilipchuk, S.P.; Giannì, A.B.; Giannobile, W.V.; Rasperini, G. 3D-Printed Scaffolds and Biomaterials: Review of Alveolar Bone Augmentation and Periodontal Regeneration Applications. Int. J. Dent. 2016, 2016, 1239842. [Google Scholar] [CrossRef]
- Asa’ad, F.; Monje, A.; Larsson, L. Scaffolds in Periodontal Tissue Engineering. In Handbook of Tissue Engineering Scaffolds:Volume One; Mozafari, M., Sefat, F., Atala, A., Eds.; Elsevier: Cambridge, UK, 2019; pp. 479–496. [Google Scholar]
- Atasoy-Zeybek, A.; Kose, G.T. Gene Therapy Strategies in Bone Tissue Engineering and Current Clinical Applications. Adv. Exp. Med. Biol. 2018, 4, 85–101. [Google Scholar]
- Hacein-Bey-Abina, S.; von Kalle, C.; Schmidt, M.; Le Deist, F.; Wulffraat, N.; McIntyre, E.; Radford, I.; Villeval, J.L.; Fraser, C.C.; Cavazzana-Calvo, M.; et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N. Engl. J. Med. 2003, 348, 255–256. [Google Scholar] [CrossRef]
- Luo, D.; Saltzman, W.M. Synthetic DNA delivery systems. Nat. Biotechnol. 2000, 18, 33–37. [Google Scholar] [CrossRef]
| 1909- Wilhelm Johannsen introduced the term “gene”. |
| 1928- Griffith performed the first experiment suggesting that bacteria are capable of transferring genetic information through a process known as “transformation”. |
| 1952- Zinder and Lederberg introduced the term “transduction” as a mechanism of genetic transfer. |
| 1968- Rogers and Pfuderer demonstrated a proof of concept for virus-mediated gene transfer. |
| 1972- Fiedman and Roblin suggested gene therapy for genetic diseases. |
| 1988- The first officially approved clinical protocol to introduce a foreign gene into humans was approved by the Recombinant DNA Advisory Committee (RAC). |
| 1990- FDA approved the first time a gene therapy trial with a therapeutic attempt in humans. |
| 1999- FDA restricted all clinical trials using gene therapy for nearly a decade because of the outcomes of the first gene-therapy-based clinical trial on ADASCID (adenosine deaminase: severe combined immune deficiency) as patients eventually developed leukemia (4/10). |
| 2003- China became the first country to approve a gene therapy based product for clinical use. |
| 2012- EMA (The European Medicines Agency) recommended for the first time a gene therapy product (Glybera) for approval in the European Union. |
| December 2017- First FDA approval of an in vivo gene-therapy product, for Luxturna from Spark Therapeutics. |
| August 2018- US National Institutes of Health advisory committee declared “gene therapies on recombinant DNA”no longer need to be reviewed before clinic studies. |
| Virus Characteristics | Retrovirus | Adenovirus | Adeno-Associated Virus | Lentivirus (HIV-1) |
|---|---|---|---|---|
| Viral genome | RNA | dsDNA | ssDNA | RNA |
| Immune response | Low | High | Moderate | Low |
| Transgene expression | Constitutitve | Transient | Constitutive/transient | Constitutive |
| Immunogenicity | Low | High | Moderate | Moderate |
| Potential pathogenicity | Low | Low | None | Low |
| Insertional mutagenesis | Yes | No | Yes/no | No |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goker, F.; Larsson, L.; Del Fabbro, M.; Asa’ad, F. Gene Delivery Therapeutics in the Treatment of Periodontitis and Peri-Implantitis: A State of the Art Review. Int. J. Mol. Sci. 2019, 20, 3551. https://doi.org/10.3390/ijms20143551
Goker F, Larsson L, Del Fabbro M, Asa’ad F. Gene Delivery Therapeutics in the Treatment of Periodontitis and Peri-Implantitis: A State of the Art Review. International Journal of Molecular Sciences. 2019; 20(14):3551. https://doi.org/10.3390/ijms20143551
Chicago/Turabian StyleGoker, Funda, Lena Larsson, Massimo Del Fabbro, and Farah Asa’ad. 2019. "Gene Delivery Therapeutics in the Treatment of Periodontitis and Peri-Implantitis: A State of the Art Review" International Journal of Molecular Sciences 20, no. 14: 3551. https://doi.org/10.3390/ijms20143551
APA StyleGoker, F., Larsson, L., Del Fabbro, M., & Asa’ad, F. (2019). Gene Delivery Therapeutics in the Treatment of Periodontitis and Peri-Implantitis: A State of the Art Review. International Journal of Molecular Sciences, 20(14), 3551. https://doi.org/10.3390/ijms20143551







