Integrated Systems Pharmacology, Urinary Metabonomics, and Quantitative Real-Time PCR Analysis to Uncover Targets and Metabolic Pathways of the You-Gui Pill in Treating Kidney-Yang Deficiency Syndrome
Abstract
:1. Introduction
2. Results
2.1. Biochemical Parameters and Histopathological Analysis
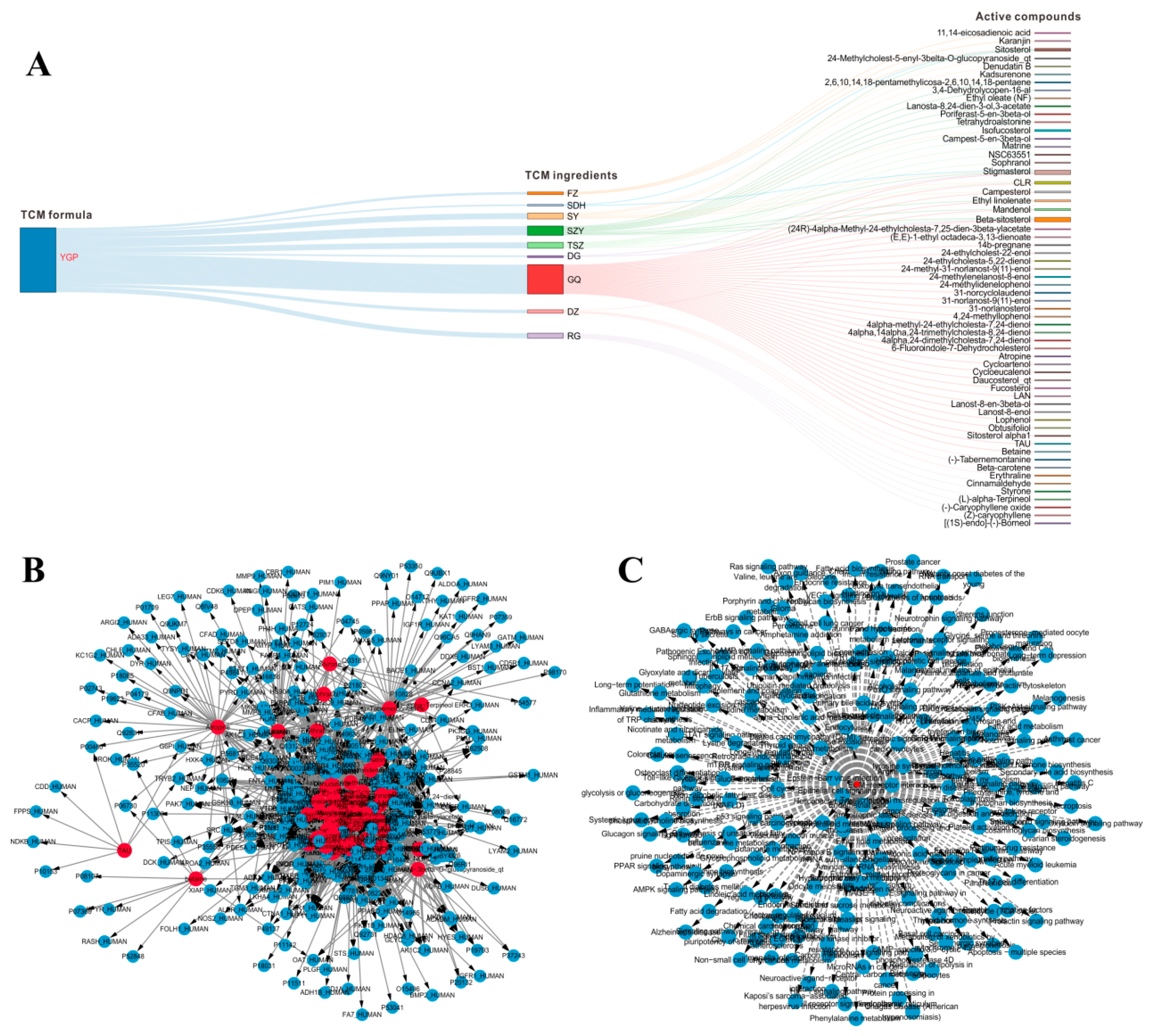
2.2. Systems Pharmacology Analysis
2.3. 1H NMR Spectra of Urine Sample
2.4. Multivariate Statistical Analysis
2.5. Metabolic Pathway Analysis
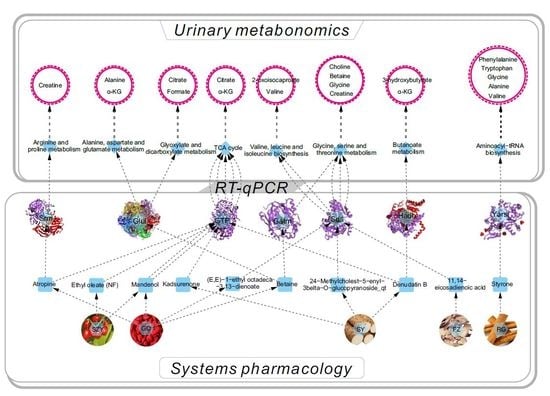
2.6. YGP Regulates KYDS-Related Differential Gene Expression
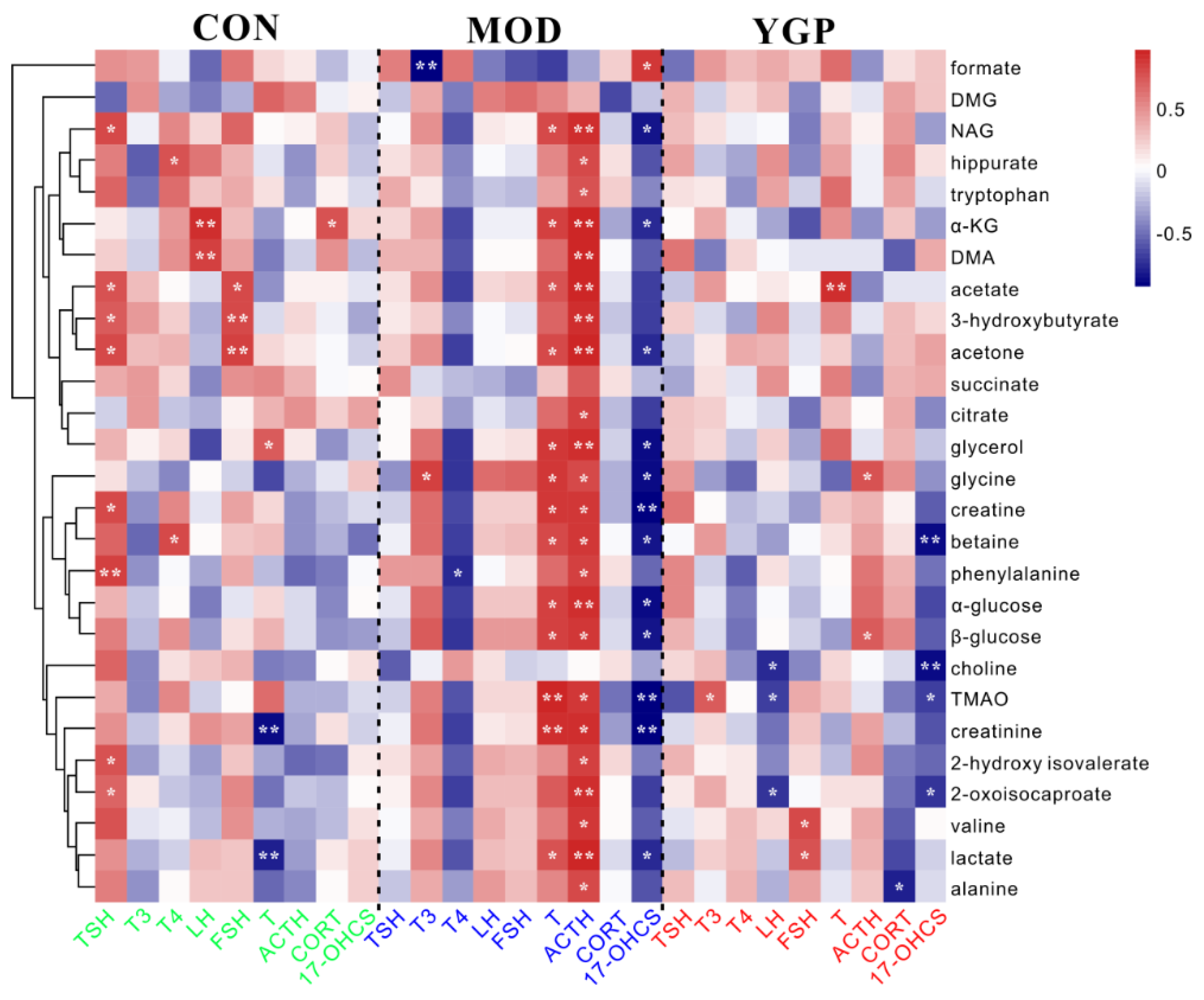
2.7. Correlation Analysis between Differential Metabolites and Biochemical Parameters
2.8. Overall Interactive Network Diagram of Herbs, Active Compounds, Targets, Pathways, and Metabolites
3. Discussion
3.1. Energy Metabolism
3.2. Oxidative Stress
3.3. Ammonia Metabolism
3.4. Amino Acid Metabolism
3.5. Fatty Acid Metabolism
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Systems Pharmacology
4.3. Preparation of YGP Solution
4.4. Animal Care and Experiments
4.5. Sample Collections
4.6. Urinary Metabonomics
4.7. Integrated Systems Pharmacology and Urinary Metabonomics Analysis
4.8. RNA Extraction and RT-qPCR Analysis
4.9. Pearson’s Correlation Analysis of 27 Metabolites and Nine Biochemical Indicators
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Unschuld, P.U. Huang Di Nei Jing Su Wen: Nature, Knowledge, Imagery in an Ancient Chinese Medical Text: With an appendix: The Doctrine of the Five Periods and Six Qi in the Huang Di Nei Jing Su Wen; University of California Press: Oakland, CA, USA, 2003; p. 187. [Google Scholar] [CrossRef]
- Zhao, L.; Wu, H.; Qiu, M.; Sun, W.; Wei, R.; Zheng, X.; Yang, Y.; Xin, X.; Zou, H.; Chen, T.; et al. Metabolic signatures of Kidney yang deficiency syndrome and protective effects of two herbal extracts in rats using GC/TOFMS. Evid.-Based Complement. Altern. Med. 2013, 2013, 540957. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Xiong, Z.; Li, J.; Zheng, S.; Huo, T.; Li, F. Metabonomic study on ‘Kidney-Yang Deficiency syndrome’ and intervention effects of Rhizoma Drynariae extracts in rats using ultra performance liquid chromatography coupled with masss pectrometry. Talanta 2011, 83, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Gang, G.; Yang, G.J. Metabonomic study on urine of rats with hydrocortisone-induced kidney deficiency syndrome. Acad. J. Second Mil. Med Univ. 2009, 29, 565–568. [Google Scholar]
- Zou, Z.J.; Gong, M.J.; Xie, Y.Y.; Wang, S.M.; Liang, S.W. Urinary metabonomic study of kidney-yang deficiency syndrome induced by hydrocortisone. Chin. J. Exp. Tradit. Med. Formulae 2012, 18, 133–136. [Google Scholar]
- Wang, L.; Cao, A.L.; Chi, Y.F.; Ju, Z.C.; Yin, P.H.; Zhang, X.M.; Peng, W. You-gui Pill ameliorates renal tubulointerstitial fibrosis via inhibition of TGF-beta/Smad signaling pathway. J. Ethnopharmacol. 2015, 169, 229–238. [Google Scholar] [CrossRef]
- Chen, R.; Wang, J.; Liao, C.; Zhang, L.; Guo, Q.; Wang, X. Exploring the biomarkers and therapeutic mechanism of kidney-yang deficiency syndrome treated by You-gui pill using systems pharmacology and serum metabonomics. RSC Adv. 2018, 8, 1098–1115. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Wang, P.E.; Ying, J.; Jin, X.; Luo, C.; Xu, T.; Xu, S.; Dong, R.; Xiao, L.; Tong, P.; et al. Yougui pills attenuate cartilage degeneration via activation of TGF-beta/Smad Signaling in chondrocyte of osteoarthritic mouse model. Front. Pharm. 2017, 8, 611. [Google Scholar] [CrossRef]
- Liu, H.; Qiu, F.; Bian, B.; Yang, X.; Zou, H.; Zhao, H.; Chen, Z.; Wang, Q.; Zhao, H.; Wang, L. Integrating qualitative and quantitative assessments of Yougui pill, an effective traditional Chinese medicine, by HPLC-LTQ-Orbitrap-MSn and UPLC-QqQ-MS/MS. Anal. Methods 2017, 9, 3485–3496. [Google Scholar] [CrossRef]
- Chen, R.; Wang, J.; Liao, C.; Ma, N.; Zhang, L.; Wang, X. 1H NMR studies on serum metabonomic changes over time in a kidney-Yang deficiency syndrome model. RSC Adv. 2017, 7, 34251–34261. [Google Scholar] [CrossRef]
- Chen, M.; Zhao, L.; Jia, W. Metabonomic study on the biochemical profiles of a hydrocortisone-induced animal model. J. Proteome Res. 2005, 4, 2391–2396. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, A.; Wang, L.; Yan, G.; Zhao, H.; Sun, H.; Zou, S.; Han, J.; Ma, C.W.; Kong, L.; et al. High-throughput chinmedomics-based prediction of effective components and targets from herbal medicine AS1350. Sci. Rep. 2016, 6, 38437. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Yang, J.; Lu, X.; Deng, Y.; Xiong, Z.; Li, F. An integrated plasma and urinary metabonomic study using UHPLC-MS: Intervention effects of Epimedium koreanum on ‘Kidney-Yang Deficiency syndrome’ rats. J. Pharm. Biomed. Anal. 2013, 76, 200–206. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, Q.; Zhao, H.; Zhou, X.; Sun, H.; Nan, Y.; Zou, S.; Ma, C.W.; Wang, X. Phenotypic characterization of nanshi oral liquid alters metabolic signatures during disease prevention. Sci. Rep. 2016, 6, 19333. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Zhou, X.; Zhao, H.; Zou, S.; Ma, C.W.; Liu, Q.; Sun, H.; Liu, L.; Wang, X. Metabolomics and proteomics technologies to explore the herbal preparation affecting metabolic disorders using high resolution masss pectrometry. Mol. Biosyst. 2017, 13, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Nan, Y.; Zhou, X.; Liu, Q.; Zhang, A.; Guan, Y.; Lin, S.; Kong, L.; Han, Y.; Wang, X. Serum metabolomics strategy for understanding pharmacological effects of ShenQi pill acting on kidney yang deficiency syndrome. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1026, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hu, C.; Dai, J.; Chen, L. Metabolomics analysis of seminal plasma in infertile males with kidney-yang deficiency: A preliminary study. Evid.-Based Complement. Altern. Med. 2015, 2015, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.Z.; Geng, L.; Zhou, H.B.; Wei, H.C.; Chen, H.D. Chinese herbal medicine Yougui Pill reduces exogenous glucocorticoid-induced apoptosis in anterior pituitary cells. Neural Regen. Res. 2016, 11, 1962–1968. [Google Scholar] [CrossRef]
- Zhou, X.H.; Zhang, A.H.; Wang, L.; Tan, Y.L.; Guan, Y.; Han, Y.; Sun, H.; Wang, X.J. Novel chinmedomics strategy for discovering effective constituents from ShenQiWan acting on ShenYangXu syndrome. Chin. J. Nat. Med. 2016, 14, 561–581. [Google Scholar] [CrossRef]
- Dong, H.; Chen, Z.; Huang, Y. Changes of LPO contents and SOD and ATPase activities of erythrocytic membranes in patients with syndrome of kidney yang deficiency. Chin. J. Microcirc. 2002, 12, 10–11. [Google Scholar]
- Chen, Y.; Li, X.; Sun, S.; Wang, M.; Wang, X.; Jiang, B.; Yu, L.; Yu, B. Effect of Yougui pill on rats with renal interstitial fibrosis. Tradit. Chin. Drug Res. Clin. Pharmacol. 2016, 27, 23–27. [Google Scholar]
- Jia, X.; Qu, Z.; Yao, K.; Lu, X. Mechanism of Yougui pills in ameliorating the free radical peroxidation injury in Vivo in rats with hypothyroidism. Tianjin J. Tradit. Chin. Med. 2008, 25, 225–228. [Google Scholar]
- Zhao, K.; Xue, P.F.; Tu, P.F. Research progress of chemical constituents and pharmacological activities of cinnamon (cinnamomumcassia). J. Inn. Mong. Med. Univ. 2013, 35, 63–74. [Google Scholar]
- Zhao, K.; Jiang, Y.; Xue, P.F.; Tu, P.F. Chemical constituents from barks of Cinnamomum cassia growing in China. Chin. Tradit. Herb. Drugs 2013, 44, 2358–2363. [Google Scholar]
- Luo, L.; Zhen, L.; Xu, Y.; Yang, Y.; Feng, S.; Wang, S.; Liang, S. (1)HNMR-based metabonomics revealed protective effect of Naodesheng bioactive extract on ischemics trokerats. J. Ethnopharmacol. 2016, 186, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zheng, L.; Luo, R.; Zhao, X.; Han, Z.; Wang, Y.; Yang, Y. A 1H NMR-based metabonomic investigation of time-dependent metabolic trajectories in a high salt-induced hypertension rat model. RSC Adv. 2015, 5, 281–290. [Google Scholar] [CrossRef]
- Pan, S.; Chen, A.; Han, Z.; Wang, Y.; Lu, X.; Yang, Y. (1)H NMR-based metabonomic study on the effects of Epimedium on glucocorticoid-induced osteoporosis. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1038, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Liao, P.; Wu, H.; Li, X.; Pei, F.; Li, W.; Wu, Y. Metabolic profiling studies on the toxicological effects of realgar in rats by(1)H NMR spectroscopy. Toxicol. Appl. Pharm. 2009, 234, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.Y.; Yang, K.M.; Yang, L.; Miao, Z.X.; Wang, Y.H.; Zhu, H.B. A (1)H NMR-Based metabonomic investigation of time-related metabolic trajectories of the plasma, urine and liver extracts of hyperlipidemic hamsters. PLoS ONE 2013, 8, e66786. [Google Scholar] [CrossRef]
- Xu, W.; Wang, H.; Chen, G.; Li, W.; Xiang, R.; Pei, Y. (1)H NMR-based metabonomics study on the toxicity alleviation effect of other traditional Chinese medicines in NiuhuangJiedu tablet to realgar (As2S2). J. Ethnopharmacol. 2013, 148, 88–98. [Google Scholar] [CrossRef]
- Saric, J.; Li, J.V.; Wang, Y.L.; Keiser, J.; Bundy, J.G.; Holmes, E.; Utzinger, J. Metabolic profiling of an Echinostoma caproni infection in the mouse for biomarker discovery. PLoS Negl. Trop. Dis. 2008, 2, e254. [Google Scholar] [CrossRef]
- Li, Y.S.; Wang, Q.; Yuan, Z.J. NMR-based metabonomics studies on serum and urine of yang-deficiency constitution. Chem. J. Chin. Univ. 2011, 32, 2521–2527. [Google Scholar]
- Sun, L.; Bartlam, M.; Liu, Y.; Pang, H.; Rao, Z. Crystal structure of the pyridoxal-5’-phosphate-dependent serine dehydratase from human liver. Protein Sci. 2005, 14, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Li, C.S.; Zhang, G.X.; Shi, T.R.; Dong, X.X.; Sun, P.; Li, D.D.; Xu, H.Z.; Chen, K.J. Experimental study on effect of the water extract of dogwood fruits on the liver and testis in rats model of kidney-yang deficiency. China J. Chin. Mater. Med. 2003, 28, 743–746. [Google Scholar]
- Lu, D.; Wo, X.; Wo, L.; Li, Y.; Tang, L.; Yang, Z. Effects of warm and tonify kidney-yang herbs on liver mitochondria proteome of kidney-yang deficiency rats. Zhongguo Zhong Yao Za Zhi 2009, 34, 1251–1256. [Google Scholar] [PubMed]
- Tan, Y.; Lv, C.; Zhao, H.Y. Influence of white prepared lateral root of aconite on blood biochemical indicators in normal and kidney Yang deficientrats. Chin. J. Inf. Tradit. Chin. Med. 2010, 17, 31–33. [Google Scholar]
- Tan, Y.; Liu, X.; Lu, C.; He, X.; Li, J.; Xiao, C.; Jiang, M.; Yang, J.; Zhou, K.; Zhang, Z.; et al. Metabolic profiling reveals therapeutic biomarkers of processed Aconitum carmichaeli Debx in treating hydrocortisone induced kidney-yang deficiency syndromerats. J. Ethnopharmacol. 2014, 152, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Humm, A.; Fritsche, E.; Steinbacher, S.; Huber, R. Crystal structure and mechanism of human L-arginine: Glycine amidinotransferase: A mitochondrial enzyme involved in creatine biosynthesis. EMBO J. 1997, 16, 3373–3385. [Google Scholar] [CrossRef]
- Farshidfar, F.; Pinder, M.A.; Myrie, S.B. Creatine supplementation and skeletal muscle metabolism for building muscle mass-review of the potential mechanisms of action. Curr. Protein Pept. Sci. 2017, 18, 1273–1287. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, X.; Dai, W.; Lv, Y.; Yan, S.; Zhang, W. A combined HPLC-PDA and HPLC-MS method for quantitative and qualitative analysis of 10 major constituents in the traditional Chinese medicine Zuo Gui Wan. J. Pharm. Biomed. Anal. 2009, 49, 931–936. [Google Scholar] [CrossRef]
- Fons, C.; Campistol, J. Creatine defects and central nervous system. Semin. Pediatr. Neurol. 2016, 23, 285–289. [Google Scholar] [CrossRef]
- Fan, Y.P.; Song, L.J.; Gong, H.Y.; Wang, L.; Ye, M.; Zhou, L. Effect of Zuogui Pills and Yougui Pills on expression of IFN-γ and MMP-9 in central nervous system of EAE rats. China J. Tradit. Chin. Med. Pharm. 2009, 24, 1446–1450. [Google Scholar]
- Ji, X.; Liu, H.; An, C.; Wang, Y.; Zhao, H.; Zhang, Q.; Li, M.; Qi, F.; Chen, Z.; Wang, X.; et al. You-Gui pills promote nerve regeneration by regulating netrin1, DCC and Rho family GTPases RhoA, Racl, Cdc42 in C57BL/6 mice with experimental autoimmune encephalomyelitis. J. Ethnopharmacol. 2016, 187, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Kou, S.; Zheng, Q.; Wang, Y.; Zhao, H.; Zhang, Q.; Li, M.; Qi, F.; Fang, L.; Liu, L.; Ouyang, J.; et al. Zuo-Gui and You-Gui pills, two traditional Chinese herbal formulas, downregulated the expression of NogoA, NgR, and RhoA in rats with experimental autoimmune encephalomyelitis. J. Ethnopharmacol. 2014, 158, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhao, C.; Zhang, A.; Zhang, J.; Wang, X.; Sun, X.; Sun, Z.; Wang, X. High-throughput metabolomics used to identify potential therapeutic targets of Guizhi Fuling Wan against endometriosis of cold coagulation and blood stasis. RSC Adv. 2018, 8, 19238–19250. [Google Scholar] [CrossRef] [Green Version]
- Rider, J.E.; Hacker, A.; Mackintosh, C.A.; Pegg, A.E.; Woster, P.M.; Casero, R.A. Spermine and spermidine mediate protection against oxidative damage caused by hydrogen peroxide. Amino Acids 2007, 33, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.H.; Yie, S.M.; Zhen, X.; Den, Y.L.; Liang, X.; Hu, X.; Li, L.M.; Li, Q.J.; Cao, S.; Lu, H. Effect of You Gui Wan on mouse sperm fertilising ability in vivo and in vitro. Andrologia 2014, 46, 283–289. [Google Scholar] [CrossRef]
- Wu, H.; Min, J.; Ikeguchi, Y.; Zeng, H.; Dong, A.; Loppnau, P.; Pegg, A.E.; Plotnikov, A.N. Structure and mechanism of spermidine synthases. Biochemistry 2007, 46, 8331–8339. [Google Scholar] [CrossRef]
- Sun, J.; Wu, X.; Bao, J.; Ouyang, G.; Zhang, P.; Xu, Q.; Ma, B.; Zhang, Q. Pharmaceutical effects and mechanisms of total flavones from Semen cuscutae onoligoasthenospermia induced by hydrocortisone in rats. West China J. Pharm. Sci. 2016, 31, 14–17. [Google Scholar]
- Yang, J.; Wang, Y.; Bao, Y.; Guo, J. The total flavones from Semen cuscutae reverse the reduction of testosterone level and the expression of and rogen receptor gene in kidney-yang deficient mice. J. Ethnopharmacol. 2008, 119, 166–171. [Google Scholar] [CrossRef]
- Lu, A.; Li, D.; Yi, J.; Huang, F.; Liu, S.; Xia, S.J. The comparative research of the relation on lipid peroxidation and antioxidation with spleen-yang deficiency and kidney-yang deficiency in rat. China J. Basic Med. Tradit. Chin. Med. 2000, 6, 15–17. [Google Scholar]
- Eisenberg, D.; Almassy, R.J.; Janson, C.A.; Chapman, M.S.; Suh, S.W.; Cascio, D.; Smith, W.W. Some evolutionary relationships of the primary biological catalysts glutamine synthetase and RuBisCO. Cold Spring Harb. Symp. Quant. Biol. 1987, 52, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Krajewski, W.W.; Collins, R.; Holmberg-Schiavone, L.; Jones, T.A.; Karlberg, T.; Mowbray, S.L. Crystal structures of mammalian glutamine synthetases illustrate substrate-induced conformational changes and provide opportunities for drug and herbicide design. J. Mol. Biol. 2008, 375, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Poon, H.F.; StClair, D.; Keller, J.N.; Pierce, W.M.; Klein, J.B.; Markesbery, W.R. Redox proteomics identification of oxidatively modified hippocampal proteins in mild cognitive impairment: Insights into the development of Alzheimer’s disease. Neurobiol. Dis. 2006, 22, 223–232. [Google Scholar] [CrossRef]
- Wei, N.; Shi, Y.; Truong, L.N.; Fisch, K.M.; Xu, T.; Gardiner, E.; Fu, G.; Hsu, Y.S.; Kishi, S.; Su, A.I.; et al. Oxidative stress diverts tRNA synthetase to nucleus for protection against DNA damage. Mol. Cell 2014, 56, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Q.; Cui, Z.J.; Wei, D.L.; Zhao, W.Q.; Zhang, L.; Liu, T.L. On the chemical components of volatile oil of Cortex Cinnamomi. Acad. J. Shanghai Univ. Tradit. Chin. Med. 2003, 17, 49–51. [Google Scholar]
- Wilcken, B.; Leung, K.C.; Hammond, J.; Kamath, R.; Leonard, J.V. Pregnancy and fetal long-chain 3-hydroxyacyl coenzyme A dehydrogenase deficiency. Lancet 1993, 341, 407–408. [Google Scholar] [CrossRef]
- Turner, N.; Cooney, G.J.; Kraegen, E.W.; Bruce, C.R. Fatty acid metabolism, energy expenditure and insulin resistance in muscle. J. Endocrinol. 2014, 220, 61–79. [Google Scholar] [CrossRef]
- Scafidi, S.; Fiskum, G.; Lindauer, S.L.; Bamford, P.; Shi, D.; Hopkins, I.; McKenna, M.C. Metabolism of acetyl-L-carnitine for energy and neurotransmitter synthesis in the immature rat brain. J. Neurochem. 2010, 114, 820–831. [Google Scholar] [CrossRef] [PubMed]
- Al-Majed, A.A.; Sayed-Ahmed, M.M.; Al-Omar, F.A.; Al-Yahya, A.A.; Aleisa, A.M.; Al-Shabanah, O.A. Carnitine esters prevent oxidative stress damage and energy depletion following transient forebrain ischaemia in the rat hippocampus. Clin. Exp. Pharm. Physiol. 2006, 33, 725–733. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, H.L.; Zhang, A.H.; Zhou, X.H.; Wang, X.Q.; Han, Y.; Yan, G.L.; Liu, L.; Wang, X.J. Network pharmacology combined with functional metabolomics discover bile acid metabolism as a promising target for mirabilite against colorectal cancer. RSC Adv. 2018, 8, 30061–30070. [Google Scholar] [CrossRef] [Green Version]
- Pang, H.Q.; Yue, S.J.; Tang, Y.P.; Chen, Y.Y.; Tan, Y.J.; Cao, Y.J.; Shi, X.Q.; Zhou, G.S.; Kang, A.; Huang, S.L.; et al. Integrated metabolomics and network pharmacology approach to explain possible action mechanisms of Xin-Sheng-Hua granule for treating anemia. Front. Pharm. 2018, 9, 165. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, W.; Huang, C.; Li, Y.; Yu, H.; Wang, Y.; Duan, J.; Ling, Y. A novel chemometric method for the prediction of human oral bioavailability. Int. J. Mol. Sci. 2012, 13, 6964–6982. [Google Scholar] [CrossRef] [PubMed]
- Tattersall, M.H.; Sodergren, J.E.; Dengupta, S.K.; Trites, D.H.; Modest, E.J.; Frei, E., 3rd. Pharmacokinetics of actinoymcin D in patients with malignant melanoma. Clin. Pharm. 1975, 17, 701–708. [Google Scholar]
- Tao, W.; Xu, X.; Wang, X.; Li, B.; Wang, Y.; Li, Y.; Yang, L. Network pharmacology-based prediction of the active ingredients and potential targets of Chinese herbal Radix Curcumae formula for application to cardiovascular disease. J. Ethnopharmacol. 2013, 145, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Madden, J.C. Silico Approaches for Predicting Adme Properties; Springer: Dordrecht, The Netherlands, 2010; pp. 283–304. [Google Scholar]
- Yang, H.; Zhang, W.; Huang, C.; Zhou, W.; Yao, Y.; Wang, Z.; Li, Y.; Xiao, W.; Wang, Y. A novel systems pharmacology model for herbal medicine injection: A case using Reduning injection. BMC Complement. Altern. Med. 2014, 14, 430. [Google Scholar] [CrossRef] [PubMed]
- Zidan, A.M.; Hemeida, A.A.; Hussein, A.M.; Hashim, M.H. Glycogen synthase kinase 3b prediction as primary cellular target to mediate anti-hepatitis C effect of nitazoxanide. Int. Res. J. Pharm. 2013, 4, 37–39. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Z.; Sun, J. Gene chip study on cerebral gene of effect of Jinkui Shenqiwan and Youguiwan on mouse model of kidney-yang asthenia with syndrome disproved according to therapeutic efficacy of drugs used. China J. Chin. Mater. Med. 2009, 34, 1124–1128. [Google Scholar]
- Chen, R.; Liao, C.; Guo, Q.; Wu, L.; Zhang, L.; Wang, X. Combined systems pharmacology and fecal metabonomics to study the biomarkers and therapeutic mechanism of type 2 diabetic nephropathy treated with Astragalus and Leech. RSC Adv. 2018, 8, 27448–27463. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Wu, J.; Li, J.V.; Zhou, N.; Tang, H.; Wang, Y. Gut microbiota composition modifies fecal metabolic profiles in mice. J. Proteome Res. 2013, 12, 2987–2999. [Google Scholar] [CrossRef]







| Thyroid Gland | Testis | Adrenal Gland | |||||||
|---|---|---|---|---|---|---|---|---|---|
| TSH (UIU/mL) | T3 (nmol/L) | T4 (nmol/L) | LH (MIu/mL) | FSH (mIU/mL) | T (ng/dL) | ACTH (ng/mL) | CORT (ng/mL) | 17-OHCS (nmol/mL) | |
| CON | 5.58 ± 0.41 | 6.11 ± 0.47 | 84.05 ± 2.81 | 3.16 ± 0.13 | 4.07 ± 0.160 | 178.71 ± 1.93 | 44.76 ± 3.33 | 150.67 ± 2.95 | 4.25 ± 0.46 |
| MOD | 4.30 ± 0.31 ** | 4.33 ± 049 ** | 44.58 ± 3.40 ** | 1.46 ± 0.31 ** | 2.32 ± 0.31 ** | 21.85 ± 1.44 ** | 28.95 ± 1.90 ** | 65.53 ± 4.63 ** | 3.69 ± 0.37 * |
| YGP | 5.12 ± 0.46 ## | 5.51 ± 0.45 ## | 62.87 ± 2.54 ## | 2.64 ± 0.08 ## | 3.71 ± 0.66 ## | 138.26 ± 6.54 ## | 37.47 ± 1.56 ## | 120.14 ± 4.38 ## | 4.29 ± 0.57 # |
| No | Gene | Primer Sequence |
|---|---|---|
| 1 | Phosphoenolpyruvate carboxykinase (GTP) | Forward: 5′-CTGCATAACGGTCTGGACTTC-3′ |
| Reverse: 5′-CAGCAACTGCCCGTACTCC-3′ | ||
| 2 | Tyrosyl-tRNA synthetase (Yars) | Forward: 5′-GCTGCATCTTATCACCCGGAA-3′ |
| Reverse: 5′-GATCTTGGACATGGGTACAAAGT-3′ | ||
| 3 | Spermidine synthase (Srm) | Forward: 5′-ACATCCTCGTCTTCCGCAGTA-3′ |
| Reverse: 5′-GGCAGGTTGGCGATCATCT-3′ | ||
| 4 | L-serine dehydratase (Sds) | Forward: 5′-GAAGACCCCACTTCGTGACAG-3′ |
| Reverse: 5′-TCTTGCAGAGATGCCCAATGC-3′ | ||
| 5 | 3-hydroxyacyl-CoA dehydrogenase (Hadh) | Forward: 5′-TCAAGCATGTGACCGTCATCG-3′ |
| Reverse: 5′-TGGATTTTGCCAGGATGTCTTC-3′ | ||
| 6 | Glutamine synthetase (Glul) | Forward: 5′-TGAACAAAGGCATCAAGCAAATG-3′ |
| Reverse: 5′-CAGTCCAGGGTACGGGTCTT-3′ | ||
| 7 | Glycine amidinotransferase (Gatm) | Forward: 5′-GCTTCCTCCCGAAATTCCTGT-3′ |
| Reverse: 5′-CCTCTAAAGGGTCCCATTCGT-3′ | ||
| 8 | Alcohol dehydrogenase 1C (Alcohol-1C) | Forward: 5′-ATGGGCACCGCTGGAAAAG-3′ |
| Reverse: 5′-TAACACGGACTTCCTTAGCCT-3′ | ||
| 9 | Alcohol dehydrogenase class-3 (Alcohol-3C) | Forward: 5′-AGTTCGGATTAAGATCCTTGCCA-3′ |
| Reverse: 5′-ACTTTCCACAATTCCAGCACC-3′ | ||
| 10 | Betaine-homocysteine S-methyltransferase 1 (BHSM) | Forward: 5′-TGTATGGGCTGTCGAAGTCTT-3′ |
| Reverse: 5′-CATGGTCTGCAAGCTAGTCCA-3′ | ||
| 11 | Cystathionine beta-synthase (CBS) | Forward: 5′-CCAGGCACCTGTGGTCAAC-3′ |
| Reverse: 5′-GGTCTCGTGATTGGATCTGCT-3′ | ||
| 12 | β-actins | Forward: 5′-GGAGATTACTGCCCTGGCTCCTA-3′ |
| Reverse: 5′-GACTCATCGTACTCCTGCTTGCTG-3′ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, R.; Wang, J.; Zhan, R.; Zhang, L.; Wang, X. Integrated Systems Pharmacology, Urinary Metabonomics, and Quantitative Real-Time PCR Analysis to Uncover Targets and Metabolic Pathways of the You-Gui Pill in Treating Kidney-Yang Deficiency Syndrome. Int. J. Mol. Sci. 2019, 20, 3655. https://doi.org/10.3390/ijms20153655
Chen R, Wang J, Zhan R, Zhang L, Wang X. Integrated Systems Pharmacology, Urinary Metabonomics, and Quantitative Real-Time PCR Analysis to Uncover Targets and Metabolic Pathways of the You-Gui Pill in Treating Kidney-Yang Deficiency Syndrome. International Journal of Molecular Sciences. 2019; 20(15):3655. https://doi.org/10.3390/ijms20153655
Chicago/Turabian StyleChen, Ruiqun, Jia Wang, Runhua Zhan, Lei Zhang, and Xiufeng Wang. 2019. "Integrated Systems Pharmacology, Urinary Metabonomics, and Quantitative Real-Time PCR Analysis to Uncover Targets and Metabolic Pathways of the You-Gui Pill in Treating Kidney-Yang Deficiency Syndrome" International Journal of Molecular Sciences 20, no. 15: 3655. https://doi.org/10.3390/ijms20153655
APA StyleChen, R., Wang, J., Zhan, R., Zhang, L., & Wang, X. (2019). Integrated Systems Pharmacology, Urinary Metabonomics, and Quantitative Real-Time PCR Analysis to Uncover Targets and Metabolic Pathways of the You-Gui Pill in Treating Kidney-Yang Deficiency Syndrome. International Journal of Molecular Sciences, 20(15), 3655. https://doi.org/10.3390/ijms20153655