Poly(2,6-Dimethyl-1,4-Phenylene Oxide)-Based Hydroxide Exchange Separator Membranes for Zinc–Air Battery
Abstract
:1. Introduction
2. Results and Discussion
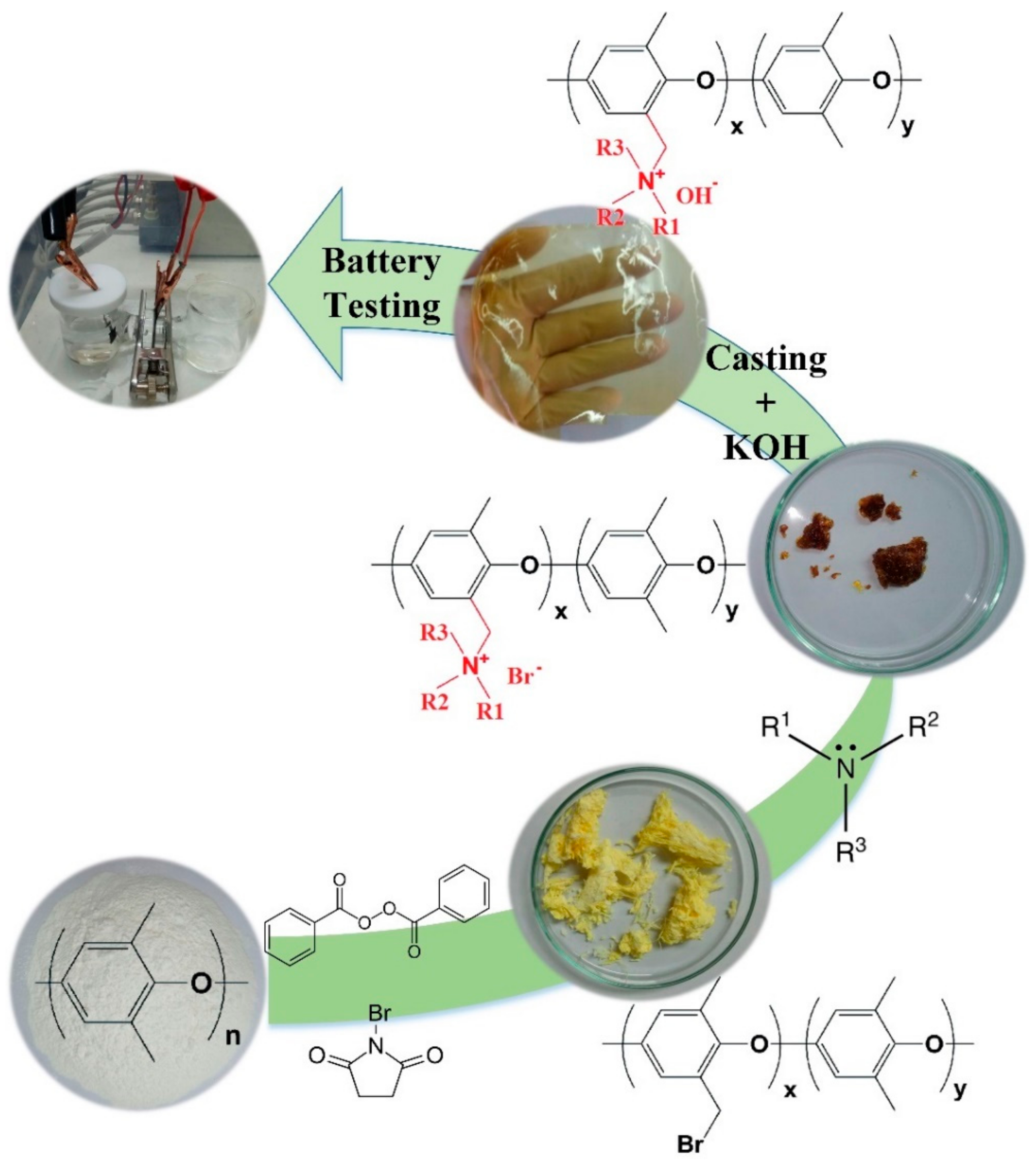
2.1. Separator Membrane Synthesis
2.2. Structural Changes
2.2.1. Proton Nuclear Magnetic Resonance
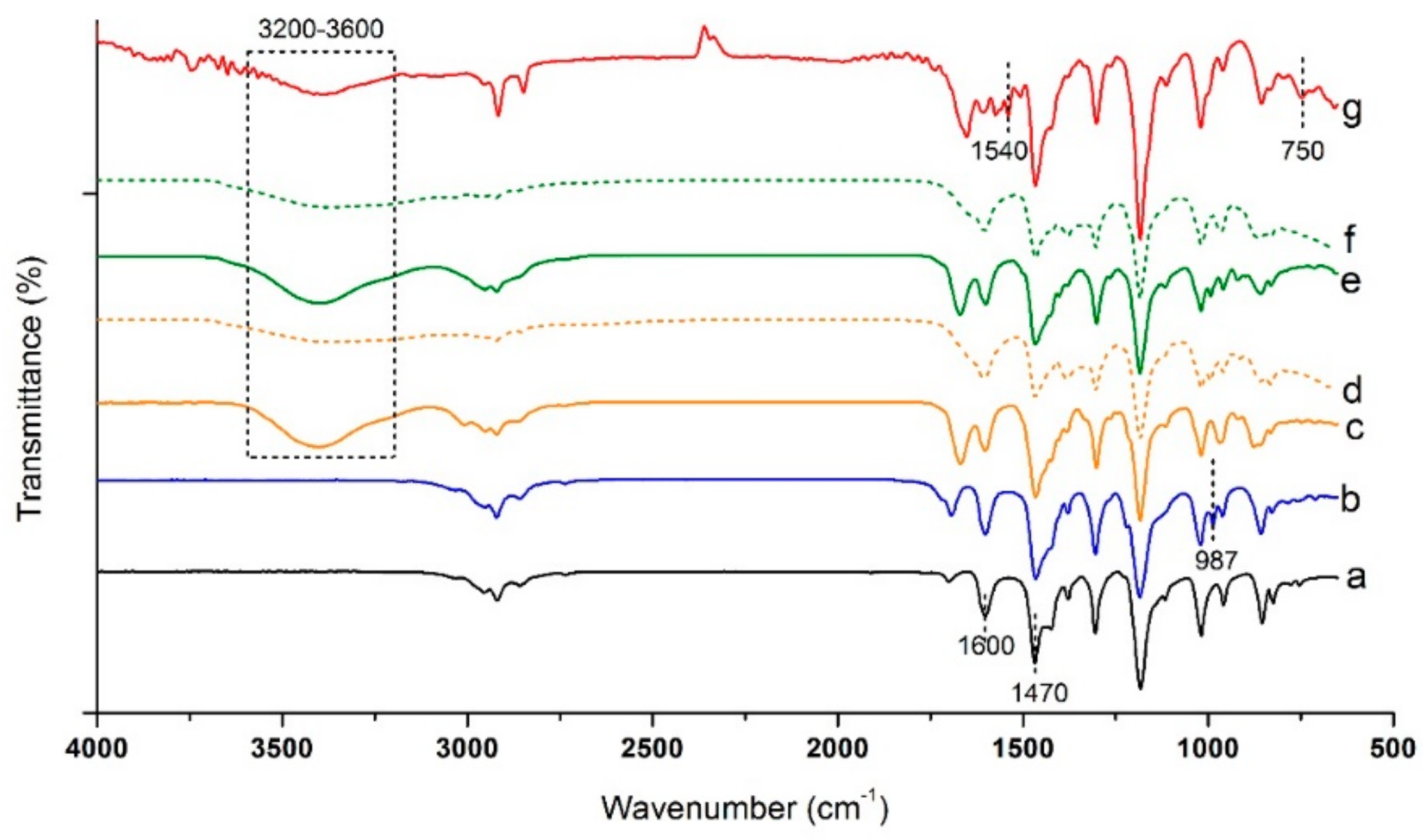
2.2.2. Fourier-Transform Infrared Spectroscopy
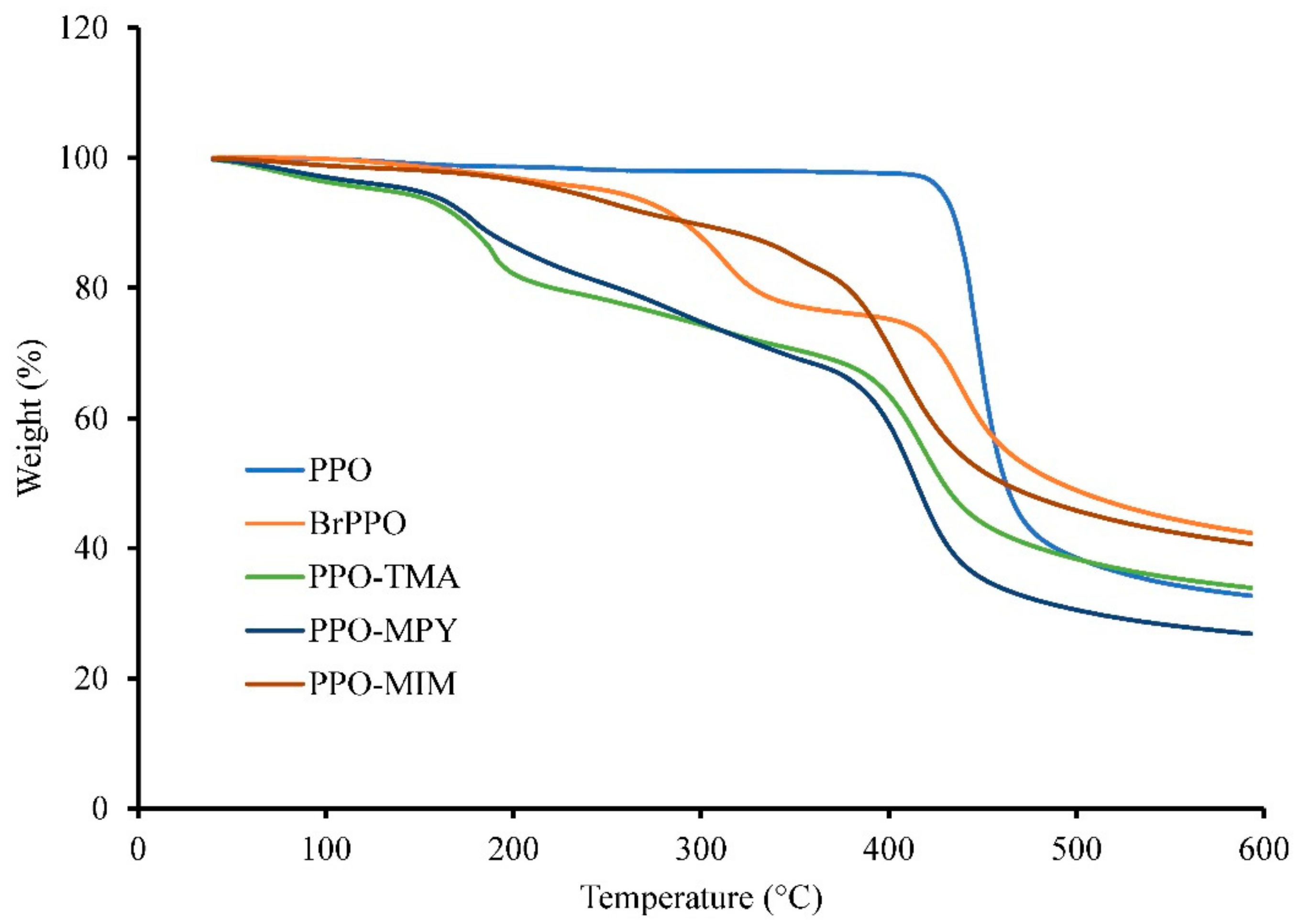
2.3. Thermal Properties
2.4. Electrochemical Characterization
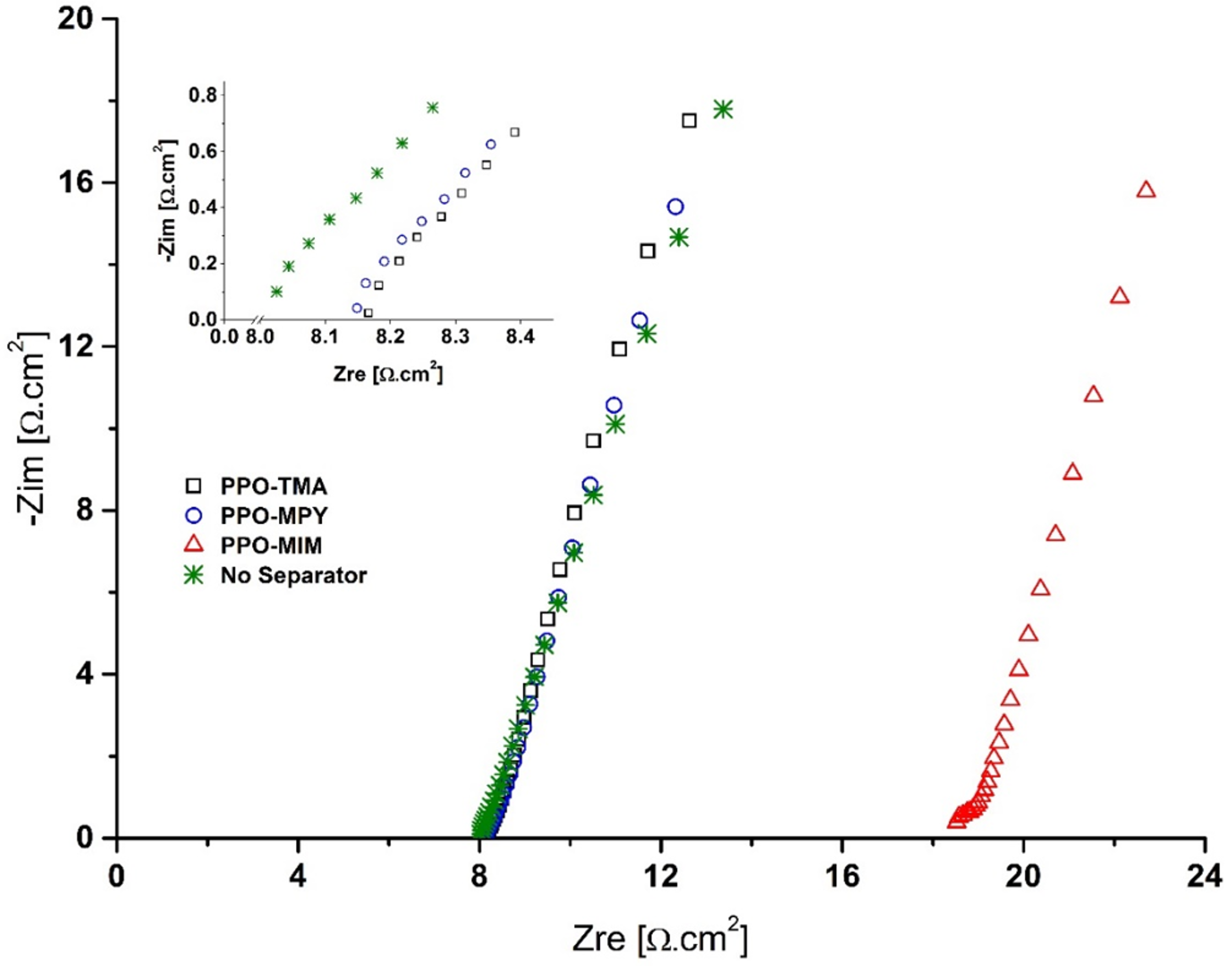
2.4.1. Ionic Conductivity
2.4.2. Zincate Crossover
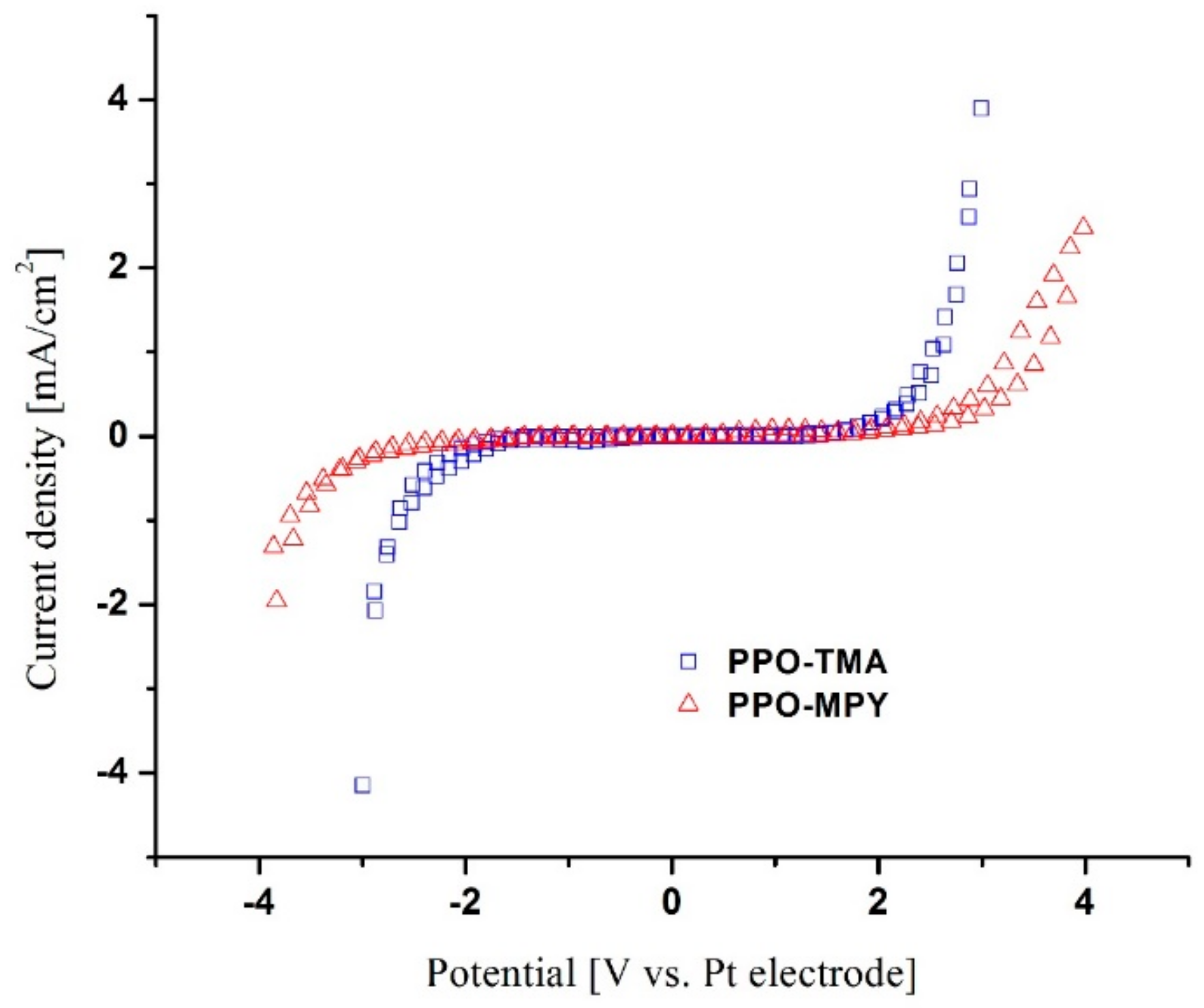
2.4.3. Electrochemical Stability
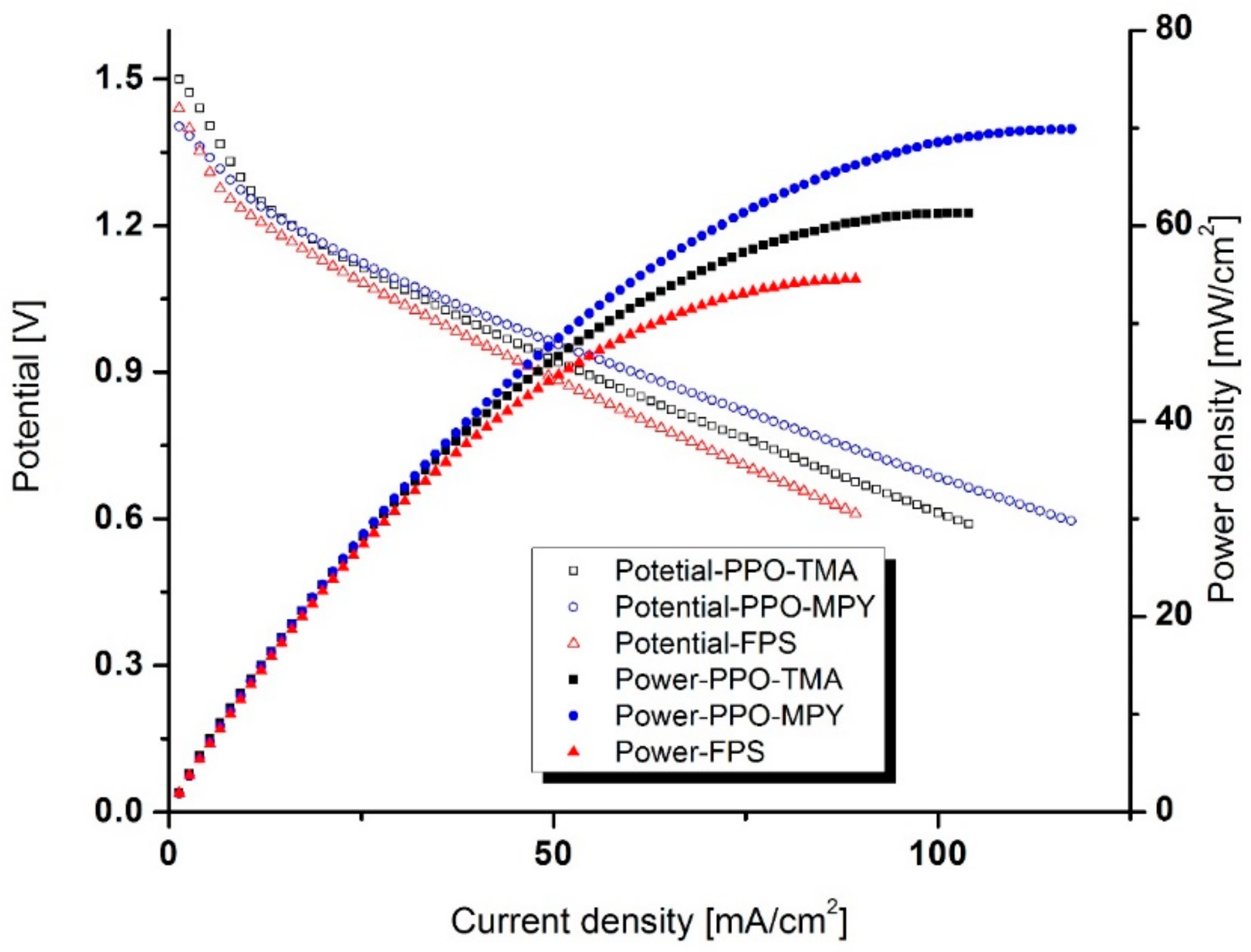
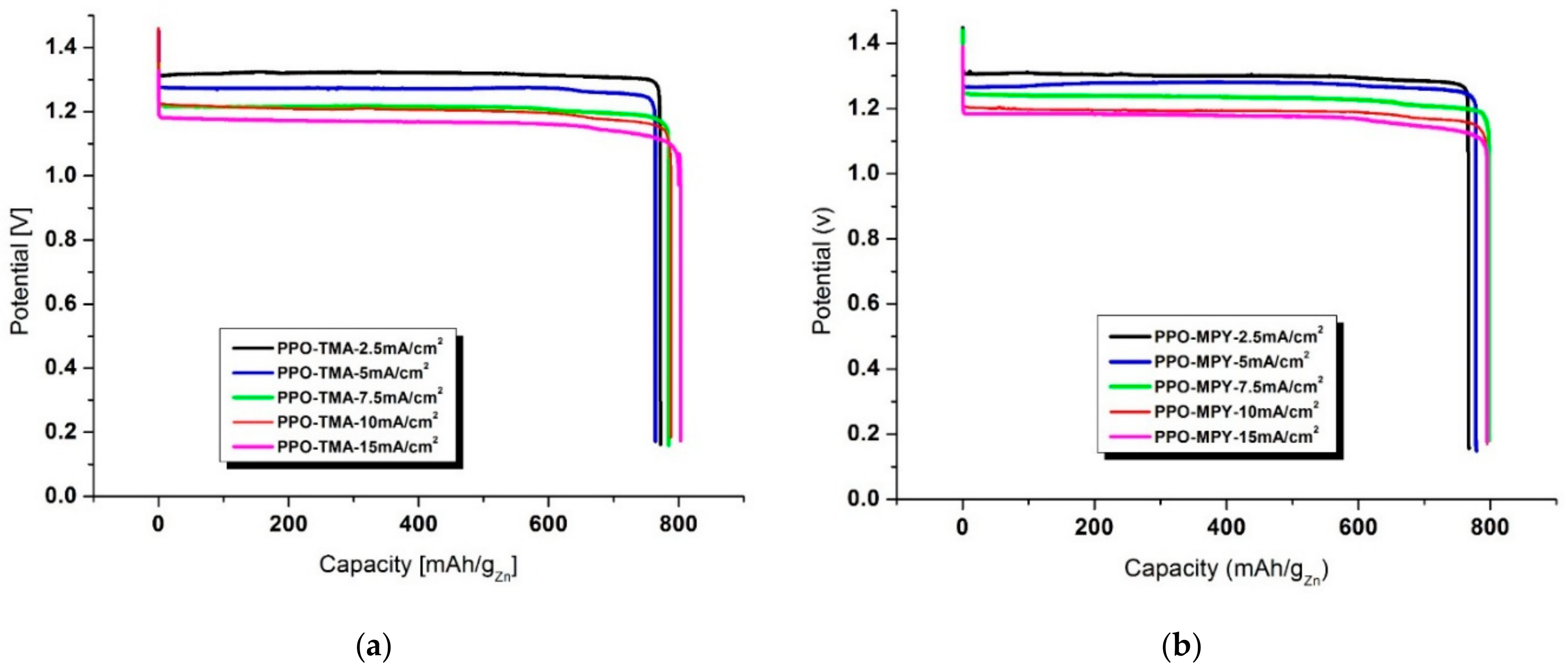
2.5. Discharge Performance
3. Materials and Methods
3.1. Materials
3.2. Separator Membrane Synthesis
3.3. Structural/Physicochemical Characterization
3.4. Electrochemical Characterization
3.5. Discharge Performance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| +FPS | Filter paper-bases separator |
| MIM | 1-Methylimidazolium |
| MPY | 1-Methylpyrolinine |
| TMA | Trimethylamine |
| PPO | Poly (2,6-dimethyl-1,4-phenylene oxide) |
| PPO–MIM | Quaternized PPO using 1-methylimidazolium |
| PPO–MPY | Quaternized PPO using 1-methylpyrolinine |
| PPO–TMA | Quaternized PPO using trimethylamine |
References
- Hosseini, S.; Han, S.J.; Arponwichanop, A.; Yonezawa, T.; Kheawhom, S. Ethanol as an electrolyte additive for alkaline zinc-air flow batteries. Sci. Rep. 2018, 8, 11273. [Google Scholar] [CrossRef] [PubMed]
- Lao-atiman, W.; Bumroongsil, K.; Arpornwichanop, A.; Bumroongsakulsawat, P.; Olaru, S.; Kheawhom, S. Model-Based Analysis of an Integrated Zinc-Air Flow Battery/Zinc Electrolyzer System. Front. Energy Res. 2019, 7, 15. [Google Scholar] [CrossRef]
- Liu, S.; Han, W.; Cui, B.; Liu, X.; Sun, H.; Zhang, J.; Lefler, M.; Licht, S. Rechargeable Zinc Air Batteries and Highly Improved Performance through Potassium Hydroxide Addition to the Molten Carbonate Eutectic Electrolyte. J. Electrochem. Soc. 2018, 165, A149–A154. [Google Scholar] [CrossRef] [Green Version]
- Fang, G.; Zhou, J.; Pan, A.; Liang, S. Recent Advances in Aqueous Zinc-Ion Batteries. Acs Energy Lett. 2018, 3, 2480–2501. [Google Scholar] [CrossRef]
- Li, Y.; Dai, H. Recent advances in zinc-air batteries. Chem. Soc. Rev. 2014, 43, 5257–5275. [Google Scholar] [CrossRef]
- Fu, J. Material Design and Engineering for Polymer Electrolyte Membrane Zinc-Air Batteries; UWSpace: Ontario, Canada, 2018. [Google Scholar]
- Dewi, E.L.; Oyaizu, K.; Nishide, H.; Tsuchida, E. Cationic polysulfonium membrane as separator in zinc–air cell. J. Power Sources 2003, 115, 149–152. [Google Scholar] [CrossRef]
- Lee, H.-J.; Lim, J.-M.; Kim, H.-W.; Jeong, S.-H.; Eom, S.-W.; Hong, Y.T.; Lee, S.-Y. Electrospun polyetherimide nanofiber mat-reinforced, permselective polyvinyl alcohol composite separator membranes: A membrane-driven step closer toward rechargeable zinc–air batteries. J. Membr. Sci. 2016, 499, 526–537. [Google Scholar] [CrossRef]
- Kim, H.-W.; Lim, J.-M.; Lee, H.-J.; Eom, S.-W.; Hong, Y.T.; Lee, S.-Y. Artificially engineered, bicontinuous anion-conducting/-repelling polymeric phases as a selective ion transport channel for rechargeable zinc-air battery separator membranes. J. Mater. Chem. A 2016, 4, 3711–3720. [Google Scholar] [CrossRef]
- Pan, J.; Chen, C.; Li, Y.; Wang, L.; Tan, L.; Li, G.; Tang, X.; Xiao, L.; Lu, J.; Zhuang, L. Constructing ionic highway in alkaline polymer electrolytes. Energy Environ. Sci. 2014, 7, 354–360. [Google Scholar] [CrossRef]
- Zhu, L.; Pan, J.; Wang, Y.; Han, J.; Zhuang, L.; Hickner, M.A. Multication Side Chain Anion Exchange Membranes. Macromolecules 2016, 49, 815–824. [Google Scholar] [CrossRef]
- Zhou, J.; Zuo, P.; Liu, Y.; Yang, Z.; Xu, T. Ion exchange membranes from poly(2,6-dimethyl-1,4-phenylene oxide) and related applications. Sci. China Chem. 2018, 61, 1062–1087. [Google Scholar] [CrossRef]
- Li, N.; Yan, T.; Li, Z.; Thurn-Albrecht, T.; Binder, W.H. Comb-shaped polymers to enhance hydroxide transport in anion exchange membranes. Energy Environ. Sci. 2012, 5, 7888–7892. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, Y.; Ye, N.; Zhang, D.; Yang, J.; He, R. Alkali Resistant Anion Exchange Membranes Based on Saturated Heterocyclic Quaternary Ammonium Cations Functionalized Poly(2,6-dimethyl-1,4-phenylene oxide)s. J. Electrochem. Soc. 2018, 165, F350–F356. [Google Scholar] [CrossRef]
- Varcoe, J.R.; Atanassov, P.; Dekel, D.R.; Herring, A.M.; Hickner, M.A.; Kohl, P.A.; Kucernak, A.R.; Mustain, W.E.; Nijmeijer, K.; Scott, K.; et al. Anion-exchange membranes in electrochemical energy systems. Energy Environ. Sci. 2014, 7, 3135–3191. [Google Scholar] [CrossRef] [Green Version]
- Msomi, P.F.; Nonjola, P.; Ndungu, P.G.; Ramonjta, J. Quaternized poly(2.6 dimethyl-1.4 phenylene oxide)/polysulfone blend composite membrane doped with ZnO-nanoparticles for alkaline fuel cells. J. Appl. Polym. Sci. 2018, 135, 45959. [Google Scholar] [CrossRef]
- Tongwen, X.; Weihua, Y. Fundamental studies of a new series of anion exchange membranes: Membrane preparation and characterization. J. Membr. Sci. 2001, 190, 159–166. [Google Scholar] [CrossRef]
- Zhu, L.; Zimudzi, T.J.; Wang, Y.; Yu, X.; Pan, J.; Han, J.; Kushner, D.I.; Zhuang, L.; Hickner, M.A. Mechanically Robust Anion Exchange Membranes via Long Hydrophilic Cross-Linkers. Macromolecules 2017, 50, 2329–2337. [Google Scholar] [CrossRef]
- Yang, J.; Liu, C.; Hao, Y.; He, X.; He, R. Preparation and investigation of various imidazolium-functionalized poly(2,6-dimethyl-1,4-phenylene oxide) anion exchange membranes. Electrochim. Acta 2016, 207, 112–119. [Google Scholar] [CrossRef]
- Dang, H.-S.; Jannasch, P. A comparative study of anion-exchange membranes tethered with different hetero-cycloaliphatic quaternary ammonium hydroxides. J. Mater. Chem. A 2017, 5, 21965–21978. [Google Scholar] [CrossRef] [Green Version]
- Si, Z.; Sun, Z.; Gu, F.; Qiu, L.; Yan, F. Alkaline stable imidazolium-based ionomers containing poly(arylene ether sulfone) side chains for alkaline anion exchange membranes. J. Mater. Chem. A 2014, 2, 4413–4421. [Google Scholar] [CrossRef]
- Yang, C.-C.; Lin, S.-J. Alkaline composite PEO–PVA–glass-fibre-mat polymer electrolyte for Zn–air battery. J. Power Sources 2002, 112, 497–503. [Google Scholar] [CrossRef]
- Vassal, N.; Salmon, E.; Fauvarque, J.F. Electrochemical properties of an alkaline solid polymer electrolyte based on P(ECH-co-EO). Electrochim. Acta 2000, 45, 1527–1532. [Google Scholar] [CrossRef]
- Hosseini, S.; Lao-atiman, W.; Han, S.J.; Arpornwichanop, A.; Yonezawa, T.; Kheawhom, S. Discharge Performance of Zinc-Air Flow Batteries Under the Effects of Sodium Dodecyl Sulfate and Pluronic F. Sci. Rep. 2018, 8, 14909. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.M.; Lin, S.J.; You, J.H.; Yang, C.C. Study of high-anionic conducting sulfonated microporous membranes for zinc-air electrochemical cells. Mater. Chem. Phys. 2008, 112, 798–804. [Google Scholar] [CrossRef]

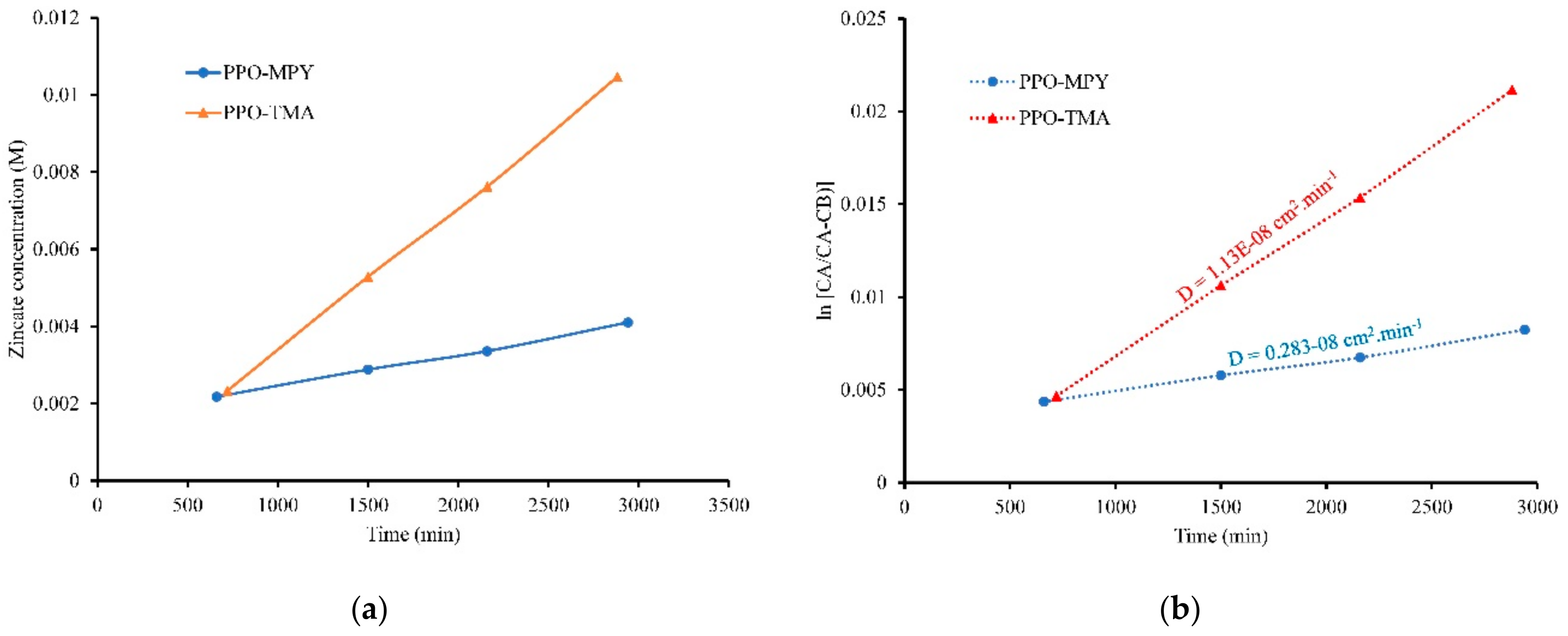
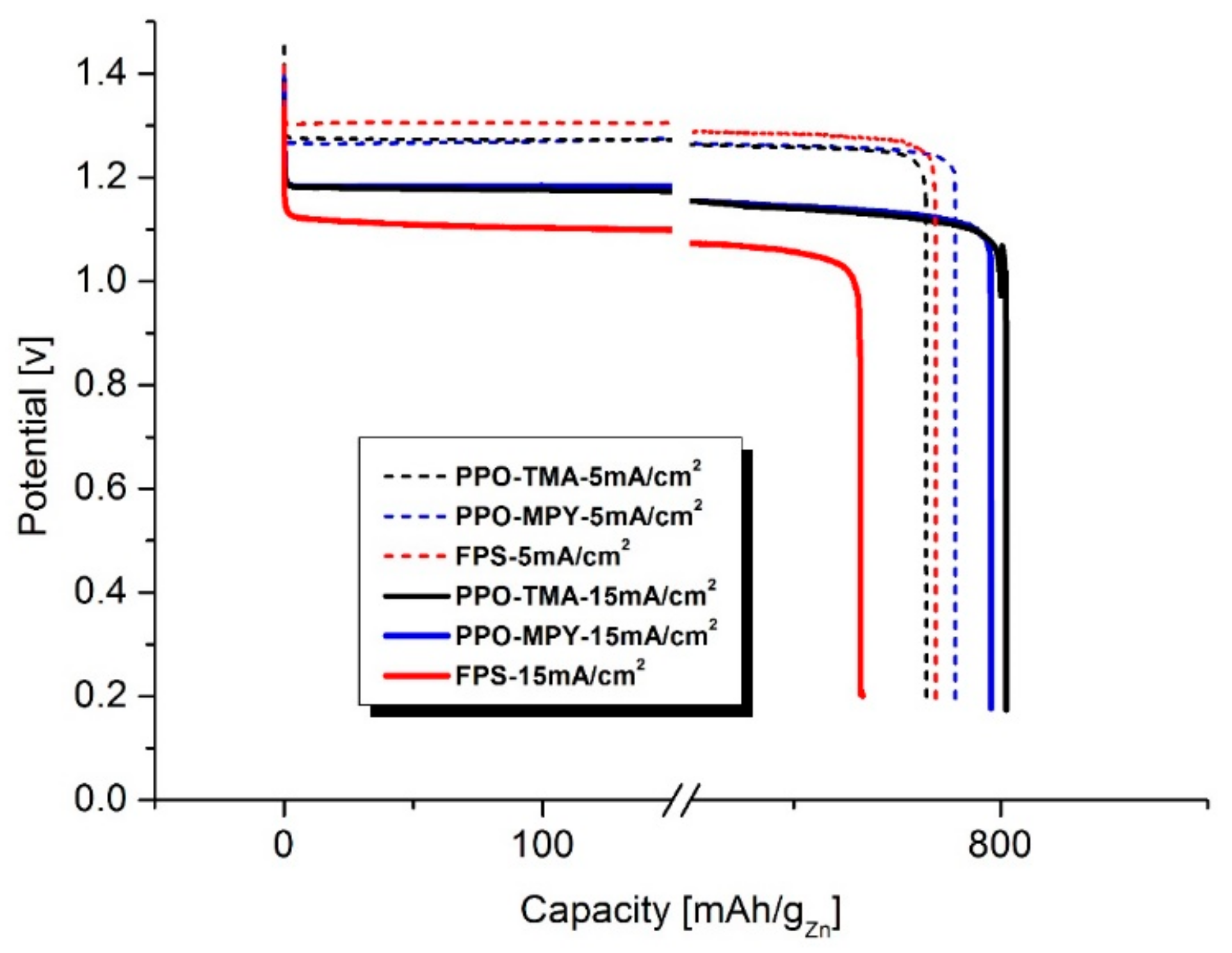
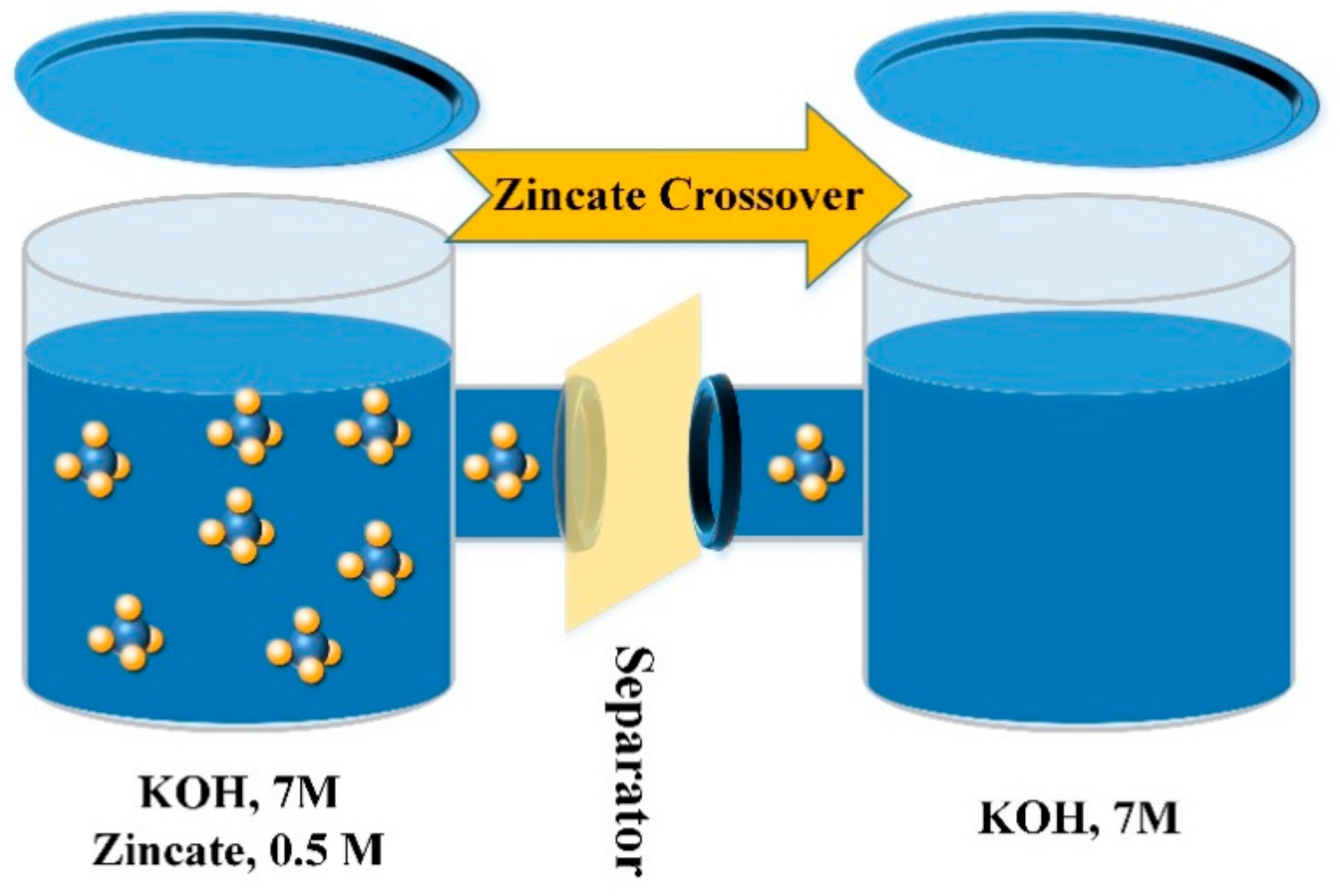
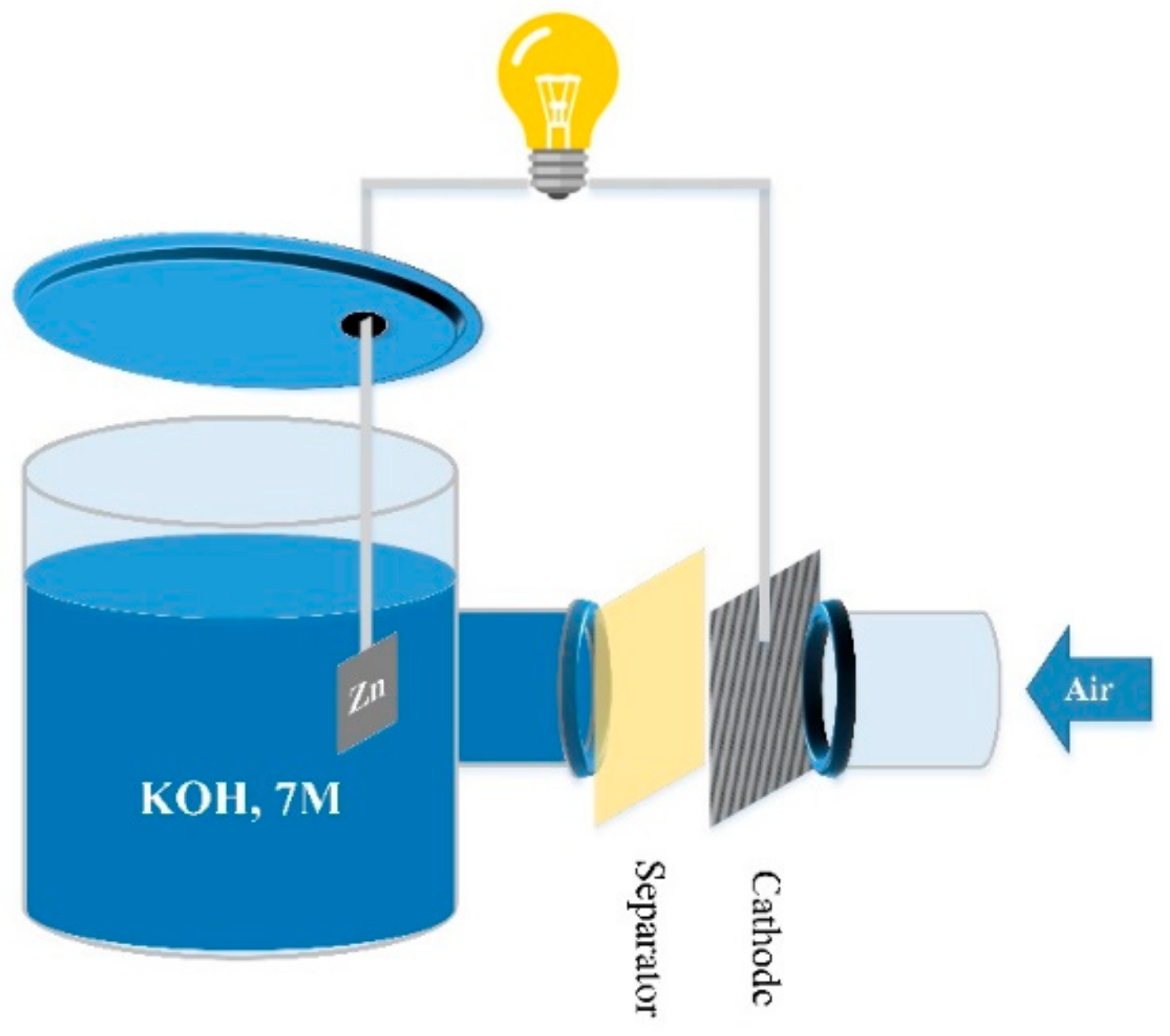
| Sample | DI Water | KOH, 7 M | Thickness (μm) | Area (cm2) | Rb (Ω) 2 | Ionic Conductivity (mS/cm)—σ | Zincate Diffusion Coefficient (×10−8 cm2/min)—D | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Uptake ΔW (wt%) | Area Change ΔA (%) | Volume Change ΔV (%) | Uptake ΔW (wt%) | Area Change ΔA (%) | Volume Change ΔV (%) | ||||||
| PPO–TMA | 89 | 65 | 119 | 31 | 11 | 39 | 50 | 1.766 | 0.1630 | 17.37 | 1.13 |
| PPO–MPy | 78 | 41 | 76 | 30 | 11 | 39 | 40 | 1.766 | 0.1394 | 16.25 | 0.28 |
| PPO–MIM 1 | 13 | 14 | 30 | 3 | 0 | 0 | 30 | 1.766 | 5.8014 | 0.29 | N/A |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbasi, A.; Hosseini, S.; Somwangthanaroj, A.; Mohamad, A.A.; Kheawhom, S. Poly(2,6-Dimethyl-1,4-Phenylene Oxide)-Based Hydroxide Exchange Separator Membranes for Zinc–Air Battery. Int. J. Mol. Sci. 2019, 20, 3678. https://doi.org/10.3390/ijms20153678
Abbasi A, Hosseini S, Somwangthanaroj A, Mohamad AA, Kheawhom S. Poly(2,6-Dimethyl-1,4-Phenylene Oxide)-Based Hydroxide Exchange Separator Membranes for Zinc–Air Battery. International Journal of Molecular Sciences. 2019; 20(15):3678. https://doi.org/10.3390/ijms20153678
Chicago/Turabian StyleAbbasi, Ali, Soraya Hosseini, Anongnat Somwangthanaroj, Ahmad Azmin Mohamad, and Soorathep Kheawhom. 2019. "Poly(2,6-Dimethyl-1,4-Phenylene Oxide)-Based Hydroxide Exchange Separator Membranes for Zinc–Air Battery" International Journal of Molecular Sciences 20, no. 15: 3678. https://doi.org/10.3390/ijms20153678
APA StyleAbbasi, A., Hosseini, S., Somwangthanaroj, A., Mohamad, A. A., & Kheawhom, S. (2019). Poly(2,6-Dimethyl-1,4-Phenylene Oxide)-Based Hydroxide Exchange Separator Membranes for Zinc–Air Battery. International Journal of Molecular Sciences, 20(15), 3678. https://doi.org/10.3390/ijms20153678








