Intergeneric Relationships within the Family Salicaceae s.l. Based on Plastid Phylogenomics
Abstract
1. Introduction
2. Results
2.1. Characteristics of the Plastid Genomes
2.2. Repeat Sequence Analysis
2.3. Inverted Repeats and Genome Comparison
2.4. Divergence Hotspots of Plastid Genomes
2.5. Phylogenetic Analysis
3. Discussion
3.1. Features of Plastid Genomes
3.2. Repeat Sequence Variations
3.3. Comparative Analyses
3.4. Divergence Hotspot Analysis
3.5. Phylogenetic Relationships
4. Materials and Methods
4.1. Sampling and Genome Sequencing
4.2. Plastid Genome Assembly and Annotation
4.3. Repeat Sequence Analysis
4.4. Genome Comparative Analysis
4.5. Sequence Divergence Analysis
4.6. Phylogenetic Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ohashi, H. Salicaceae of Japan. Sci. Rep. 2001, 40, 269–396. [Google Scholar]
- Chase, M.W.; Zmarzty, S.; Dolores Lledó, M.; Wurdack, K.J.; Fay, S.M.F. When in Doubt, Put It in Flacourtiaceae: A Molecular Phylogenetic Analysis Based on Plastid rbcL DNA Sequences. Kew Bull. 2002, 57, 141–181. [Google Scholar] [CrossRef]
- Leskinen, E.; Alström-Rapaport, C. Molecular phylogeny of Salicaceae and closely related Flacourtiaceae: Evidence from 5.8 S, ITS 1 and ITS 2 of the rDNA. Plant Syst. Evol. 1999, 215, 209–227. [Google Scholar] [CrossRef]
- Hou, J.; Ye, N.; Zhang, D.; Chen, Y.; Fang, L.; Dai, X.; Yin, T. Different autosomes evolved into sex chromosomes in the sister genera of Salix and Populus. Sci. Rep. 2015, 5, 9076. [Google Scholar] [CrossRef] [PubMed]
- Kersten, B.; Pakull, B.; Groppe, K.; Lueneburg, J.; Fladung, M. The sex-linked region in Populus tremuloides Turesson 141 corresponds to a pericentromeric region of about two million base pairs on P. trichocarpa chromosome 19. Plant Biol. 2014, 16, 411–418. [Google Scholar] [CrossRef]
- Li, R.J.; Gao, X.; Li, L.M.; Liu, X.L.; Wang, Z.Y.; Lü, S.Y. De novo Assembly and Characterization of the Fruit Transcriptome of Idesia polycarpa Reveals Candidate Genes for Lipid Biosynthesis. Front. Plant Sci. 2016, 7, 801. [Google Scholar] [CrossRef] [PubMed]
- Abrahamson, L.P.; Robison, D.J.; Volk, T.A.; White, E.H.; Neuhauser, E.F.; Benjamin, W.H.; Peterson, J.M. Sustainability and environmental issues associated with willow bioenergy development in New York (U.S.A.). Biomass Bioenergy 1998, 15, 17–22. [Google Scholar]
- Braatne, J.H.; Hinckly, T.M.; Stettler, R.F. Influence of soil water on the physiological and morphological components of plant water balance in Populus trichocarpa, Populus deltoides and their F1 hybrids. Tree Physiol. 1992, 11, 325–340. [Google Scholar] [CrossRef]
- Besnard, G.; Hernández, P.; Khadari, B.; Dorado, G.; Savolainen, V. Genomic profiling of plastid DNA variation in the Mediterranean olive tree. BMC Plant Biol. 2011, 11, 80. [Google Scholar] [CrossRef]
- Dexter, K.G.; Terborgh, J.W.; Cunningham, C.W. Historical effects on beta diversity and community assembly in Amazonian trees. Proc. Natl. Acad. Sci. USA 2012, 109, 7787–7792. [Google Scholar] [CrossRef]
- Howe, C.J.; Barbrook, A.C.; Lila Koumandou, V.; Nisbet, R.E.; Symington, H.A.; Wightman, T.F. Evolution of the chloroplast genome. Philos. Trans. R Soc. Lond. B Biol. Sci. 2003, 358, 99–107. [Google Scholar] [CrossRef]
- Liu, L.X.; Li, R.; Worth, J.R.P.; Li, X.; Li, P.; Cameron, K.M.; Fu, C.X. The Complete Chloroplast Genome of Chinese Bayberry (Morella rubra, Myricaceae): Implications for Understanding the Evolution of Fagales. Front. Plant Sci. 2017, 8, 968. [Google Scholar] [CrossRef]
- Wicke, S.; Schneeweiss, G.M.; Depamphilis, C.W.; Kai, F.M.; Quandt, D. The evolution of the plastid chromosome in land plants: Gene content, gene order, gene function. Plant Mol. Biol. 2011, 76, 273–297. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Khan, I.A.; Heinze, B.; Azim, M.K. The Chloroplast Genome Sequence of Date Palm (Phoenix dactylifera L. cv. ’Aseel’). Plant Mol. Biol. Rep. 2012, 30, 666–678. [Google Scholar] [CrossRef]
- Bendich, A.J. Circular chloroplast chromosomes: The grand illusion. Plant Cell 2004, 16, 1661–1666. [Google Scholar] [CrossRef]
- Chumley, T.W.; Palmer, J.D.; Mower, J.P.; Fourcade, H.M.; Calie, P.J.; Boore, J.L.; Jansen, R.K. The complete chloroplast genome sequence of Pelargonium x hortorum: Organization and evolution of the largest and most highly rearranged chloroplast genome of land plants. Mol. Biol. Evol. 2006, 23, 2175–2190. [Google Scholar] [CrossRef] [PubMed]
- Knox, E.B. The dynamic history of plastid genomes in the Campanulaceae sensu lato is unique among angiosperms. Proc. Natl. Acad. Sci. USA 2014, 111, 11097–11102. [Google Scholar] [CrossRef]
- Palmer, J.D. Chloroplast DNA exists in two orientations. Nature 1983, 301, 92–93. [Google Scholar] [CrossRef]
- Gurdon, C.; Maliga, P. Two Distinct Plastid Genome Configurations and Unprecedented Intraspecies Length Variation in the accD Coding Region in Medicago truncatula. DNA Res. 2014, 21, 417–427. [Google Scholar] [CrossRef]
- Vieira, L.D.N.; Helisson, F.; Marcelo, R.; Fraga, H.P.D.F.; Cardoso, R.L.A.; Emanuel Maltempi, D.S.; Fábio, D.O.P.; Rubens Onofre, N.; Miguel Pedro, G. The complete chloroplast genome sequence of Podocarpus lambertii: Genome structure, evolutionary aspects, gene content and SSR detection. PLoS ONE 2014, 9, e90618. [Google Scholar] [CrossRef]
- Stein, D.B.; Palmer, J.D.; Thompson, W.F. Structural evolution and flip-flop recombination of chloroplast DNA in the fern genus Osmunda. Curr. Genet. 1986, 10, 835–841. [Google Scholar] [CrossRef]
- Tangphatsornruang, S.; Sangsrakru, D.; Chanprasert, J.; Uthaipaisanwong, P.; Yoocha, T.; Jomchai, N.; Tragoonrung, S. The Chloroplast Genome Sequence of Mungbean (Vigna radiata) Determined by High-throughput Pyrosequencing: Structural Organization and Phylogenetic Relationships. DNA Res. 2009, 17, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Jun, W.; Yongping, Y.; Chuanzhu, F.; Jiahui, C. Phylogenomic Analysis and Dynamic Evolution of Chloroplast Genomes in Salicaceae. Front. Plant Sci. 2017, 8, 1050. [Google Scholar] [CrossRef] [PubMed]
- Zong, D.; Gan, P.; Zhou, A.; Zhang, Y.; Zou, X.; Duan, A.; Song, Y.; He, C. Plastome Sequences Help to Resolve Deep-Level Relationships of Populus in the Family Salicaceae. Front. Plant Sci. 2019, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Zong, D.; Zhou, A.; Zhang, Y.; Zou, X.; Li, D.; Duan, A.; He, C. Characterization of the complete chloroplast genomes of five Populus species from the western Sichuan plateau, southwest China: Comparative and phylogenetic analyses. PeerJ 2019, 7, e6386. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.I.; Hefer, C.A.; Kolosova, N.; Douglas, C.J.; Cronk, Q.C. Whole plastome sequencing reveals deep plastid divergence and cytonuclear discordance between closely related balsam poplars, Populus balsamifera and P. trichocarpa (Salicaceae). New Phytol. 2014, 204, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Eckenwalder, E.J. Taxonomic Signal and Noise in Multivariate Interpopulational Relationships in Populus mexicana (Salicaceae). Syst. Bot. 1996, 21, 261–271. [Google Scholar] [CrossRef]
- Chen, J.H.; Sun, H.; Wen, J.; Yang, Y.P. Molecular Phylogeny of Salix L. (Salicaceae) Inferred from Three Chloroplast Datasets and Its Systematic Implications. Taxon 2010, 59, 29–37. [Google Scholar] [CrossRef]
- Lauron-Moreau, A.; Pitre, F.E.; Argus, G.W.; Labrecque, M.; Brouillet, L. Phylogenetic relationships of American willows (Salix L., Salicaceae). PLoS ONE 2015, 10, e0121965. [Google Scholar] [CrossRef]
- Wu, J.; Nyman, T.; Wang, D.C.; Argus, G.W.; Yang, Y.P.; Chen, J.H. Phylogeny of Salix subgenus Salix s.l. (Salicaceae): Delimitation, biogeography, and reticulate evolution. BMC Evol. Biol. 2015, 15, 31. [Google Scholar] [CrossRef]
- Lei, Z.; Xi, Z.X.; Wang, M.C.; Guo, X.Y.; Ma, T. Plastome phylogeny and lineage diversification of Salicaceae with focus on poplars and willows. Ecol. Evol. 2018, 8, 7817–7823. [Google Scholar]
- Palmer, J.D. Plastid Chromosomes; Structure and Evolution. In The Molecular Biology of Plastids; Bogorad, L., Vasil, I.K., Eds.; Academic Press: San Diego, CA, USA, 1911; pp. 5–53. [Google Scholar]
- Jansen, R.K.; Raubeson, L.A.; Boore, J.L.; Depamphilis, C.W.; Chumley, T.W.; Haberle, R.C.; Wyman, S.K.; Alverson, A.J.; Peery, R.; Herman, S.J. Methods for Obtaining and Analyzing Whole Chloroplast Genome Sequences. Methods Enzym. 2005, 395, 348–383. [Google Scholar]
- Kim, K.J.; Jansen, R.K. ndhF sequence evolution and the major clades in the sunflower family. Proc. Natl. Acad. Sci. USA 1995, 92, 10379–10383. [Google Scholar] [CrossRef]
- Henry, R.J.; Henry, R.J. Plant diversity and evolution: Genotypic and phenotypic variation in higher plants. Biologist 2005, 121. [Google Scholar]
- Dugas, D.V.; Hernandez, D.; Koenen, E.J.M.; Schwarz, E.; Straub, S.; Hughes, C.E.; Jansen, R.K.; Nageswara-Rao, M.; Staats, M.; Trujillo, J.T. Mimosoid legume plastome evolution: IR expansion, tandem repeat expansions, and accelerated rate of evolution inclpP. Sci. Rep. 2015, 5, 16958. [Google Scholar] [CrossRef]
- Millen, R.S.; Olmstead, R.G.; Adams, K.L.; Palmer, J.D.; Lao, N.T.; Heggie, L.; Kavanagh, T.A.; Hibberd, J.M.; Gray, J.C.; Morden, C.W. Many parallel losses of infA from chloroplast DNA during angiosperm evolution with multiple independent transfers to the nucleus. Plant Cell 2001, 13, 645–658. [Google Scholar] [CrossRef]
- Thomas, F.; Massenet, O.; Dorne, A.M.; Briat, J.F.; Mache, R. Expression of the rpl23, rpl2 and rps19 genes in spinach chloroplasts. Nucleic Acids Res. 1988, 16, 2461–2472. [Google Scholar] [CrossRef]
- Downie, S.R.; Olmstead, R.G.; Zurawski, G.; Soltis, D.E.; Palmer, J.D. Six independent loss of the chloroplast DNA rpl2 intron in Dicotyledons: Molecular and phylogenetic implications. Evolution 1991, 45, 1245–1259. [Google Scholar]
- Xiong, A.S.; Peng, R.H.; Zhuang, J.; Gao, F.; Zhu, B.; Fu, X.Y.; Xue, Y.; Jin, X.F.; Tian, Y.S.; Zhao, W. Gene duplication, transfer, and evolution in the chloroplast genome. Biotechnol. Adv. 2009, 27, 340–347. [Google Scholar] [CrossRef]
- Haberle, R.C.; Fourcade HMBoore, J.L.; Jansen, R.K. Extensive rearrangements in the chloroplast genome of Trachelium caeruleum are associated with repeats and tRNA genes. J. Mol. Evol. 2008, 66, 350–361. [Google Scholar] [CrossRef]
- Hae-Lim, L.; Jansen, R.K.; Chumley, T.W.; Ki-Joong, K. Gene relocations within chloroplast genomes of Jasminum and Menodora (Oleaceae) are due to multiple, overlapping inversions. Mol. Biol. Evol. 2007, 24, 1161–1180. [Google Scholar]
- Hipkins, V.D.; Marshall KANeale, D.B.; Rottmann, W.H.; Strauss, S.H. A mutation hotspot in the chloroplast genome of a conifer (Douglas-fir: Pseudotsuga) is caused by variability in the number of direct repeats derived from a partially duplicated tRNA gene. Curr. Genet. 1995, 27, 572–579. [Google Scholar] [CrossRef]
- Lenka, D.; Jan, K.; Cestmír, V.; Václav, P. TrnL- trnF intergenic spacer and trnL intron define major clades within Luzula and Juncus (Juncaceae): Importance of structural mutations. J. Mol. Evol. 2004, 59, 1–10. [Google Scholar]
- Lin, C.P.; Wu, C.S.; Huang, Y.Y.; Chaw, S.M. The complete chloroplast genome of Ginkgo biloba reveals the mechanism of inverted repeat contraction. Genome Biol. Evol. 2012, 4, 374–381. [Google Scholar] [CrossRef]
- Vijverberg, K.; Bachmann, K. Molecular evolution of a tandemly repeated trnF (GAA) gene in the chloroplast genomes of Microseris (Asteraceae) and the use of structural mutations in phylogenetic analyses. Mol. Biol. Evol. 1999, 16, 1329–1340. [Google Scholar] [CrossRef][Green Version]
- Jiao, Y.; Jia, H.M.; Li, X.W.; Chai, M.L.; Jia, H.J.; Chen, Z.; Wang, G.Y.; Chai, C.Y.; Weg, E.V.D.; Gao, Z.S. Development of simple sequence repeat (SSR) markers from a genome survey of Chinese bayberry (Myrica rubra). BMC Genom. 2012, 13, 201. [Google Scholar] [CrossRef]
- Kaundun, S.S.; Matsumoto, S. Heterologous nuclear and chloroplast microsatellite amplification and variation in tea, Camellia sinensis. Genome 2002, 45, 1041–1048. [Google Scholar] [CrossRef]
- Powell, W.; Morgante, M.; Mcdevitt, R.; Vendramin, G.G.; Rafalski, J.A. Polymorphic simple sequence repeat regions in chloroplast genomes: Applications to the population genetics of pines. Proc. Natl. Acad. Sci. USA 1995, 92, 7759–7763. [Google Scholar] [CrossRef]
- Pauwels, M.V.X.; Godé, C.; Frérot, H.; Castric, V.; Saumitou-Laprade, P. Nuclear and chloroplast DNA phylogeography reveals vicariance among European populations of the model species for the study of metal tolerance, Arabidopsis halleri (Brassicaceae). New Phytol. 2012, 193, 916–928. [Google Scholar] [CrossRef]
- Yeong Deuk, J.; Jongsun, P.; Jungeun, K.; Wonho, S.; Cheol-Goo, H.; Yong-Hwan, L.; Byoung-Cheorl, K. Complete sequencing and comparative analyses of the pepper (Capsicum annuum L.) plastome revealed high frequency of tandem repeats and large insertion/deletions on pepper plastome. Plant Cell Rep. 2011, 30, 217–229. [Google Scholar]
- Cosner, M.E.; Jansen, R.K.; Palmer, J.D.; Downie, S.R. The highly rearranged chloroplast genome of Trachelium caeruleum (Campanulaceae): Multiple inversions, inverted repeat expansion and contraction, transposition, insertions/deletions, and several repeat families. Curr. Genet. 1997, 31, 419–429. [Google Scholar] [CrossRef]
- Mao-Lun, W.; Blazier, J.C.; Madhumita, G.; Jansen, R.K. Reconstruction of the ancestral plastid genome in Geraniaceae reveals a correlation between genome rearrangements, repeats, and nucleotide substitution rates. Mol. Biol. Evol. 2014, 31, 645–659. [Google Scholar]
- Ogihara, Y.; Terachi, T.; Sasakuma, T. Intramolecular recombination of chloroplast genome mediated by short direct-repeat sequences in wheat species. Proc. Natl. Acad. Sci. USA 1988, 85, 8573–8577. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, T.; Duan, D.; Yang, J.; Feng, L.; Zhao, G. Comparative Analysis of the Complete Chloroplast Genomes of Five Quercus Species. Front. Plant Sci. 2016, 7, 959. [Google Scholar] [CrossRef]
- Daniell, H.; Lin, C.S.; Ming, Y.; Chang, W.J. Chloroplast genomes: Diversity, evolution, and applications in genetic engineering. Genome Biol. 2016, 17, 134. [Google Scholar] [CrossRef]
- Wu, F.H.; Chan, M.T.; Liao, D.C.; Hsu, C.T.; Lee, Y.W.; Daniell, H.; Duvall, M.R.; Lin, C.S. Complete chloroplast genome ofOncidiumGower Ramsey and evaluation of molecular markers for identification and breeding in Oncidiinae. BMC Plant Biol. 2010, 10, 68. [Google Scholar] [CrossRef]
- Ki-Joong, K.; Hae-Lim, L. Widespread occurrence of small inversions in the chloroplast genomes of land plants. Mol. Cells 2005, 19, 104–113. [Google Scholar]
- Bouchenak-Khelladi, Y.; Maurin, O.; Hurter, J.; van der Bank, M. The evolutionary history and biogeography of Mimosoideae (Leguminosae): An emphasis on African acacias. Mol. Phylogen. Evol. 2010, 57, 495–508. [Google Scholar] [CrossRef]
- Miller, J.T.; Seigler, D. Evolutionary and taxonomic relationships of Acacia s.l. (Leguminosae: Mimosoideae). Aust. Syst. Bot. 2012, 25, 217–224. [Google Scholar] [CrossRef]
- Givnish, T.J.; Spalink, D.; Ames, M.; Lyon, S.P.; Hunter, S.J.; Zuluaga, A.; Iles, W.J.D.; Clements, M.A.; Arroyo, M.T.K.; Leebens-Mack, J. Orchid phylogenomics and multiple drivers of their extraordinary diversification. Proc. Biol. Sci. 2015, 282, 2108–2111. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Ma, P.F.; Li, D.Z. High-throughput sequencing of six bamboo chloroplast genomes: Phylogenetic implications for temperate woody bamboos (Poaceae: Bambusoideae). PLoS ONE 2011, 6, e20596. [Google Scholar] [CrossRef]
- Barba-Montoya, J.; Dos Reis, M.; Schneider, H.; Donoghue, P.C.J.; Yang, Z. Constraining uncertainty in the timescale of angiosperm evolution and the veracity of a Cretaceous Terrestrial Revolution. New Phytol. 2018, 218, 819–834. [Google Scholar] [CrossRef]
- Xi, Z.; Ruhfel, B.R.; Schaefer, H.; Amorim, A.M.; Sugumaran, M.; Wurdack, K.J.; Endress, P.K.; Matthews, M.L.; Stevens, P.F.; Mathews, S. Phylogenomics and a posteriori data partitioning resolve the Cretaceous angiosperm radiation Malpighiales. Proc. Natl. Acad. Sci. USA 2012, 109, 17519–17524. [Google Scholar] [CrossRef]
- Wurdack, K.J.; Davis, C.C. Malpighiales phylogenetics: Gaining ground on one of the most recalcitrant clades in the angiosperm tree of life. Am. J. Bot. 2009, 96, 1551–1570. [Google Scholar] [CrossRef]
- Cai, L.; Xi, Z.; Amorim, A.M.; Sugumaran, M.; Rest, J.S.; Liang, L.; Davis, C.C. Widespread ancient whole genome duplications in Malpighiales coincide with Eocene global climatic upheaval. New Phytol. 2017, 221, 565–576. [Google Scholar] [CrossRef]
- Diffey, J.M. Phylogenetic Relationships of Salicaceae Based on Analyses of Nuclear DNA Data. Honor. Theses 2017, 5, 21. [Google Scholar]
- Group, T.A.P.; Chase, M.W.; Christenhusz, M.J.M.; Fay, M.F.; Byng, J.W.; Judd, W.S.; Soltis, D.E.; Mabberley, D.J.; Sennikov, A.N.; Soltis, P.S.; et al. An update of the angiosperm phylogeny group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–21. [Google Scholar]
- Allen, G.C.; Flores-Vergara, M.A.; Krasynanski, S.; Kumar, S.; Thompson, W.F. A modified protocol for rapid DNA isolation from plant tissues using cetyltrimethylammonium bromide. Nat. Protoc. 2006, 1, 2320–2325. [Google Scholar] [CrossRef]
- Dierckxsens, N.; Mardulyn, P.; Smits, G. NOVOPlasty: De novo assembly of organelle genomes from whole genome data. Nucleic Acids Res. 2017, 45, e18. [Google Scholar]
- Matthew, K.; Richard, M.; Amy, W.; Steven, S.H.; Matthew, C.; Shane, S.; Simon, B.; Alex, C.; Sidney, M.; Chris, D. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar]
- Huang, D.I.; Cronk, Q.C.B. Plann: A Command-Line Application for Annotating Plastome Sequences. Appl. Plant Sci. 2015, 3, apps.1500026. [Google Scholar] [CrossRef]
- Marc, L.; Oliver, D.; Sabine, K.; Ralph, B. OrganellarGenomeDRAW--a suite of tools for generating physical maps of plastid and mitochondrial genomes and visualizing expression data sets. Nucleic Acids Res. 2013, 41, W575–W581. [Google Scholar]
- Thiel, T.; Michalek, W.; Varshney, R.K.; Graner, A. Exploiting EST databases for the development and characterization of gene-derived SSR-markers in barley (Hordeum vulgare L.). Appl. Genet. 2003, 106, 411–422. [Google Scholar] [CrossRef]
- Kurtz, S.; Choudhuri, J.V.; Ohlebusch, E.; Schleiermacher, C.; Stoye, J.; Giegerich, R. REPuter: The manifold applications of repeat analysis on a genomic scale. Nucleic Acids Res. 2001, 29, 4633–4642. [Google Scholar] [CrossRef]
- Benson, G. Tandem repeats finder: A program to analyze DNA sequences. Nucleic Acids Res. 1999, 27, 573–580. [Google Scholar] [CrossRef]
- Frazer, K.A.; Pachter, L.; Poliakov, A.; Rubin, E.M.; Dubchak, I. VISTA: Computational tools for comparative genomics. Nucleic Acids Res. 2004, 32, W273–W279. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2012, 28, 1647. [Google Scholar] [CrossRef]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef]
- Alexandros, S. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar]
- Yang, Z. PAML 4: Phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 2007, 24, 1586–1591. [Google Scholar] [CrossRef]
- Rannala, B.; Yang, Z. Inferring speciation times under an episodic molecular clock. Syst. Biol. 2007, 56, 453–466. [Google Scholar] [CrossRef]
- Huang, C.H.; Sun, R.; Hu, Y.; Zeng, L.; Zhang, N.; Cai, L.; Zhang, Q.; Koch, M.A.; Al-Shehbaz, I.; Edger, P.P. Resolution of Brassicaceae Phylogeny Using Nuclear Genes Uncovers Nested Radiations and Supports Convergent Morphological Evolution. Mol. Biol. Evol. 2016, 33, 394–412. [Google Scholar] [CrossRef]
- Hermant, M.; Hennion, F.; Bartish, I.V.; Yguel, B.; Prinzing, A. Disparate relatives: Life histories vary more in genera occupying intermediate environments. Perspect. Plant Ecol. Evol. Syst. 2012, 14, 283–301. [Google Scholar] [CrossRef]
- Baraloto, C.; Chave, J. Using functional traits and phylogenetic trees to examine the assembly of tropical tree communities. J. Ecol. 2012, 100, 690–701. [Google Scholar] [CrossRef]
- Vanneste, K.; Baele, G.; Maere, S.; Yves, V.D.P. Analysis of 41 plant genomes supports a wave of successful genome duplications in association with the Cretaceous-Paleogene boundary. Genome Res. 2014, 24, 1334–1347. [Google Scholar] [CrossRef]
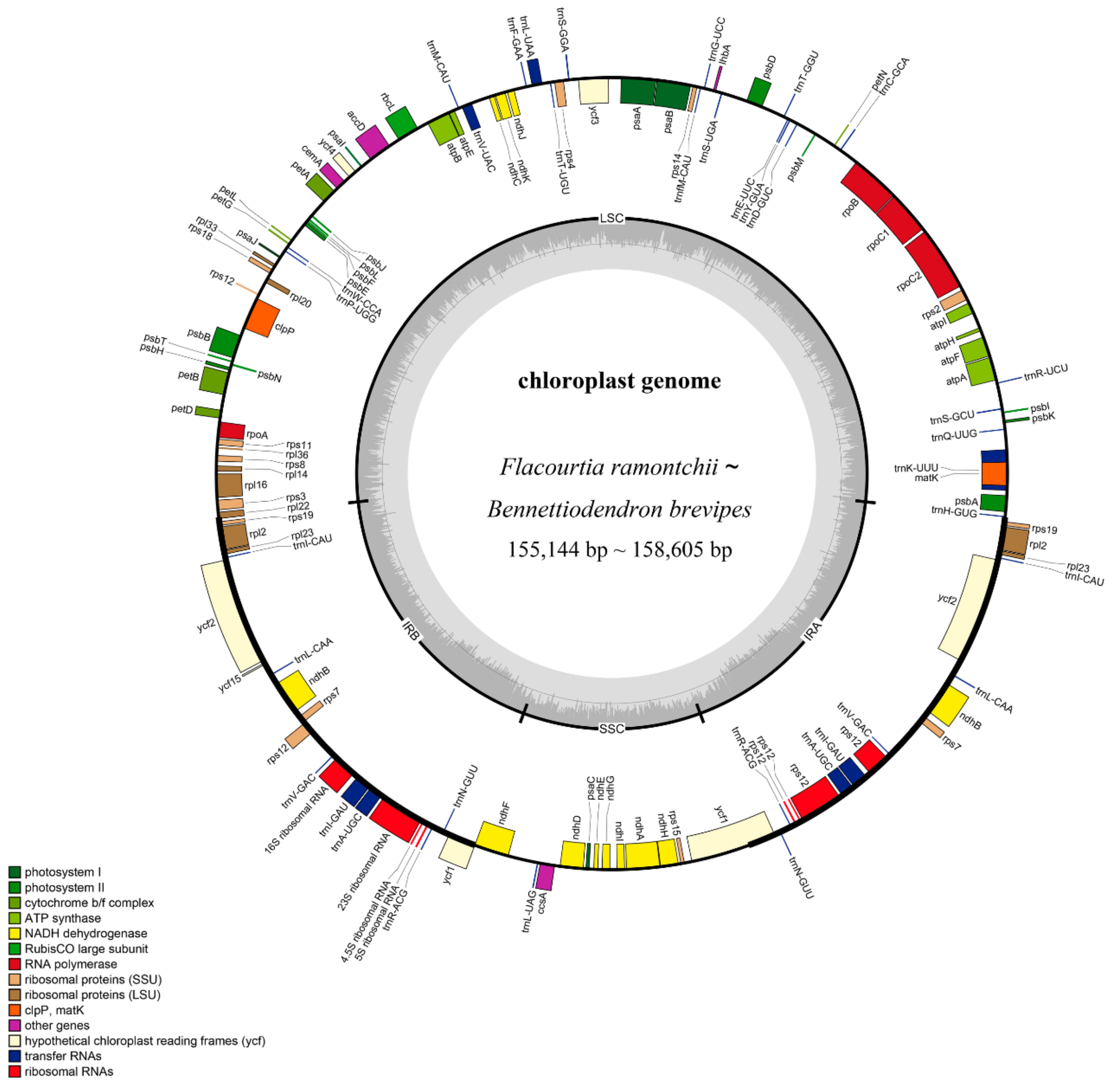




| Species | No. of Total Genes | Genome Size (bp) | LSC Length (bp) | SSC Length (bp) | IR Length (bp) | GC Content (%) | Protein-Coding Genes | No. of tRNAs Genes | No. of rRNAs Genes | Genes with Introns | GenBank Accesion Number |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Populus euphratica * | 131 | 157,839 | 85,858 | 16,649 | 27,666 | 36.5 | 86(8) | 37(7) | 8(4) | 20(5) | MK267314 |
| Salix interior * | 127 | 156,620 | 85,979 | 16,305 | 27,168 | 37.0 | 80(5) | 37(7) | 8(4) | 21(5) | NC_024681 |
| Bennettiodendron leprosipes | 131 | 157,872 | 85,643 | 16,439 | 27,895 | 36.7 | 86(8) | 37(7) | 8(4) | 21(5) | MK281360 |
| Bennettiodendron brevipes | 131 | 158,605 | 86,350 | 16,501 | 27,877 | 36.7 | 86(8) | 37(7) | 8(4) | 21(5) | MK046729 |
| Idesia polycarpa Maxim * | 130 | 157,017 | 84,787 | 16,512 | 27859 | 36.7 | 86(8) | 36(7) | 8(4) | 19(5) | NC_032060 |
| Olmediella betschleriana | 131 | 158,171 | 85,783 | 16,588 | 27,900 | 36.7 | 85(8) | 37(7) | 8(4) | 20(5) | MK044831 |
| Carrierea calycina | 131 | 158,085 | 84,663 | 17,570 | 27,926 | 36.8 | 86(8) | 37(7) | 8(4) | 21(5) | MK263737 |
| Itoa orientalis | 131 | 157,875 | 84,495 | 17,742 | 27,819 | 36.7 | 86(8) | 37(7) | 8(4) | 21(5) | MN078146 |
| Poliothyrsis sinensis * | 129 | 156,680 | 84,793 | 16,605 | 27641 | 36.8 | 85(8) | 37(7) | 8(4) | 19(5) | NC_037412 |
| Flacourtia inermis | 131 | 155,535 | 83,706 | 16,577 | 27,626 | 36.9 | 86(8) | 37(7) | 8(4) | 21(5) | MN078138 |
| Flacourtia ramontchii | 131 | 155,144 | 83,391 | 16,591 | 27,581 | 36.9 | 86(8) | 37(7) | 8(4) | 21(5) | MN078145 |
| Flacourtia rukam | 131 | 155,582 | 83,854 | 16,598 | 27,565 | 36.9 | 86(8) | 37(7) | 8(4) | 21(5) | MK281365 |
| Xylosma congestum | 131 | 155,986 | 84,180 | 16,650 | 27,578 | 36.8 | 86(8) | 37(7) | 8(4) | 21(5) | MN078135 |
| Scolopia chinensis | 131 | 157,292 | 84,464 | 17,716 | 27,556 | 36.8 | 86(8) | 37(7) | 8(4) | 21(5) | MN078144 |
| Scolopia saeva | 131 | 155,832 | 84,062 | 16,436 | 27,667 | 36.9 | 86(8) | 37(7) | 8(4) | 21(5) | MN078143 |
| Dovyalis caffra | 131 | 155,182 | 83,593 | 16,683 | 27,453 | 37.0 | 86(8) | 37(7) | 8(4) | 21(5) | MN078137 |
| Homalium racemosum | 131 | 156,961 | 85,818 | 16,547 | 27,298 | 36.6 | 86(8) | 37(7) | 8(4) | 21(5) | MN078136 |
| Homalium cochinchinense | 131 | 155,339 | 83,701 | 16,566 | 27,536 | 36.8 | 86(8) | 37(7) | 8(4) | 21(5) | MN078140 |
| Banara guianensis | 131 | 158,577 | 85,863 | 17,334 | 27,690 | 36.5 | 86(8) | 37(7) | 8(4) | 21(5) | MH937752 |
| Prockia crucis | 131 | 157,406 | 85,622 | 17,024 | 27,380 | 36.7 | 86(8) | 37(7) | 8(4) | 21(5) | MN078147 |
| Azara serrata | 131 | 158,306 | 85,059 | 17,889 | 27,697 | 36.5 | 86(8) | 37(7) | 8(4) | 21(5) | MH719101 |
| Abatia parviflora | 131 | 156,426 | 84,800 | 16,724 | 27,451 | 36.5 | 86(8) | 37(7) | 8(4) | 21(5) | MN078139 |
| Casearia decandra Jacq | 131 | 156,809 | 84,890 | 17,037 | 27,441 | 36.8 | 85(8) | 37(7) | 8(4) | 21(5) | MN078142 |
| Casearia velutina | 131 | 156,008 | 84,446 | 17,220 | 27,171 | 36.8 | 85(8) | 37(7) | 8(4) | 21(5) | MN078141 |
| Passiflora laurifolia ** | 130 | 151,422 | 85,411 | 13,511 | 26,250 | 37.0 | 77(4) | 37(7) | 8(4) | 20(5) | NC_038121 |
| Passiflora ligularis ** | 128 | 150,827 | 85,471 | 13,502 | 25,927 | 37.0 | 77(4) | 36(7) | 8(4) | 19(5) | NC_038122 |
| Category | Gene | Type Gene | ||||||
|---|---|---|---|---|---|---|---|---|
| Self-replication | Ribosomal RNA | rrn16 | rrn23 | rrn4.5 | rrn5 | |||
| Transfer RNA | trnA-UGC * | trnfM-CAU | trnI-GAU * | trnM-CAU | trnR-ACG | trnS-UGA | ||
| trnC-GCA | trnG-GCC | trnK-UUU * | trnN-GUU | trnW-CCA | trnT-GGU | |||
| trnD-GUC | trnG-UCC * | trnL-CAA | trnY-GUA | trnR-UCU | trnT-UGU | |||
| trnE-UUC | trnH-GUG | trnL-UAA * | trnP-UGG | trnS-GCU | trnV-GAC | |||
| trnF-GAA | trnI-CAU | trnL-UAG | trnQ-UUG | trnS-GGA | trnV-UAC * | |||
| Small ribosomal units | rps11 | rps12 * | rps14 | rps15 | rps18 | |||
| rps19 | rps2 | rps3 | rps4 | rps7 | rps8 | |||
| Large ribosomal units | rpl14 | rpl16 * | rpl2 * | rpl20 | rpl22 | rpl23 | ||
| rpl33 | rpl36 | |||||||
| RNA polymerase subunits | rpoA | rpoB | rpoC1 * | rpoC2 | ||||
| Photosynthesis genes | NADH dehydrogenase | ndhA * | ndhB* | ndhC | ndhD | ndhE | ndhF | |
| ndhG | ndhH | ndhI | ndhJ | ndhK | ||||
| photosystem I | psaA | psaB | psaC | psaI | psaJ | ycf3 * | ycf4 | |
| photosystem II | psbA | psbB | psbC | psbD | psbE | psbF | psbH | |
| psbI | psbJ | psbK | psbL | psbM | psbN | psbT | ||
| psbZ | ||||||||
| cytochrome b/f complex | petA | petB * | petD | petG | petL | petN | ||
| ATP synthase | atpA | atpB | atpE | atpF* | atpH | atpI | ||
| Large subunit of rubisco | rbcL | |||||||
| Other genes | Maturase | matK | ||||||
| Protease | clpP * | |||||||
| Acetyl-CoA-carboxylase subunit | accD | |||||||
| Envelope membrane protein | cemA | |||||||
| Component of TIC complex | ycf1 | |||||||
| c-type cytochrome synthesis | ccsA | |||||||
| Unknown | hypothetical genes reading frames | ycf2 | ||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.-M.; Wang, D.-Y.; Zhang, L.; Kang, M.-H.; Lu, Z.-Q.; Zhu, R.-B.; Mao, X.-X.; Xi, Z.-X.; Ma, T. Intergeneric Relationships within the Family Salicaceae s.l. Based on Plastid Phylogenomics. Int. J. Mol. Sci. 2019, 20, 3788. https://doi.org/10.3390/ijms20153788
Li M-M, Wang D-Y, Zhang L, Kang M-H, Lu Z-Q, Zhu R-B, Mao X-X, Xi Z-X, Ma T. Intergeneric Relationships within the Family Salicaceae s.l. Based on Plastid Phylogenomics. International Journal of Molecular Sciences. 2019; 20(15):3788. https://doi.org/10.3390/ijms20153788
Chicago/Turabian StyleLi, Meng-Meng, De-Yan Wang, Lei Zhang, Ming-Hui Kang, Zhi-Qiang Lu, Ren-Bin Zhu, Xing-Xing Mao, Zhen-Xiang Xi, and Tao Ma. 2019. "Intergeneric Relationships within the Family Salicaceae s.l. Based on Plastid Phylogenomics" International Journal of Molecular Sciences 20, no. 15: 3788. https://doi.org/10.3390/ijms20153788
APA StyleLi, M.-M., Wang, D.-Y., Zhang, L., Kang, M.-H., Lu, Z.-Q., Zhu, R.-B., Mao, X.-X., Xi, Z.-X., & Ma, T. (2019). Intergeneric Relationships within the Family Salicaceae s.l. Based on Plastid Phylogenomics. International Journal of Molecular Sciences, 20(15), 3788. https://doi.org/10.3390/ijms20153788





