Early Sex Differences in the Immune-Inflammatory Responses to Neonatal Ischemic Stroke
Abstract
1. Introduction
2. Results
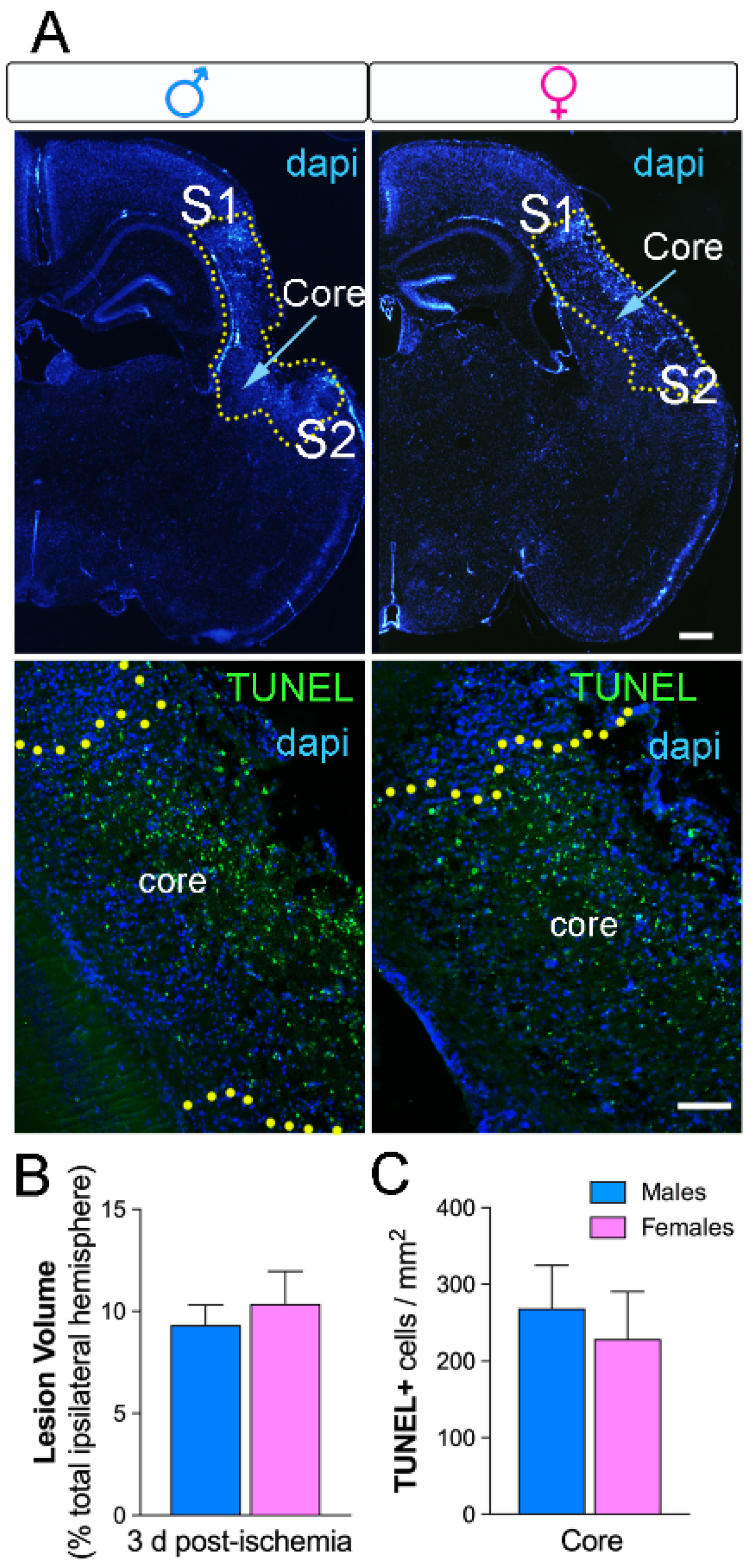
2.1. No Sex Differences in Neuronal Loss and Lesion Size Three Days after Ischemia
2.2. Sex Differences in Microglial Genes Three Days after Ischemia
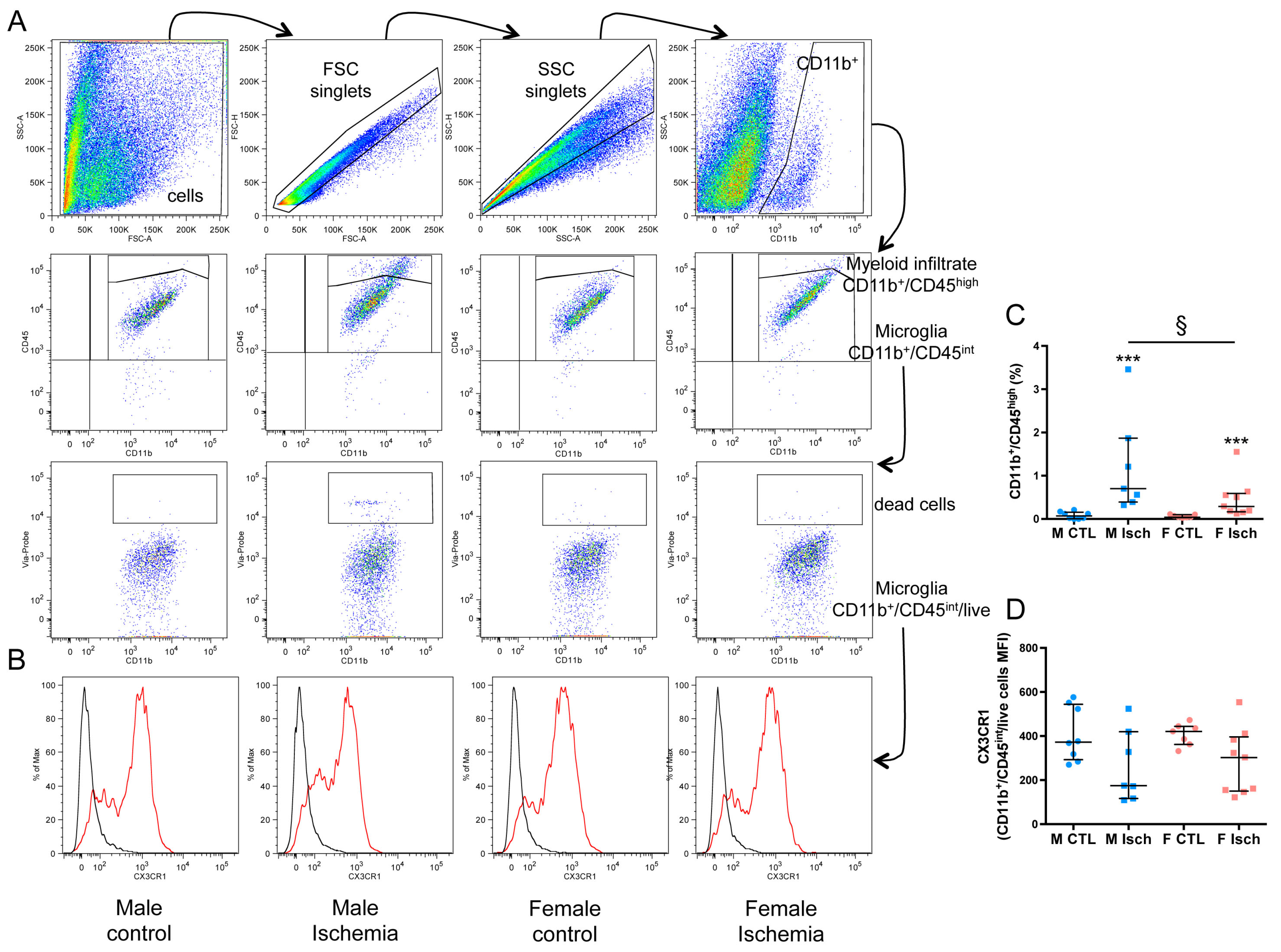
2.3. Sex Differences in Microglial Phenotypes Three Days after Ischemia
2.4. Higher Myeloid Infiltrate in Males Versus Females Three Days after Ischemia
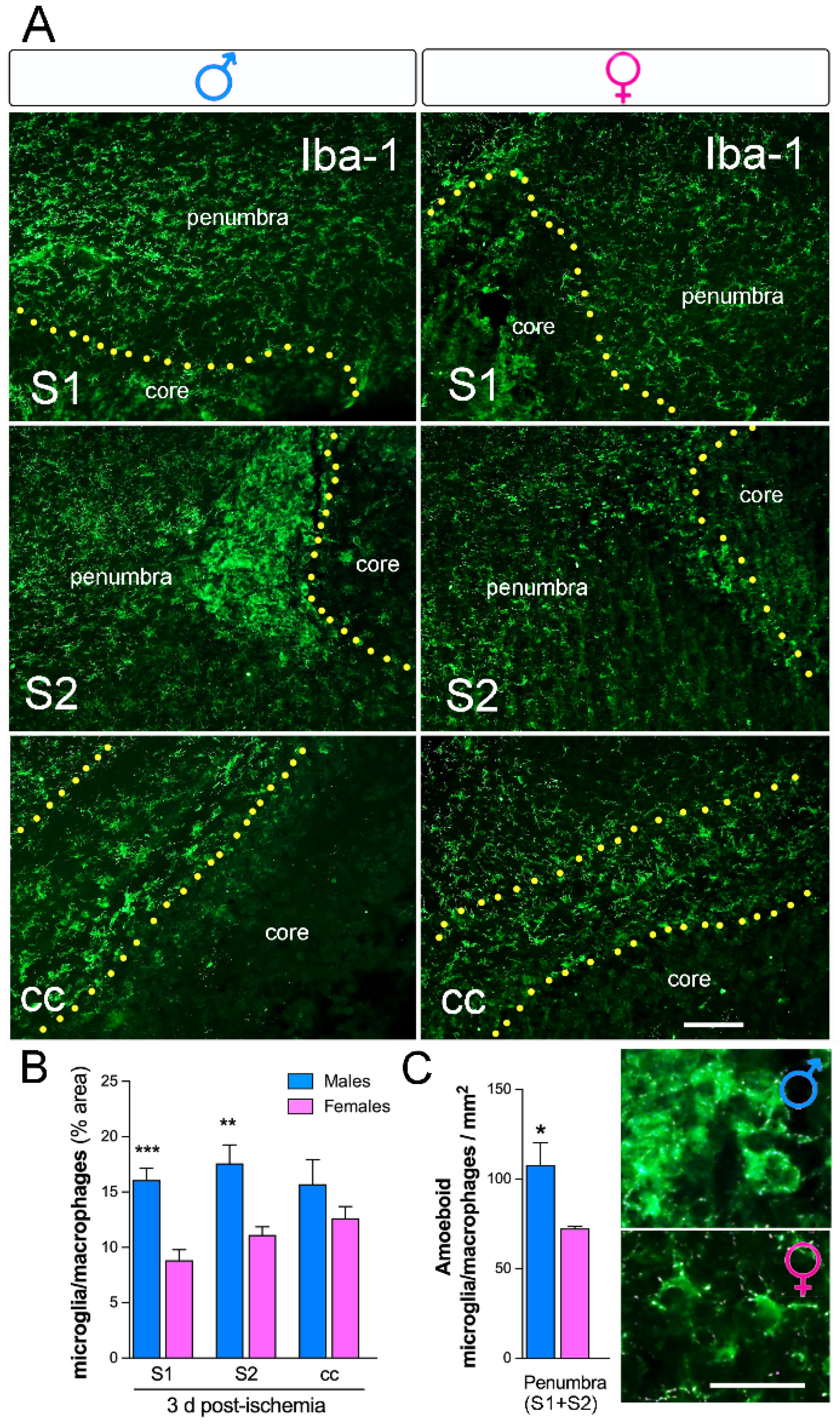
2.5. Increased Microglia Immunoreactivity in Males Versus Females Three Days after Ischemia
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Neonatal Ischemia
4.3. Brain Tissue Dissociation and Magnetic-Activated Cell Sorting (MACS)
4.4. Real-Time Quantitative Reverse Transcriptase Polymerase Chain Reaction
4.5. Flow Cytometry
4.6. Immunohistochemical Techniques
4.7. Fluorescent In Situ Hybridization Combined with Immunofluorescence Staining
4.8. Densitometry and Quantitative Analysis of Immunofluorescence and FISH
4.9. Cell Death Assay
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nelson, K.B. Perinatal ischemic stroke. Stroke 2007, 38, 742–745. [Google Scholar] [CrossRef] [PubMed]
- Coq, J.O.; Delcour, M.; Massicotte, V.S.; Baud, O.; Barbe, M.F. Prenatal ischemia deteriorates white matter, brain organization, and function: Implications for prematurity and cerebral palsy. Dev. Med. Child. Neurol. 2016, 58, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Golomb, M.R.; Dick, P.T.; MacGregor, D.L.; Curtis, R.; Sofronas, M.; de Veber, G.A. Neonatal arterial ischemic stroke and cerebral sinovenous thrombosis are more commonly diagnosed in boys. J. Child. Neurol. 2004, 19, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Golomb, M.R.; Fullerton, H.J.; Nowak-Gottl, U.; Deveber, G. Male predominance in childhood ischemic stroke: Findings from the international pediatric stroke study. Stroke 2009, 40, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Renolleau, S.; Fau, S.; Charriaut-Marlangue, C. Gender-related differences in apoptotic pathways after neonatal cerebral ischemia. Neuroscientist 2008, 14, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Lenz, K.M.; McCarthy, M.M. A starring role for microglia in brain sex differences. Neuroscientist 2015, 21, 306–321. [Google Scholar] [CrossRef] [PubMed]
- Gur, R.C.; Mozley, P.D.; Resnick, S.M.; Gottlieb, G.L.; Kohn, M.; Zimmerman, R.; Herman, G.; Atlas, S.; Grossman, R.; Berretta, D.; et al. Gender differences in age effect on brain atrophy measured by magnetic resonance imaging. Proc. Natl. Acad. Sci. USA 1991, 88, 2845–2849. [Google Scholar] [CrossRef]
- Charriaut-Marlangue, C.; Besson, V.C.; Baud, O. Sexually Dimorphic Outcomes after Neonatal Stroke and Hypoxia-Ischemia. Int. J. Mol. Sci. 2017, 19, 61. [Google Scholar] [CrossRef]
- Kanazawa, M.; Nimomiya, I.; Hatakeyama, M.; Takahashi, T.; Shimohata, T. Microglia and monocytes/macrophages polarization reveal novel therapeutic mechanism against stroke. Int. J. Mol. Sci. 2017, 18, 2135. [Google Scholar] [CrossRef]
- Murray, K.N.; Girard, S.; Holmes, W.M.; Parkes, L.M.; Williams, S.R.; Parry-Jones, A.R.; Allan, S.M. Systemic inflammation impairs tissue reperfusion through endothelin-dependent mechanisms in cerebral ischemia. Stroke 2014, 45, 3412–3419. [Google Scholar] [CrossRef]
- Moretti, R.; Leger, P.L.; Besson, V.C.; Csaba, Z.; Pansiot, J.; Di Criscio, L.; Gentili, A.; Titomanlio, L.; Bonnin, P.; Baud, O.; et al. Sildenafil, a cyclic GMP phosphodiesterase inhibitor, induces microglial modulation after focal ischemia in the neonatal mouse brain. J. Neuroinflamm. 2016, 13, 95. [Google Scholar] [CrossRef] [PubMed]
- Charriaut-Marlangue, C.; Leconte, C.; Csaba, Z.; Chafa, L.; Pansiot, J.; Talatizi, M.; Simon, K.; Moretti, R.; Marchand-Leroux, C.; Baud, O.; et al. Sex differences in the effects of PARP inhibition on microglial phenotypes following neonatal stroke. Brain Behav. Immun. 2018, 73, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Mulligan, M.K.; Nowak, T.S., Jr. Substrain- and sex-dependent differences in stroke vulnerability in C57BL/6 mice. J. Cereb. Blood Flow Metab. 2019, 39, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Faber, J.E.; Moore, S.M.; Lucitti, J.L.; Aghajanian, A.; Zhang, H. Sex Differences in the Cerebral Collateral Circulation. Transl. Stroke Res. 2017, 8, 273–283. [Google Scholar] [CrossRef]
- Mirza, M.A.; Ritzel, R.; Xu, Y.; McCullough, L.D.; Liu, F. Sexually dimorphic outcomes and inflammatory responses in hypoxic-ischemic encephalopathy. J. Neuroinflam. 2015, 12, 32. [Google Scholar] [CrossRef]
- Smith, P.L.P.; Mottahedin, A.; Svedin, P.; Mohn, C.J.; Hagberg, H.; Ek, J.; Mallard, C. Peripheral myeloid cells contribute to brain injury in male neonatal mice. J. Neuroinflamm. 2018, 15, 301. [Google Scholar] [CrossRef] [PubMed]
- Benjelloun, N.; Renolleau, S.; Represa, A.; Ben-Ari, Y.; Charriaut-Marlangue, C. Inflammatory responses in the cerebral cortex after ischemia in the P7 neonatal Rat. Stroke 1999, 30, 1916–1923. [Google Scholar] [CrossRef]
- Nazmi, A.; Albertsson, A.M.; Rocha-Ferreira, E.; Zhang, X.; Vontell, R.; Zelco, A.; Rutherford, M.; Zhu, C.; Nilsson, G.; Mallard, C.; et al. Lymphocytes Contribute to the Pathophysiology of Neonatal Brain Injury. Front. Neurol. 2018, 9, 159. [Google Scholar] [CrossRef]
- Rahimian, R.; Cordeau, P., Jr.; Kriz, J. Brain Response to Injuries: When Microglia Go Sexist. Neuroscience 2018, 45, 14–23. [Google Scholar] [CrossRef]
- Sheiner, E.; Levy, A.; Katz, M.; Hershkovitz, R.; Leron, E.; Mazor, M. Gender does matter in perinatal medicine. Fetal Diagn. Ther. 2004, 19, 366–369. [Google Scholar] [CrossRef]
- Dunn, L.; Prior, T.; Greer, R.; Kumar, S. Gender specific intrapartum and neonatal outcomes for term babies. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 185, 19–22. [Google Scholar]
- Darmency-Stamboul, V.; Chantegret, C.; Ferdynus, C.; Mejean, N.; Durand, C.; Sagot, P. Antenatal factors associated with perinatal arterial ischemic stroke. Stroke 2012, 43, 2307–2312. [Google Scholar] [CrossRef] [PubMed]
- Bruce-Keller, A.J.; Dimayuga, F.O.; Reed, J.L.; Wang, C.; Angers, R.; Wilson, M.E.; Dimayuga, V.M.; Scheff, S.W. Gender and estrogen manipulation do not affect traumatic brain injury in mice. J. Neurotrauma 2007, 24, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Dimayuga, F.O.; Reed, J.L.; Carnero, G.A.; Wang, C.; Dimayuga, E.R.; Dimayuga, V.M.; Perger, A.; Wilson, M.E.; Keller, J.N.; Bruce-Keller, A.J. Estrogen and brain inflammation: Effects on microglial expression of MHC, costimulatory molecules and cytokines. J. Neuroimmunol. 2005, 161, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Al Mamun, A.; Yu, H.; Romana, S.; Liu, F. Inflammatory Responses are Sex Specific in Chronic Hypoxic-Ischemic Encephalopathy. Cell Transplant. 2018, 27, 1328–1339. [Google Scholar] [CrossRef] [PubMed]
- Herz, J.; Köster, C.; Crasmöller, M.; Abberger, H.; Hansen, W.; Felderhoff-Müser, U.; Bendix, I. Peripheral T Cell Depletion by FTY720 Exacerbates Hypoxic-Ischemic Brain Injury in Neonatal Mice. Front. Immunol. 2018, 9, 1696. [Google Scholar] [CrossRef] [PubMed]
- Denker, S.P.; Ji, S.; Dingman, A.; Lee, S.Y.; Derugin, N.; Wendland, M.F.; Vexler, Z.S. Macrophages are comprised of resident brain microglia not infiltrating peripheral monocytes acutely after neonatal stroke. J. Neurochem. 2007, 100, 893–904. [Google Scholar] [CrossRef]
- Ritzel, R.M.; Patel, A.R.; Grenier, J.M.; Crapser, J.; Verma, R.; Jellison, E.R.; McCullough, L.D. Functional differences between microglia and monocytes after ischemic stroke. J. Neuroinflamm. 2015, 12, 106. [Google Scholar] [CrossRef]
- Wattananit, S.; Tornero, D.; Graubardt, N.; Memanishvili, T.; Monni, E.; Tatarishvili, J.; Miskinyte, G.; Ge, R.; Ahlenius, H.; Lindvall, O.; et al. Monocyte-Derived Macrophages Contribute to Spontaneous Long-Term Functional Recovery after Stroke in Mice. J. Neurosci. 2016, 36, 4182–4195. [Google Scholar] [CrossRef]
- Lenz, K.M.; Nugent, B.M.; Haliyur, R.; McCarthy, M.M. Microglia are essential to masculinization of brain and behavior. J. Neurosci. 2013, 33, 2761–2772. [Google Scholar] [CrossRef]
- Savman, K.; Heyes, M.P.; Svedin, P.; Karlsson, A. Microglia/macrophage-derived inflammatory mediators galectin-3 and quinolinic acid are elevated in cerebrospinal fluid from newborn infants after birth asphyxia. Transl. Stroke Res. 2013, 4, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Wolf, Y.; Yona, S.; Kim, K.W.; Jung, S. Microglia, seen from the CX3CR1 angle. Front. Cell Neurosci. 2013, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Wyss-Coray, T.; Mucke, L. Inflammation in neurodegenerative disease-a double-edged sword. Neuron 2002, 35, 419–432. [Google Scholar] [CrossRef]
- Zabel, M.K.; Zhao, L.; Zhang, Y.; Gonzalez, S.R.; Ma, W.; Wang, X.; Fariss, R.N.; Wong, W.T. Microglial phagocytosis and activation underlying photoreceptor degeneration is regulated by CX3CL1-CX3CR1 signaling in a mouse model of retinitis pigmentosa. Glia 2016, 64, 1479–1491. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Gan, Y.; Liu, Q.; Yin, Q.; Shi, J.; Shi, F.-D. CX3CR1 deficiency suppresses activation and neurotoxicity of microglia/macrophage in experimental ischemic stroke. J. Neuroinflamm. 2014, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Hoogland, I.C.; Houbolt, C.; van Westerloo, D.J.; van Gool, W.A.; van de Beek, D. Systemic inflammation and microglial activation: Systematic review of animal experiments. J. Neuroinflamm. 2015, 12, 114. [Google Scholar] [CrossRef] [PubMed]
- Perego, C.; Fumagalli, S.; Zanier, E.R.; Carlino, E.; Panini, N.; Erba, E.; De Simoni, M.G. Macrophages are essential for maintaining a M2 protective response early after ischemic brain injury. Neurobiol. Dis. 2016, 96, 284–293. [Google Scholar] [CrossRef]
- Luchena, C.; Zuazo-Ibarra, J.; Alberdi, E.; Matute, C.; Capetillo-Zarate, E. Contribution of Neurons and Glial Cells to Complement-Mediated Synapse Removal during Development, Aging and in Alzheimer’s Disease. Mediat. Inflamm. 2018, 2018, 12. [Google Scholar] [CrossRef]
- Morrison, H.W.; Filosa, J.A. Sex differences in astrocyte and microglia responses immediately following middle cerebral artery occlusion in adult mice. Neuroscience 2016, 339, 85–99. [Google Scholar] [CrossRef]
- Hu, X.; Li, P.; Guo, Y.; Wang, H.; Leak, R.K.; Chen, S.; Gao, Y.; Chen, J. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke 2012, 43, 3063–3070. [Google Scholar] [CrossRef]
- Ransohoff, R.M. A polarizing question: Do M1 and M2 microglia exist? Nat. Neurosci. 2016, 19, 987–991. [Google Scholar] [CrossRef]
- Cherry, J.D.; Olschowka, J.A.; O’Banion, M.K. Neuroinflammation and M2 microglia: The good, the bad, and the inflamed. J. Neuroinflamm. 2014, 11, 98. [Google Scholar] [CrossRef]
- Peruzzotti-Jametti, L.; Donega, M.; Giusto, E.; Mallucci, G.; Marchetti, B.; Pluchino, S. The role of the immune system in central nervous system plasticity after acute injury. Neuroscience 2014, 283, 210–221. [Google Scholar] [CrossRef]
- Krishnan, M.L.; Van Steenwinckel, J.; Schang, A.L.; Yan, J.; Arnadottir, J.; Le Charpentier, T.; Csaba, Z.; Dournaud, P.; Cipriani, S.; Auvynet, C.; et al. Integrative genomics of microglia implicates DLG4 (PSD95) in the white matter development of preterm infants. Nat. Commun. 2017, 8, 428. [Google Scholar] [CrossRef] [PubMed]
- Fe Lanfranco, M.; Loane, D.J.; Mocchetti, I.; Burns, M.P.; Villapol, S. Combination of Fluorescent in situ Hybridization (FISH) and Immunofluorescence Imaging for Detection of Cytokine Expression in Microglia/Macrophage Cells. Bio-Protocol 2017, 7, e2608. [Google Scholar] [CrossRef] [PubMed]
- Villapol, S.; Loane, D.J.; Burns, M.P. Sexual dimorphism in the inflammatory response to traumatic brain injury. Glia 2017, 65, 1423–1438. [Google Scholar] [CrossRef]
- McCarthy, M.M. Sex differences in neuroimmunity as an inherent risk factor. Neuropsychopharmacology 2019, 44, 38–44. [Google Scholar] [CrossRef]


| Microglial Cells | M CTL | M Isch | F CTL | F Isch | p |
|---|---|---|---|---|---|
| Kruskal–Wallis | |||||
| Test | |||||
| % CD11b+/CD45 int (microglia) | 9.3 | 9.5 | 7 | 7.7 | 0.2588 |
| (8.7–11.1) | (7.6–9.7) | (6.6–8.4) | (5.3–10.1) | ||
| (n) | (8) | (7) | (7) | (9) | |
| % Dead cells in CD11b+/CD45 int | 1.3 | 2.6 | 0.8 | 2.4 | 0.014 |
| (0.7–2.3) | (2.0–3.3) | (0.6–1.0) | (1.5–2.7) | ||
| (n) | (5) | (4) | (4) | (5) | |
| * p = 0.0159 | |||||
| % CD11b+/CD45 high (myeloid infiltrate) | 0.07 | 0.7 | 0.04 | 0.3 | <0.0001 |
| (0.0005–0.2) | (0.4–2) | (0.03–0.1) | (0.2–0.6) | ||
| (n) | (8) | (7) | (7) | (9) | |
| * p = 0.0003 | * p = 0.0002 | ||||
| § p = 0.0418 | |||||
| CD11b Mean Fluorescence Intensity (MFI) | 3123 | 4036 | 3195 | 4548 | 0.0082 |
| (3006–3424) | (3142–4527) | (2995–3506) | (3715–4896) | ||
| (n) | (6) | (6) | (7) | (9) | |
| * p = 0.0079 | |||||
| % CX3CR1+ cells in CD11b+/CD45 int | 59.6 | 46.2 | 68.7 | 63.9 | 0.0359 |
| (52.1–65.9) | (33.4–61.2) | (63.4–72.3) | (42.2–69 0) | ||
| (n) | (8) | (7) | (7) | (9) | |
| § p = 0.0541 | |||||
| CX3CR1 MFI in CD11b+/CD45 int | 373 | 175 | 421 | 302 | 0.1004 |
| (293–544) | (116–419) | (362–444) | (150–397) | ||
| (n) | (8) | (7) | (7) | (9) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villapol, S.; Faivre, V.; Joshi, P.; Moretti, R.; Besson, V.C.; Charriaut-Marlangue, C. Early Sex Differences in the Immune-Inflammatory Responses to Neonatal Ischemic Stroke. Int. J. Mol. Sci. 2019, 20, 3809. https://doi.org/10.3390/ijms20153809
Villapol S, Faivre V, Joshi P, Moretti R, Besson VC, Charriaut-Marlangue C. Early Sex Differences in the Immune-Inflammatory Responses to Neonatal Ischemic Stroke. International Journal of Molecular Sciences. 2019; 20(15):3809. https://doi.org/10.3390/ijms20153809
Chicago/Turabian StyleVillapol, Sonia, Valerie Faivre, Pooja Joshi, Raffaella Moretti, Valerie C. Besson, and Christiane Charriaut-Marlangue. 2019. "Early Sex Differences in the Immune-Inflammatory Responses to Neonatal Ischemic Stroke" International Journal of Molecular Sciences 20, no. 15: 3809. https://doi.org/10.3390/ijms20153809
APA StyleVillapol, S., Faivre, V., Joshi, P., Moretti, R., Besson, V. C., & Charriaut-Marlangue, C. (2019). Early Sex Differences in the Immune-Inflammatory Responses to Neonatal Ischemic Stroke. International Journal of Molecular Sciences, 20(15), 3809. https://doi.org/10.3390/ijms20153809






