1. Introduction
Pluripotent stem cells (PSCs), such as embryonic stem cells (ESCs) or induced pluripotent stem cells (iPSCs), are characterized by unique properties, i.e., the ability of extensive proliferation, self-renewal, as well as multidirectional differentiation enabling generation of all mammalian cell types. For this reason, PSCs are considered as a potential source of cells that could be transplanted into defective tissues or organs to replace lost, damaged or malfunctioning cells. The first clinical trials using PSC derivatives have already been conducted and reported [
1]. Among conditions that could be treated with PSC derivatives are skeletal muscle dysfunctions which may result inter alia from severe physical trauma or progression of skeletal muscle diseases, such as muscular dystrophies or spinal muscle atrophy. To reach this aim PSCs must be converted into functional myogenic cells with high efficiency and, preferentially, under defined culture conditions.
PSCs present naive myogenic potential as they form skeletal muscle tissue in vivo, during teratoma formation or chimeric animal development [
2,
3]. However, despite numerous attempts, efficient in vitro conversion of PSCs into functional myogenic cells had been challenging until factors involved in embryonic myogenesis were used to promote PSC myogenic differentiation [
4]. Activation of canonical Wnt, followed by fibroblast growth factor 2 (FGF-2) treatment led to the derivation of myogenic cells with high efficiency, i.e., nearly 90% of cells derived from mouse or human ESCs synthesized myogenic markers—such as Pax3, the marker of skeletal muscle precursor cells formed during embryonic myogenesis, or myosin heavy chains (MHC)—characteristic of already committed and differentiated myogenic cells [
5]. However, the generation of such cells required 50-day long culture [
5]. Moreover, their myogenic potential has not been verified in vivo. In the study by Chal and co-workers, the activation of canonical Wnt signaling with simultaneous BMP4 inhibition enabled the conversion of PSCs into multinucleated striated myofibers, i.e., basic structural and functional skeletal muscle units, which were accompanied by cells expressing Pax7, i.e., marker characteristic of satellite cells, skeletal muscle stem cells responsible for muscle growth and regeneration. Transplantation of Pax7-positive cells into
mdx mice, the animal model of Duchenne muscular dystrophy (DMD), led to the appearance of dystrophin, a protein which is not present in DMD muscle, causing myofiber membrane fragility and muscle injuries. Moreover, transplanted cells settled in the satellite cell niche, which is crucial for effective and long lasting cell therapy for dysfunctional skeletal muscles [
6]. Altogether, several protocols have already demonstrated that myogenic differentiation of PSCs can be successfully supported by myogenesis regulators such as FGF-2, Wnts, and other factors [
7]. However, indicated methods are usually time-consuming and, therefore, should be further improved to be even more efficient, faster, safer, and conducted under defined culture conditions.
In the current study, we focused on the influence of interleukin 4 (IL-4) on the fate of PSCs, mainly their competence for myogenic conversion. IL-4 plays an important role in myogenesis as it is engaged in recruiting myoblasts—i.e., mononuclear committed myogenic cells—into nascent multinuclear myotubes, leading to the increase in nuclei number in myotubes, their growth, and eventually myofiber formation. This process is critical for proper development and maintenance of functional skeletal muscles because, as mentioned above, multinucleated myofibers are their basic units. In skeletal muscle, IL-4 acts through the type II receptor, comprised of IL-4Rα and IL-13Rα1 [
8]. Lack of either IL-4 or IL-4Rα receptor subunit in mouse skeletal muscle results in the decreased number of nuclei in myofibers, and—as a consequence—smaller and thinner muscles of such knockout mice [
8]. Similar effect was observed during postnatal development of mice lacking serum response factor (SRF), a transcription factor regulating expression of different genes crucial for proper muscle function such as dystrophin, muscle creatinine kinase, or insulin-like growth factor 1 (IGF-1). Disruption of the
Srf gene results in strong downregulation of IL-4 expression, impairing myoblast recruitment to myotubes and myofibers, delayed postnatal muscle growth, and decreased muscle mass [
9]. The role of IL-4 is not limited to the skeletal muscle cells. This cytokine is also important for immune cells, as it modulates inter alia proliferation of B and T cells [
9,
10,
11,
12]. It was also shown that IL-4 promotes fusion of bone marrow derived mesenchymal stem cells (BM-MSCs) with myoblasts during their co-culture [
13]. However, the mechanism by which IL-4 influences different cell types is only partially understood. It was reported that IL-4 induces expression of vascular cell adhesion molecule 1 (VCAM1) in eosinophils and basophils [
14] and increases the expression of integrins and other cell membrane proteins in B cells [
15] and macrophages [
16]. Elevated expression of β1 and β3 integrins was also detected in myogenic cells treated with IL-4 which, as a result, were characterized by enhanced ability to migrate both in vitro and in vivo during skeletal muscle regeneration [
17]. All these reports suggest that IL-4-mediated modulation of cells’ ability to adhere, migrate and fuse may be linked with the changes in the expression of cell membrane proteins, induced by this cytokine.
Our previous study showed that undifferentiated mouse ESCs may fuse with myoblasts during their co-culture. As a result of it, ESC-derived nuclei found in hybrid myotubes formed by both ESCs and myoblasts are characterized by the presence of myogenic regulatory factors (MRFs), i.e., markers characteristic of committed myogenic cells [
18]. However, this process occurs with a very low efficiency: hybrid myotubes make up to no more than 1% of all myotubes found in the co-culture of ESCs and myoblasts. In the same study, we also showed that undifferentiated ESCs lack proteins important for skeletal muscle myoblast fusion such as VCAM1 and M-cadherin, which may limit their competence for fusion [
18]. On the basis of previously described data on IL-4’s role in myogenic and other cells, we investigated the influence of this cytokine on PSCs to verify whether IL-4 may enhance ESC ability to fuse with myoblasts as well as other features of these cells, e.g., proliferation rate or their gene expression profile. We analyzed both undifferentiated ESCs and cells cultured in embryoid bodies (EBs), i.e., three dimensional aggregates formed by PSCs differentiating in suspension culture, in medium lacking self-renewal promoting factors, such as leukemia inhibitory factor (LIF).
3. Discussion
In our previous study, we showed that undifferentiated mouse ESCs fuse with skeletal muscle myoblasts, which leads to the appearance of MRFs, such as MyoD and myogenin, in ESC-derived nuclei localized in hybrid myotubes [
18]. Fusion of ESCs with myoblasts was a prerequisite for their myogenic conversion as no significant effect was noticed for ESCs which did not participate in hybrid myotube formation. Thus, factors released by differentiating myoblasts and present in culture medium promoting myoblast differentiation were not sufficient to induce myogenic conversion of ESCs until other factors—e.g., pre-treatment with 5-azactidine, hemimethylating agent—were used. Currently available protocols enable efficient myogenic conversion of PSCs by modulating crucial pathways regulating myogenesis, such as those dependent on Wnt, FGF-2, or BMP-4 [
4]. However, while development of functional skeletal muscle during mouse embryogenesis takes circa 10 days, abovementioned protocols usually require much longer—e.g., 5 times as much time—to generate myogenic cells from PSCs [
5]. Moreover, in many protocols animal-derived reagents are used while generation of clinically-relevant human cells should be conducted under defined and animal-free conditions. For this reason, further improvement of protocols leading to myogenic conversion of PSCs is needed. To address this issue, we investigated IL-4 as a potential booster of myogenic conversion for mouse ESCs.
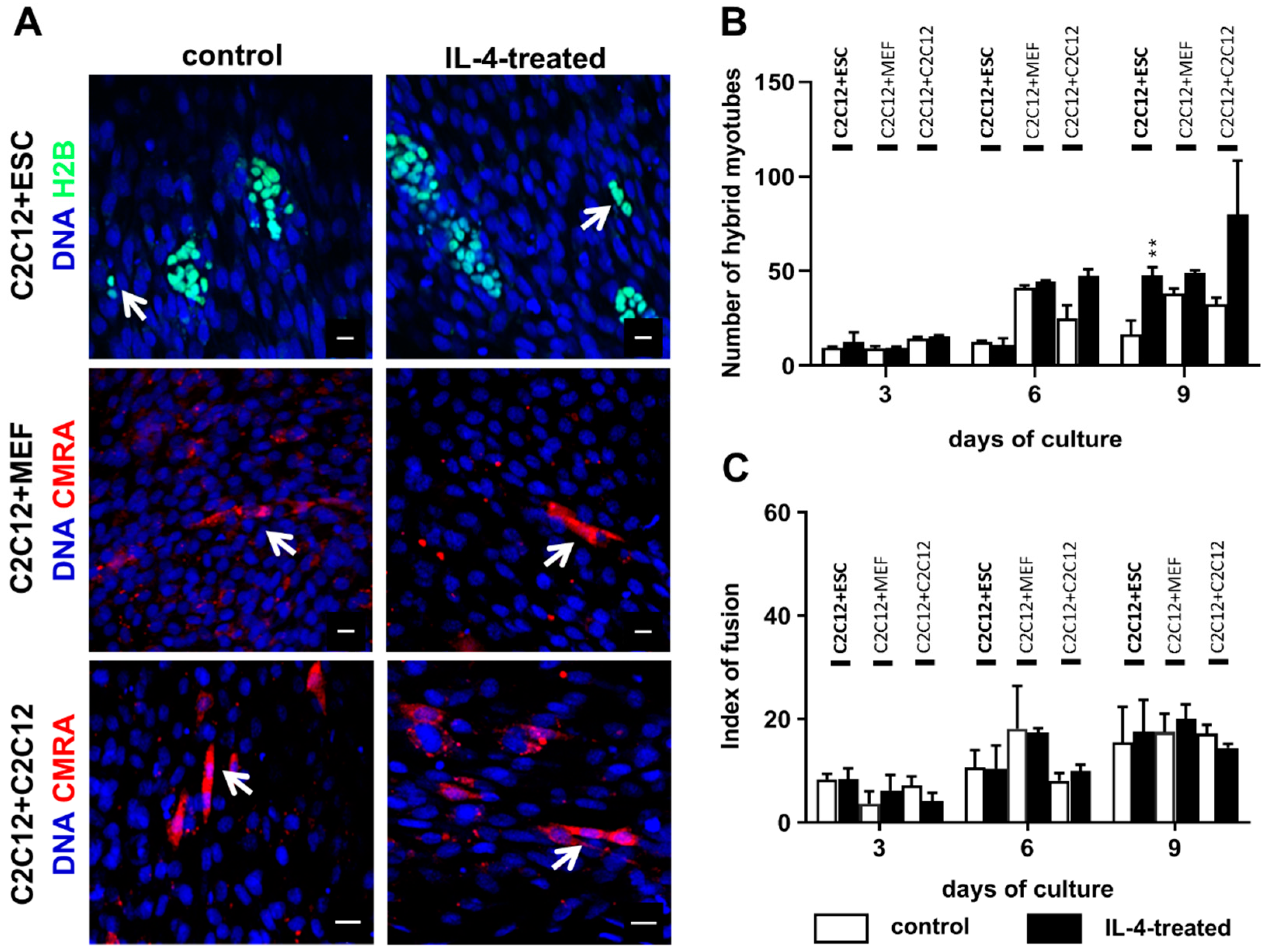
As mentioned before, fusion between undifferentiated ESCs and myoblasts leading to formation of hybrid myotubes occurs with very low frequency (no higher than 1%; [
18]). Since IL-4 promotes fusion of myoblasts with nascent myotubes, we hypothesized that co-culture of undifferentiated ESCs and myoblasts in the presence of this cytokine may enhance formation of hybrid myotubes. Initial experiments confirmed the presence of IL-4 type II receptor subunits in myoblasts, which is in line with the previous report [
8] and revealed the presence of both subunits in undifferentiated as well as differentiating ESCs. We also showed that ESCs do not express IL-4, while secretion of this cytokine significantly increases during myoblast differentiation progression, together with the increase in IL-4Rα subunit expression. Our results partially stay in agreement with a report by Schmitt and co-workers who showed that IL-4 is not produced either in undifferentiated or differentiating ESCs, while the receptor for this cytokine appears in differentiating but not undifferentiated ESCs [
27]. However, the type of IL-4 receptor analyzed in the indicated study was not specified. We found that γC, the subunit of IL-4 type I receptor, is not expressed in undifferentiated ESCs, so this receptor type is indeed not present in these cells in contrast to type II receptor.
The co-culture of undifferentiated ESCs and myoblasts in the presence of IL-4 resulted in a higher number of hybrid myotubes than in the control variant, but it should be noted that such myotubes were still scarce and made up to no more than 3% of all myotubes formed in the co-culture. We also noticed the increase in hybrid myotube number in the IL-4-treated co-culture of proliferating and differentiating myoblasts in comparison to the control, but due to high standard deviations observed differences were not statistically significant. IL-4 did not influence the co-culture of myoblasts with MEFs, which is in agreement with the fact that we did not detect IL-4 receptor in MEFs. We also showed that IL-4 has no impact on proliferation of any cell types used in the experiments—i.e., undifferentiated and differentiating ESCs, MEFs, and myoblasts—cultured separately as well as in the co-cultures with C2C12 myoblasts. To our knowledge, the lack of IL-4 influence on PSC and MEF proliferation has not been described yet, while Lafreniere and co-workers already reported that IL-4 does not impact human myoblast proliferation [
17]. Altogether, these results indicate that in contrast to immune cells, such as T cells, B cells, or mast cells [
9,
10,
11,
28], IL-4 does not influence the proliferation of PSCs, MEFs or skeletal muscle myoblasts. Although this statement has not been verified by us, one can hypothesize that the influence of IL-4 on cell proliferation may depend on the type of IL-4 receptor present in the cell membrane. While immune cells express IL-4 type I receptors, both ESCs and myoblasts are characterized by the presence of IL-4 type II receptor.
Since IL-4 had no significant effect on the fusion index in the co-culture of ESCs and myoblasts, but at the same time moderately elevated the number of hybrid myotubes formed by these cells, we concluded that this effect is mainly linked to the IL-4 impact on ESCs. To further address this issue, we cultured undifferentiated ESCs in the presence or absence of IL-4 under defined culture conditions—i.e., in the medium containing SR instead of serum. Moreover, we included ESCs differentiating in EBs in this analysis, as the effect of IL-4 action was noticed only after 9 days of the ESC-myoblast co-culture when first stages of ESC differentiation could have already occurred. Since several reports already described the influence of IL-4 on cell membrane proteins, e.g., [
13,
14,
15,
16,
17], we performed analysis of selected genes encoding cell membrane proteins but did not observe any significant effects except for transient elevation of
Vcam1 expression in undifferentiated ESCs. Changes in gene expression were, however, not accompanied by the synthesis of VCAM1 protein in IL-4-treated ESCs. Surprisingly, we found that IL-4 treatment results in the significant increase in the expression level of mesodermal and early myogenic markers:
Msgn1,
Pdgfra,
Pax3, and
Pax7 in differentiating ESCs. IL-4 effect was mainly visible in EB5 and EB7, which is in agreement with reports indicating appearance of the first PSC-derived myogenic cells in EBs cultured for 5 and 7 days [
2]. Since IL-4 also decreased the levels of late myogenic regulators such as
Myf-5 and
MyoD in differentiating ESCs, we speculated that this cytokine may enhance early stages of myogenic conversion of PSCs. Decrease in
Myf-5 and
MyoD expression in IL-4-treated ESCs turned out to be transient as the expression of these genes increased in outgrowths derived from such EBs and was even higher than in control—i.e., untreated EBs (data not shown).
Nevertheless, our results indicate that IL-4 affects competence of mouse ESCs for myogenic conversion by increasing their participation in hybrid myotube formation during co-culture with C2C12 myoblasts, as well as by elevating the expression level of genes encoding mesodermal and early myogenic markers—such as
Msgn1,
Pax3, and
Pax7. Since the observed effect was rather modest, IL-4 treatment is not sufficient to induce robust PSC myogenic differentiation alone but it could be implemented to other protocols to further improve such conversion. To our knowledge, this effect has not been reported so far, while the increased frequency of fusion between myoblasts and stem cells co-cultured in the presence of IL-4 was already described for bone marrow mesenchymal stem cells (BM-MSCs) [
13]. Interestingly, our recent research revealed that pre-treatment of BM-MSCs with IL-4 results in higher frequency of hybrid myotube formation in the subsequent co-culture of these cells with myoblasts but does not increase the expression of selected cell membrane factors engaged in adhesion and fusion (Kasprzycka et al., submitted). This may indicate that IL-4 may impact different types of stem cells by the same, yet unknown mechanism, enhancing their competence for fusion and myogenic conversion.
4. Materials and Methods
4.1. Animals
Animal care and all experimental procedures were approved by the Local Ethics Committee No. 1 in Warsaw, Poland, according to the European Union Directive on the approximation in laws, regulations, and administrative provisions of the Member States regarding protection of animals used for experimental and scientific purposes (permit number: 300/2012; 24 May 2012; Poland).
4.2. Cell Culture
Mouse embryonic fibroblasts (MEFs) were isolated from 13.5-day-old embryos obtained after mating C57BL6N mice. MEFs were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 4.5 mg/L glucose (Thermo Fisher Scientific, Waltham, MA, USA), supplemented with 10% heat inactivated fetal bovine serum (FBS, Thermo Fisher Scientific) as well as 50 U/mL penicillin (Thermo Fisher Scientific) and 50 µg/mL streptomycin (Thermo Fisher Scientific; hereafter referred to as antibiotics). After reaching confluence MEFs were inactivated with mitomycin C (0.01 mg/mL in MEF culture medium; Sigma-Aldrich) and next used as a feeder layer for ESC culture. Mouse ESCs constitutively expressing histone H2B fused with GFP (hereafter referred to as ESCs-GFP) were provided by Dr. Kat Hadjantonakis, Memorial Sloan Kettering Cancer Center New York [
29].
Inactivated MEFs were plated onto culture dishes coated with 1% gelatin (Sigma-Aldrich) in DMEM with 4.5 mg/L glucose (Thermo Fisher Scientific), supplemented with 10% heat inactivated FBS, and antibiotics. Next, GFP-expressing ESCs were seeded onto a MEF layer and cultured in medium consisting of Knockout DMEM (Thermo Fisher Scientific) supplemented with 15% ESC-qualified FBS (Thermo Fisher Scientific), 0.1 mM nonessential amino acids (Thermo Fisher Scientific), 2 mM L-glutamine (Thermo Fisher Scientific), 0.1 mM β-mercaptoethanol (Sigma-Aldrich, St. Louis, MO, USA), antibiotics, and 500 U/mL leukemia inhibitory factor (LIF; Chemicon) to maintain them in undifferentiated pluripotent state.
C2C12 myoblasts (European Collection of Cell Cultures ECACC no. 91031101, passage no. 13) were cultured in medium supporting their proliferation, i.e., high-glucose DMEM supplemented with 10% FBS and antibiotics. After 3 days of culture, this medium was switched to the one promoting myoblast differentiation and fusion—i.e., DMEM supplemented with 5% horse serum (HS, Thermo Fisher Scientific), and antibiotics [
30].
Primary mouse myoblasts were obtained as a result of differentiation of satellite cells isolated from hind limb muscles: flexor digitorum brevis, extensor digitorum longus, tibialis anterior, and Soleus, as described previously [
31]. Briefly, muscles were dissected from the hind limb of 3-month-old C57BL6N males. Isolated muscles were digested with 0.2% type I collagenase (Sigma Aldrich) in low-glucose DMEM at 37 °C for 90 min. Single myofibers were obtained by gentle trituration of muscle after digestion. Next, satellite cells were liberated from myofibers with a 22 G needle and plated onto dishes coated with Matrigel (BD Biosciences), diluted 1:10 in low-glucose DMEM. Satellite cell derived primary myoblasts were cultured in low-glucose DMEM supplemented with 10% HS, 20% FBS, 0.5% Chicken Embryo Extract (SeraLab, Haywards Heath, West Sussex, UK), and antibiotics on matrigel-coated dishes under standard conditions (37 °C, 5% CO
2).
4.3. In Vitro Differentiation of ESCs
GFP-expressing ESCs were differentiated in embryoid bodies (EBs). EBs were generated using the hanging drops technique. ESCs were separated from MEFs by the pre-plating procedure. Briefly, ESC and MEF suspension was plated on gelatin-coated dishes and incubated at 37 °C for 20 min. After the incubation, unattached ESCs were transferred to a new culture plate. The procedure was repeated thrice. Next, 30 μL drops of medium for ESC culture, described above but devoid of LIF and supplemented with 15% Serum Replacement (SR, Thermo Fisher Scientific) instead of FBS and containing 800 ESCs were placed onto the bottom of covers of culture dishes filled with phosphate-buffered saline (PBS). At day 2 of culture in hanging drops, EBs were transferred to low-adhesive culture dishes (Medlab, Raszyn, Poland), allowing their culture in suspension for further 3 or 5 days. After 2, 5, of 7 days of differentiation, EBs, referred to as EB2, EB5, or EB7, respectively, were collected for further analysis.
4.4. ESC and C2C12 Myoblast Co-Culture
Before co-culture experiments, GFP-expressing ESCs were separated from MEFs by the preplating method. Next, 15 × 103 ESCs were collected and seeded onto 15 × 103 fusing C2C12 myoblasts (at day 4 of C2C12 myoblast culture). Differentiating C2C12 myoblasts cultured with 15 × 103 proliferating C2C12 myoblasts or with 15 × 103 MEFs served as a positive or negative control, respectively. In control variants, proliferating C2C12 myoblasts or MEFs were labeled with the Cell TrackerTM Orange CMRA reagent according to the manufacturer’s protocol (Thermo Fisher Scientific) prior to seeding on the layer of differentiating C2C12 myoblasts. Co-cultures were conducted in high-glucose DMEM supplemented with 5% HS and antibiotics. Cells were analyzed at day 3, 6, or 9. Cells fixed with cold methanol were stained with Giemsa and May-Grunwald dyes, while cells fixed with 3% PFA were subjected to immunostaining analysis.
4.5. IL-4 Treatment
All co-culture variants (C2C12-ESC, C2C12-C2C12, and C2C12-MEF) were cultured in high-glucose DMEM supplemented with 5% HS, antibiotics, and mouse IL-4 recombinant protein (Sigma-Aldrich) at final concentration of 5 ng/mL. Additionally, C2C12-ESC co-cultures were cultured in the presence of 5 ng/mL IL-4 and either 5 µg/mL or 10 µg/mL of anti-IL-4 antibody (I7034 Sigma Aldrich), or 5 ng/mL or 10 ng/mL of anti-IL-4Rα antibody (NBP1-00884 Novus Biologicals; Centennial, CO, USA). In control variants, cells were co-cultured in the presence of IL-4 and control antibody (donkey anti-rabbit IgG Thermo Fisher Scientific) or in the presence of antibodies only. Cells were collected and analyzed at day 3, 6, or 9 of IL-4 treatment. In all experiments, cells cultured without exogenous IL-4 served as control.
For the experiments on IL-4 influence on in vitro ESC differentiation, ESCs and EBs were cultured in the medium supplemented with 15% SR, as described above, with exogenous IL-4 at the final concentration of 5 ng/mL. Medium was changed at day 2 and 5. Additionally, ESCs and EBs were cultured in the presence of 5 ng/mL IL-4 and either 5 µg/mL or 10 µg/mL of anti-IL-4 antibody (I7034 Sigma Aldrich), or 5 ng/mL or 10 ng/mL of anti-IL-4Rα antibody (NBP1-00884 Novus Biologicals). In control variants, cells were co-cultured in the presence of IL-4 and control antibody (donkey anti-rabbit IgG Thermo Fisher Scientific) or in the presence of antibodies only. EBs were collected and analyzed at day 2, 5, or 7. ESCs were collected and analyzed at day 2 of treatment. In all experiments, cells cultured without exogenous IL-4 served as control.
4.6. RNA Isolation and qPCR Analysis
Total RNA was isolated from undifferentiated GFP-expressing ESCs, EB2, EB5, EB7, C2C12, and primary myoblasts at four stages of their culture as well as from 13.5-day-old mouse embryos using a High Pure RNA Isolation Kit (Roche) and transcribed to cDNA with RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific) according to the manufacturer’s protocols. qPCR analysis was performed using specific TaqMan
® probes (Thermo Fischer Scientific): Mm012575139 (
Il4rα), Mm00446726 (
Il13rα1), Mm02019550_s1 (
Nanog), Mm01222421 (
Flk1), Mm00440701 (
Pdgfrα), Mm00490407_s1 (
Msgn1), Mm00435493_m1 (
Pax3), Mm01354484 (
Pax7), Mm0435125 (
Myf-5), Mm00440387_m1 (
Myod), Mm00483191_m1 (
Cdh15), Mm01320970_m1 (
Vcam1), Mm01253230_m1 (
Itgb1), Mm01277951_m1 (
Itga4), Mm00475770_m1 (
Adam9), Mm00514275_g1 (
Cd9), Mm01205647_g1 (
Actb), the TaqMan Gene Expression Master Mix (Thermo Fischer Scientific) and LightCycler 96 instrument (Roche). Data were collected and analyzed with LightCycler 96 SW1.1 software (Roche). For each analysis, three independent experiments were performed. ΔΔCt analysis was performed according to Livak and Schmittgen [
32].
4.7. Index of Fusion
Control or IL-4-treated co-cultures of differentiating C2C12 myoblasts and either ESCs or MEFs or proliferating C2C12 myoblasts were fixed in cold methanol for 10 min at 4 °C at day 3, 6, or 9 of the co-culture. Next, cells were stained with Giemsa and May–Grunwald dyes according to the manufacturer’s protocol (Merck, Darmstadt, Germany) and examined with 20× objectives on Eclipse TE200 microscope (Nikon) in order to calculate the index of fusion. The index of fusion is the ratio of nuclei localized in multinucleated myotubes compared to the number of all observed nuclei. Images were taken with DXM 1200 digital camera and analyzed using NIS Elements F 2.30 software (Nikon, Tokyo, Japan). Ten representative fields of view of control and IL-4-treated co-cultures at each time point were analyzed.
4.8. Immunolocalization
To visualize IL-4, undifferentiated ESCs and myoblasts were incubated with Brefeldin A (Sigma-Aldrich) at concentration of 10 µg/mL prior to the fixation, at 37 °C for 3 h and fixed with 3% PFA at room temperature for 10 min. This procedure enables studying secreted proteins within fixed cells. Subsequently, all analyzed cell types fixed with 3% PFA were permeabilized with 0.1% Triton-X 100 (Sigma-Aldrich) at room temperature for 5 min. Nonspecific antibody binding was blocked by incubation in 3% bovine serum albumin (BSA, Sigma-Aldrich) in PBS for 1 h at room temperature. Next, primary antibodies were diluted in 0.5% BSA in PBS and incubated with cells at 4 °C overnight. Primary antibodies against the following antigens were used: IL-4Rα (diluted 1:50, Novus Biologicals NBP1-00884), IL-13Rα1 (diluted 1:50, Novus Biologicals NBP1-61690), IL-4 (diluted 1:50, Novus Biologicals NB100-64798), Pax3 (diluted 1:50, Developmental Studies Hybridoma Bank; Iowa City, IA, USA), skeletal muscle marker (12/101; diluted 1:50, Developmental Studies Hybridoma Bank). Afterwards, specimens were incubated with appropriate secondary antibodies conjugated with Alexa Fluor 594 (Thermo Fisher Scientific) diluted 1:200 in 0.5% BSA in PBS for 1.5 h, and DRAQ5 diluted 1:1000 in PBS at room temperature for 10 min. Specimens were washed twice with PBS and mounted using Fluorescent Mounting Medium (Dako, Glostrup, Denmark). The specimens were analyzed using Axiovert 100 M scanning confocal microscope (Zeiss, Jena, Germany) with LSM 510 software.
4.9. FACS Analysis
For FACS analysis, ESCs were trypsynized and purified from MEFs using the preplating method and EBs were disaggregated with Enzyme-Free Hanks’-based Cell Dissociation Buffer (Thermo Fisher Scientific). The obtained single cell suspension was incubated with 0.1% BSA and 1% FBS in PBS at room temperature for 30 min. Next, cells were incubated with primary antibodies on ice for 30 min. Antibodies against following antigens were used: IL-4Rα (diluted 1:50, Novus Biologicals NBP1-00884, Centennial, CO, USA), IL-13Rα1 (diluted 1:50, Novus Biologicals NBP1-61690), and VCAM1 (diluted 1:100, R&D Systems MAB6432, Minneapolis, MN, USA). Cells were washed with PBS once. Afterwards, cells were incubated with appropriate secondary antibody conjugated with APC-A (diluted 1:100, Thermo Fisher Scientific) at 4 °C for 30 min. Cells were fixed with 0.1% PFA at 4 °C for 10 min and analyzed with LSRFortessa cytometer (Becton Dickinson, Warsaw, Poland) and Diva 6.1 software.
4.10. IL-4 Concentration
IL-4 concentration in collected culture supernatants was studied by ELISA analysis with Quantikine® ELISA (R&D Systems, M4000B) according to the manufacturer’s protocol. The plate was analyzed with Gen5 Microplate Reader and Image software.
4.11. Statistical Analysis
All experiments were performed at least three times. Data are presented as mean ± standard deviation (SD) from three independent experiments, and Student’s t-test was used for statistical analysis, p < 0.05 was considered statistically significant and marked with asterisks.